The Positive Association between Muscle Mass and Bone Status Is Conserved in Men with Diabetes: A Retrospective Cross-Sectional and Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
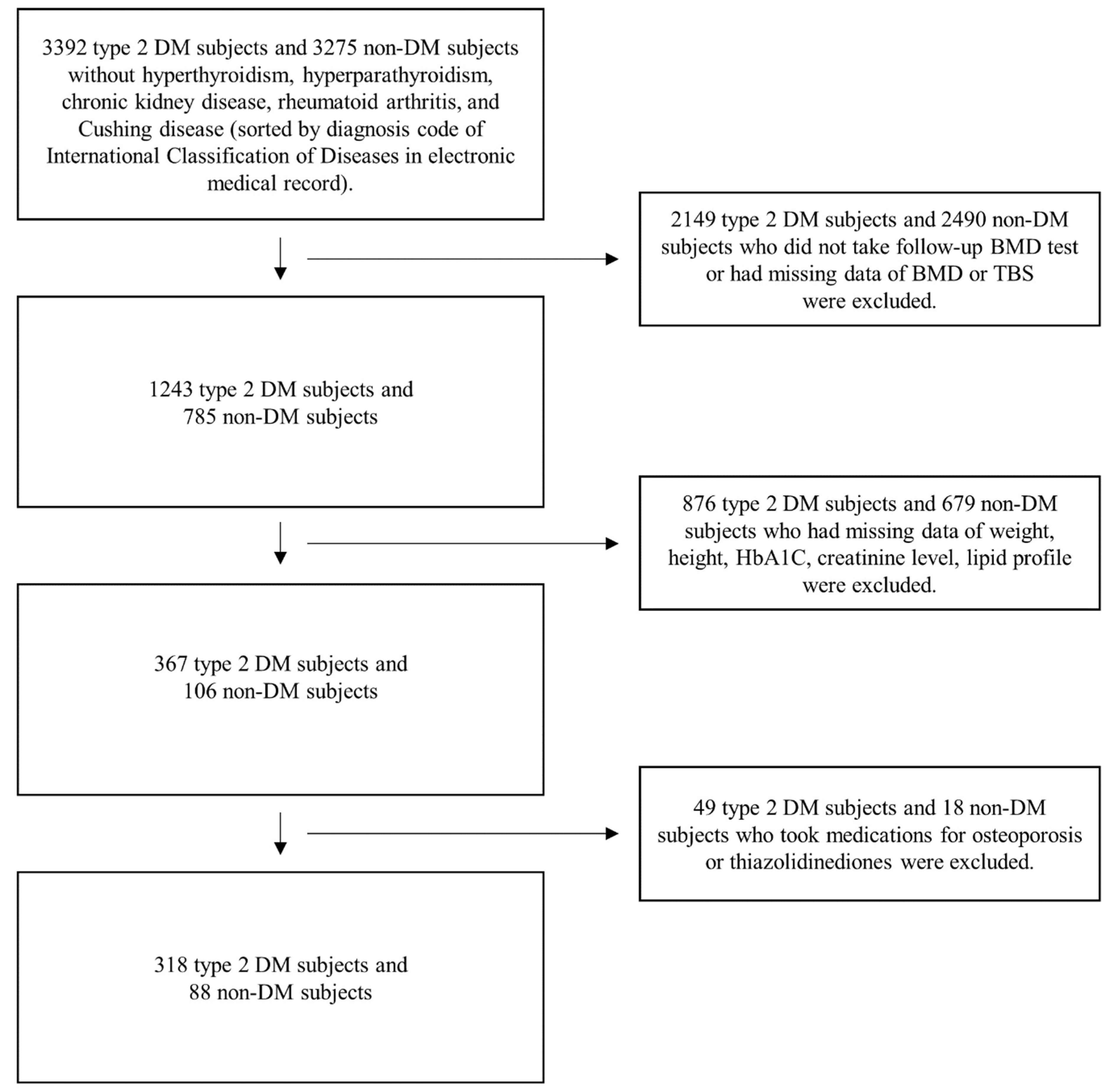
2.1. Study Design and Population
2.2. Data Collection
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Basal Characteristics of DM and Non-DM Groups
3.2. Relationship between Muscle and Bone-Related Parameters Based on Initial Measurement Data
3.3. Relationship between the Changes in Muscle Mass and Bone-Related Parameters
4. Discussion
4.1. Summary of Findings
4.2. Muscle Mass and Bone-Related Parameters in Male Group
4.3. Differences between the DM and Non-DM Groups
4.4. Association between the Changes in Muscle and Bone Parameters
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Ethgen, O.; Beaudart, C.; Buckinx, F.; Bruyere, O.; Reginster, J.Y. The Future Prevalence of Sarcopenia in Europe: A Claim for Public Health Action. Calcif. Tissue Int. 2017, 100, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; O’Neill, T.; Silman, A. The epidemiology of vertebral fractures. European Vertebral Osteoporosis Study Group. Bone 1993, 14, 89–97. [Google Scholar] [CrossRef]
- Cheung, C.L.; Ang, S.B.; Chadha, M.; Chow, E.S.; Chung, Y.S.; Hew, F.L.; Jaisamrarn, U.; Ng, H.; Takeuchi, Y.; Wu, C.H.; et al. An updated hip fracture projection in Asia: The Asian Federation of Osteoporosis Societies study. Osteoporos. Sarcopenia 2018, 4, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, O.; Beaudart, C.; Ethgen, O.; Reginster, J.Y.; Locquet, M. The health economics burden of sarcopenia: A systematic review. Maturitas 2019, 119, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Ondera, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef]
- Maurel, D.B.; Jahn, K.; Lara-Castillo, N. Muscle-Bone Crosstalk: Emerging Opportunities for Novel Therapeutic Approaches to Treat Musculoskeletal Pathologies. Biomedicines 2017, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Brotto, M.; Bonewald, L. Bone and muscle: Interactions beyond mechanical. Bone 2015, 80, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Muscles and their myokines. J. Exp. Biol. 2011, 214, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W. A role for myokines in muscle-bone interactions. Exerc. Sport Sci. Rev. 2011, 39, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Sealand, R.; Razavi, C.; Adler, R.A. Diabetes mellitus and osteoporosis. Curr. Diabetes Rep. 2013, 13, 411–418. [Google Scholar] [CrossRef]
- Bonds, D.E.; Larson, J.C.; Schwartz, A.V.; Strotmeyer, E.S.; Robbins, J.; Rodriguez, B.L.; Johnson, K.C.; Margolis, K.L. Risk of fracture in women with type 2 diabetes: The Women’s Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 2006, 91, 3404–3410. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, D.H.; Lee, E.K.; Kim, N.D.; Im, D.S.; Lee, J.; Yu, B.P.; Chung, H.Y. Age-related inflammation and insulin resistance: A review of their intricate interdependency. Arch. Pharm. Res. 2014, 37, 1507–1514. [Google Scholar] [CrossRef]
- Agostini, D.; Donati Zeppa, S.; Lucertini, F.; Annibalini, G.; Gervasi, M.; Ferri Marini, C.; Piccoli, G.; Stocchi, V.; Barbieri, E.; Sestili, P. Muscle and Bone Health in Postmenopausal Women: Role of Protein and Vitamin D Supplementation Combined with Exercise Training. Nutrients 2018, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.J.; Chang, M.C. Prevalence of Sarcopenia Among the Elderly in Korea: A Meta-Analysis. J. Prev. Med. Public Health 2021, 54, 96–102. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice, C. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Kwak, M.K.; Kim, B.J.; Kim, J.S.; Lee, S.H.; Koh, J.M. The Local and Systemic Interactions Between Muscle and Bone in Postmenopausal Korean Women. Calcif. Tissue Int. 2019, 105, 373–382. [Google Scholar] [CrossRef]
- Christiansen, B.A.; Kopperdahl, D.L.; Kiel, D.P.; Keaveny, T.M.; Bouxsein, M.L. Mechanical contributions of the cortical and trabecular compartments contribute to differences in age-related changes in vertebral body strength in men and women assessed by QCT-based finite element analysis. J. Bone Miner. Res. 2011, 26, 974–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parenteau, C.S.; Lau, E.C.; Campbell, I.C.; Courtney, A. Prevalence of spine degeneration diagnosis by type, age, gender, and obesity using Medicare data. Sci. Rep. 2021, 11, 5389. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S. Pathogenesis of Osteoporosis. Transl. Endocrinol. Metab. 2010, 1, 55–86. [Google Scholar] [PubMed]
- Heikkinen, J.; Kyllonen, E.; Kurttila-Matero, E.; Wilen-Rosenqvist, G.; Lankinen, K.S.; Rita, H.; Väänänen, H.K. HRT and exercise: Effects on bone density, muscle strength and lipid metabolism. A placebo controlled 2-year prospective trial on two estrogen-progestin regimens in healthy postmenopausal women. Maturitas 1997, 26, 139–149. [Google Scholar] [CrossRef]
- Skelton, D.A.; Phillips, S.K.; Bruce, S.A.; Naylor, C.H.; Woledge, R.C. Hormone replacement therapy increases isometric muscle strength of adductor pollicis in post-menopausal women. Clin. Sci. 1999, 96, 357–364. [Google Scholar] [CrossRef]
- Kim, I.J.; Kang, K.Y. Low Skeletal Muscle Mass is Associated with the Risk of Low Bone Mineral Density in Urban Dwelling Premenopausal Women. Calcif. Tissue Int. 2017, 101, 581–592. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Rodriguez-Manas, L.; Sinclair, A.J. Frailty, sarcopenia and diabetes. J. Am. Med. Dir. Assoc. 2014, 15, 853–859. [Google Scholar] [CrossRef]
- Garcia-Hernandez, A.; Arzate, H.; Gil-Chavarria, I.; Rojo, R.; Moreno-Fierros, L. High glucose concentrations alter the biomineralization process in human osteoblastic cells. Bone 2012, 50, 276–288. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K. Collagen cross-links as a determinant of bone quality: A possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 2010, 21, 195–214. [Google Scholar] [CrossRef]
- Aleman-Mateo, H.; Lopez Teros, M.T.; Ramirez, F.A.; Astiazaran-Garcia, H. Association between insulin resistance and low relative appendicular skeletal muscle mass: Evidence from a cohort study in community-dwelling older men and women participants. J. Gerontol. A Biol. Sci. Med. Sci 2014, 69, 871–877. [Google Scholar] [CrossRef]
- Hwang, H.; Bowen, B.P.; Lefort, N.; Flynn, C.R.; De Filippis, E.A.; Roberts, C.; Smoke, C.C.; Meyer, C.; Højlund, K.; Yi, Z. Proteomics analysis of human skeletal muscle reveals novel abnormalities in obesity and type 2 diabetes. Diabetes 2010, 59, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, M.; Saxena, M. Interleukin-1 (IL-1) family of cytokines: Role in type 2 diabetes. Clin. Chim. Acta 2012, 413, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526.E9–526.E17. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Joseph, A.M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Brennan-Speranza, T.C.; Levinger, I.; Yeap, B.B. Undercarboxylated Osteocalcin: Experimental and Human Evidence for a Role in Glucose Homeostasis and Muscle Regulation of Insulin Sensitivity. Nutrients 2018, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Fatayerji, D.; Eastell, R. Age-related changes in bone turnover in men. J. Bone Miner. Res. 1999, 14, 1203–1210. [Google Scholar] [CrossRef]
- Levinger, I.; Zebaze, R.; Jerums, G.; Hare, D.L.; Selig, S.; Seeman, E. The effect of acute exercise on undercarboxylated osteocalcin in obese men. Osteoporos. Int. 2011, 22, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.; Goodpaster, B.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Sarcopenia: Alternative definitions and associations with lower extremity function. J. Am. Geriatr Soc. 2003, 51, 1602–1609. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, H.J.; Kim, M.J.; Shin, C.S.; Cho, N.H. Fat mass is negatively associated with bone mineral content in Koreans. Osteoporos. Int. 2012, 23, 2009–2016. [Google Scholar] [CrossRef]
- Tallis, J.; James, R.S.; Seebacher, F. The effects of obesity on skeletal muscle contractile function. J. Exp. Biol. 2018, 221, jeb163840. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimpur, M.; Sharifi, F.; Nezhad, F.A.; Bagherzadeh, M.; Ostovar, A.; Shafiee, G.; Heshmat, R.; Mehrdad, N.; Razi, F.; Khashayar, P.; et al. Effect of diabetes on BMD and TBS values as determinants of bone health in the elderly: Bushehr Elderly Health program. J. Diabetes Metab. Disord. 2019, 18, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Janghorbani, M.; Van Dam, R.M.; Willett, W.C.; Hu, F.B. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am. J. Epidemiol. 2007, 166, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., 3rd; Riggs, B.L.; Leibson, C.L.; Achenbach, S.J.; Camp, J.J.; Bouxsein, M.L.; Atkinson, E.J.; Robb, R.A.; Khosla, S. A bone structural basis for fracture risk in diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 4804–4809. [Google Scholar] [CrossRef]
- Reid, I.R. Relationships among body mass, its components, and bone. Bone 2002, 31, 547–555. [Google Scholar] [CrossRef]
- Rosen, C.J.; Bouxsein, M.L. Mechanisms of disease: Is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2006, 2, 35–43. [Google Scholar] [CrossRef]
- Hans, D.; Stenova, E.; Lamy, O. The Trabecular Bone Score (TBS) Complements DXA and the FRAX as a Fracture Risk Assessment Tool in Routine Clinical Practice. Curr. Osteoporos. Rep. 2017, 15, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Williams-Russo, P.; Healey, J.H.; Szatrowski, T.P.; Schneider, R.; Paget, S.; Ales, K.; Schwartzberg, P. Clinical reproducibility of dual energy X-ray absorptiometry. J. Orthop. Res. 1995, 13, 250–257. [Google Scholar] [CrossRef]
- Haddaway, M.J.; Davie, M.W.; McCall, I.W. Bone mineral density in healthy normal women and reproducibility of measurements in spine and hip using dual-energy X-ray absorptiometry. Br. J. Radiol 1992, 65, 213–217. [Google Scholar] [CrossRef]
- Leslie, W.D.; Ward, L.M. Bone density monitoring with the total hip site: Time for a re-evaluation? J. Clin. Densitom. 2004, 7, 269–274. [Google Scholar] [CrossRef]
- Johannesdottir, F.; Poole, K.E.; Reeve, J.; Siggeirsdottir, K.; Aspelund, T.; Mogensen, B.; Jonsson, B.Y.; Sigurdsson, S.; Harris, T.B.; Gudnason, V.G.; et al. Distribution of cortical bone in the femoral neck and hip fracture: A prospective case-control analysis of 143 incident hip fractures; the AGES-REYKJAVIK Study. Bone 2011, 48, 1268–1276. [Google Scholar] [CrossRef] [Green Version]
- Achamrah, N.; Colange, G.; Delay, J.; Rimbert, A.; Folope, V.; Petit, A.; Grigioni, S.; Déchelotte, P.; Coëffier, M. Comparison of body composition assessment by DXA and BIA according to the body mass index: A retrospective study on 3655 measures. PLoS ONE 2018, 13, e0200465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | DM (n = 318) | Non-DM (n = 88) | p Value |
|---|---|---|---|
| Age (years) | 52.000 (45.750–59.250) | 46.500 (36.000–53.000) | <0.001 |
| Height (cm) | 169.600 (166.100–173.125) | 172.250 (167.550–176.275) | 0.002 |
| Weight (kg) | 73.400 (66.075–82.025) | 70.000 (61.725–76.975) | 0.002 |
| BMI (kg/m2) | 25.450 (23.700–28.025) | 24.000 (20.650–25.275) | <0.001 |
| ASMI (kg/m2) | 7.775 (7.133–8.333) | 7.341 (6.752–8.098) | 0.002 |
| Lumbar spine BMD (g/cm2) | 1.141 (1.024–1.268) | 0.994 (0.905–1.182) | <0.001 |
| Femoral neck BMD (g/cm2) | 0.962 (0.864–1.059) | 0.919 (0.829–1.028) | 0.042 |
| Total hip BMD (g/cm2) | 1.041 (0.934–1.144) | 0.951 (0.842–1.030) | <0.001 |
| Lumbar spine T-score | −0.326 (−1.300–0.730) | −1.512 (−2.265–0.027) | <0.001 |
| Femoral neck T-score | 0.090 (−0.658–0.841) | −0.161 (−0.930–0.599) | 0.050 |
| Total hip T-score | 0.766 (−0.051–1.565) | 0.074 (−0.764–0.688) | <0.001 |
| TBS | 1.458 (1.396−1.517) | 1.460 (1.407–1.520) | 0.454 |
| Creatinine (mg/dL) | 0.990 (0.900–1.140) | 1.000 (0.900–1.120) | 0.950 |
| TG (mg/dL) | 137.500 (95.750–189.750) | 98.500 (68.000–162.250) | <0.001 |
| HDL-C (mg/dL) | 42.000 (36.000–51.000) | 51.000 (43.500–60.000) | <0.001 |
| Total cholesterol (mg/dL) | 161.000 (136.000–187.000) | 170.000 (150.250–197.750) | 0.013 |
| HbA1C (%) | 7.600 (7.000–9.125) | - | - |
| Osteoporosis, n (%) | 9 (2.8%) | 14 (15.9%) | <0.001 |
| ASMI of DM (n = 318) | ASMI of Non-DM (n = 88) | |||
|---|---|---|---|---|
| Variables | γ | p | γ | p |
| Initial lumbar spine BMD | 0.163 | 0.004 | 0.313 | 0.003 |
| Initial femoral neck BMD | 0.379 | <0.001 | 0.425 | <0.001 |
| Initial total hip BMD | 0.412 | <0.001 | 0.452 | <0.001 |
| Initial TBS | 0.238 | <0.001 | 0.366 | <0.001 |
| Age | −0.256 | <0.001 | −0.030 | 0.779 |
| BMI | 0.835 | <0.001 | 0.778 | <0.001 |
| HbA1C | −0.054 | 0.335 | −0.013 | 0.903 |
| Creatinine | 0.003 | 0.956 | 0.248 | 0.020 |
| TG | 0.046 | 0.418 | 0.170 | 0.113 |
| HDL-C | −0.075 | 0.183 | −0.301 | 0.004 |
| ASMI of DM (n = 318) | ASMI of Non-DM (n = 88) | ||||||
|---|---|---|---|---|---|---|---|
| Variables | β | t | p | β | t | p | |
| Initial lumbar spine BMD | Model 1 | 0.174 | 3.047 | 0.003 | 0.302 | 2.798 | 0.006 |
| Model 2 | 0.187 | 1.779 | 0.076 | 0.481 | 2.878 | 0.005 | |
| Initial femoral neck BMD | Model 1 | 0.331 | 6.185 | <0.001 | 0.409 | 3.798 | <0.001 |
| Model 2 | 0.389 | 3.963 | <0.001 | 0.622 | 3.759 | <0.001 | |
| Initial total hip BMD | Model 1 | 0.405 | 7.554 | <0.001 | 0.428 | 4.103 | <0.001 |
| Model 2 | 0.487 | 4.957 | <0.001 | 0.663 | 4.157 | <0.001 | |
| Initial TBS | Model 1 | 0.203 | 3.623 | <0.001 | 0.357 | 3.235 | 0.002 |
| Model 2 | 0.313 | 3.052 | 0.002 | 0.464 | 2.698 | 0.008 | |
| Variables | B | Std.err | Wald | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Dependent | Independent (ΔASMI) | DM | Non-DM | DM | Non-DM | DM | Non-DM | DM | Non-DM |
| (n = 318) | (n = 88) | (n = 318) | (n = 88) | (n = 318) | (n = 88) | (n = 318) | (n = 88) | ||
| ΔLumbar spine BMD | Model 1 | 0.011 | 0.015 | 0.007 | 0.014 | 2.950 | 1.055 | 0.086 | 0.304 |
| Model 2 | 0.009 | 0.006 | 0.010 | 0.006 | 0.919 | 1.014 | 0.338 | 0.314 | |
| ΔFemoral neck BMD | Model 1 | 0.006 | 0.012 | 0.005 | 0.011 | 1.081 | 1.213 | 0.298 | 0.271 |
| Model 2 | 0.005 | 0.004 | 0.007 | 0.004 | 0.390 | 1.314 | 0.532 | 0.252 | |
| ΔTotal hip BMD | Model 1 | 0.012 | 0.015 | 0.004 | 0.011 | 7.432 | 2.003 | 0.006 | 0.157 |
| Model 2 | 0.013 | 0.009 | 0.006 | 0.005 | 5.761 | 3.407 | 0.016 | 0.065 | |
| ΔTBS | Model 1 | 0.011 | 0.019 | 0.005 | 0.005 | 4.229 | 17.399 | 0.040 | <0.001 |
| Model 2 | 0.024 | 0.020 | 0.012 | 0.005 | 3.980 | 14.431 | 0.046 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, H.U.; Han, S.J.; Kim, H.J.; Chung, Y.-S.; Kim, D.J.; Choi, Y.J. The Positive Association between Muscle Mass and Bone Status Is Conserved in Men with Diabetes: A Retrospective Cross-Sectional and Longitudinal Study. J. Clin. Med. 2022, 11, 5370. https://doi.org/10.3390/jcm11185370
Moon HU, Han SJ, Kim HJ, Chung Y-S, Kim DJ, Choi YJ. The Positive Association between Muscle Mass and Bone Status Is Conserved in Men with Diabetes: A Retrospective Cross-Sectional and Longitudinal Study. Journal of Clinical Medicine. 2022; 11(18):5370. https://doi.org/10.3390/jcm11185370
Chicago/Turabian StyleMoon, Hyun Uk, Seung Jin Han, Hae Jin Kim, Yoon-Sok Chung, Dae Jung Kim, and Yong Jun Choi. 2022. "The Positive Association between Muscle Mass and Bone Status Is Conserved in Men with Diabetes: A Retrospective Cross-Sectional and Longitudinal Study" Journal of Clinical Medicine 11, no. 18: 5370. https://doi.org/10.3390/jcm11185370
APA StyleMoon, H. U., Han, S. J., Kim, H. J., Chung, Y.-S., Kim, D. J., & Choi, Y. J. (2022). The Positive Association between Muscle Mass and Bone Status Is Conserved in Men with Diabetes: A Retrospective Cross-Sectional and Longitudinal Study. Journal of Clinical Medicine, 11(18), 5370. https://doi.org/10.3390/jcm11185370






