Can Quality Improvement Methodologies Derived from Manufacturing Industry Improve Care in Cardiac Surgery? A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
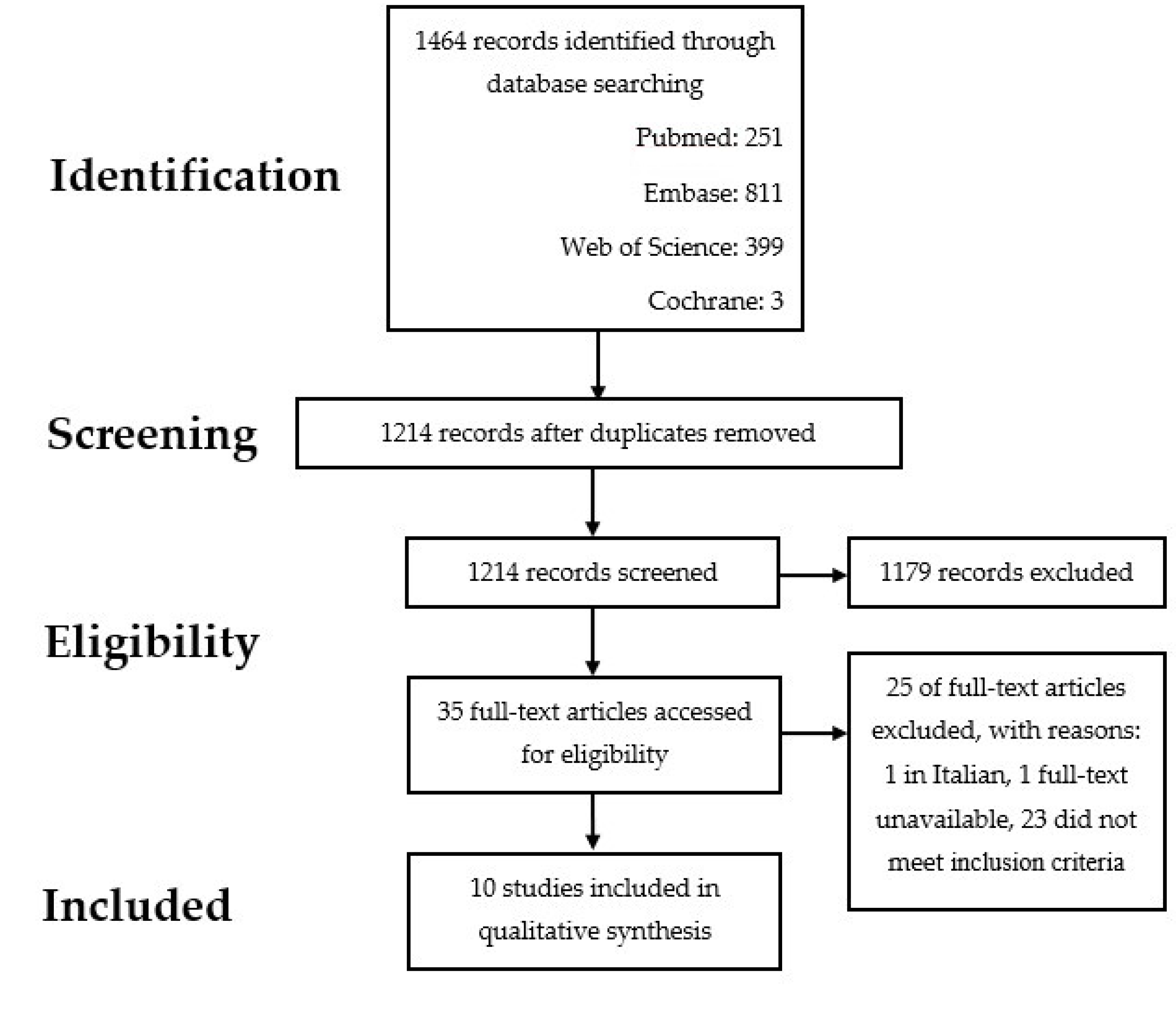
2.2. Selection Process
2.3. Data Assessment
2.4. Data Analysis
2.5. Assessment of Risk of Bias in Included Studies
2.6. Quality Improvement Methodologies
3. Results
3.1. Descriptive Synthesis of the Results
3.2. Patient Related Outcomes
3.3. Process-Related Outcomes
3.4. Patient and Staff Reported Outcomes
3.5. Financial Performance
3.6. Risk of Bias Assessment
3.7. Relevant Disclosures and Conflicts of Interests
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kaplan, R.S.; Porter, M.E. How to solve the cost crisis in health care. Harv. Bus. Rev. 2011, 89, 46–52. [Google Scholar] [PubMed]
- Fereday, S. A Guide to Quality Improvement Methods; Healthcare Quality Improvement Partnership Ltd (HQIP): London, UK, 2015. [Google Scholar]
- The Health Foundation. Quality Improvement Made Simple: What everyone should Know about Quality Improvement. The Health Foundation. 2013. Available online: https://www.health.org.uk/sites/default/files/QualityImprovementMadeSimple.pdf (accessed on 1 March 2021).
- Jones, B.; Vaux, E.; Olsson-Brown, A. How to get started in quality improvement. BMJ 2019, 364, k5408. [Google Scholar] [CrossRef] [PubMed]
- Dixon-Woods, M.; McNicol, S.; Martin, G. Ten challenges in improving quality in healthcare: Lessons from the Health Foundation’s programme evaluations and relevant literature. BMJ Qual. Saf. 2012, 21, 876–884. [Google Scholar] [CrossRef]
- De Koning, H.; Verver, J.P.; van den Heuvel, J.; Bisgaard, S.; Does, R.J. Lean Six Sigma in healthcare. J. Healthc. Qual. 2006, 28, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.C.; Blumenthal, E.Z. Implementation of Lean and Six Sigma principles in ophthalmology for improving quality of care and patient flow. Surv. Ophthalmol. 2019, 64, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Collar, R.M.; Shuman, A.G.; Feiner, S.; McGonegal, A.K.; Heidel, N.; Duck, M.; McLean, S.A.; Billi, J.E.; Healy, D.W.; Bradford, C.R. Lean management in academic surgery. J. Am. Coll. Surg. 2012, 214, 928–936. [Google Scholar] [CrossRef]
- Brown, R.; Grehan, P.; Brennan, M.; Carter, D.; Brady, A.; Moore, E.; Teeling, S.P.; Ward, M.; Eaton, D. Using Lean Six Sigma to improve rates of day of surgery admission in a national thoracic surgery department. Int. J. Qual. Health Care 2019, 31, 14–21. [Google Scholar] [CrossRef]
- Mason, S.E.; Nicolay, C.R.; Darzi, A. The use of Lean and Six Sigma methodologies in surgery: A systematic review. Surgeon 2015, 13, 91–100. [Google Scholar] [CrossRef]
- Nicolay, C.R.; Purkayastha, S.; Greenhalgh, A.; Benn, J.; Chaturvedi, S.; Phillips, N.; Darzi, A. Systematic review of the application of Quality Improvement Methodologies from the manufacturing industry to surgical healthcare. Br. J. Surg. 2012, 99, 324–335. [Google Scholar] [CrossRef]
- Cima, R.R.; Brown, M.J.; Hebl, J.R.; Moore, R.; Rogers, J.C.; Kollengode, A.; Amstutz, G.J.; Weisbrod, C.A.; Narr, B.J.; Claude Deschamps, C.; et al. Use of Lean and Six Sigma methodology to improve operating room efficiency in a high-volume tertiary-care academic medical center. J. Am. Coll. Surg. 2011, 213, 83–92. [Google Scholar] [CrossRef]
- Womack, J.P.; Jones, D.T.; Roos, D. The Machine That Changed the World; Simon and Schuster UK: London, UK, 2008. [Google Scholar]
- Liker, J. The Toyota Way: 14 Management Principles from the World’s Greatest Manufacturer: 14 Management Principles from the World’s Greatest Manufacturer; Mcgraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Engelman, D.T.; Ben Ali, W.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Fleitman, J.M.; King, T.E., Jr. Postoperative Complications among Patients Undergoing Cardiac Surgery. 2021. Available online: https://www.uptodate.com/contents/postoperative-complications-among-patients-undergoing-cardiac-surgery (accessed on 3 March 2021).
- Almashrafi, A.; Vanderbloemen, L. Quantifying the effect of complications on patient flow, costs and surgical throughputs. BMC Med. Inform. Decis. Mak. 2016, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Leiva, E.; Alvarado, P.; Dennis, R.J. Postoperative Atrial Fibrillation: Evaluation of its Economic Impact on the Costs of Cardiac Surgery. Braz. J. Cardiovasc. Surg. 2019, 34, 179–186. [Google Scholar] [CrossRef]
- Peterson, E.D.; Coombs, L.P.; Ferguson, T.B.; Shroyer, A.L.; DeLong, E.R.; Grover, F.L.; Edwards, F.H.; National Cardiac Database Investigators. Hospital variability in length of stay after coronary artery bypass surgery: Results from the Society of Thoracic Surgeon’s National Cardiac Database. Ann. Thorac. Surg. 2002, 74, 464–473. [Google Scholar] [CrossRef]
- Cowper, P.A.; DeLong, E.R.; Peterson, E.D.; Hannan, E.L.; Ray, K.T.; Racz, M.; Mark, D.B. Variability in cost of coronary bypass surgery in New York State: Potential for cost savings. Am. Heart J. 2002, 143, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Ferket, B.S.; Oxman, J.M.; Iribarne, A.; Gelijns, A.C.; Moskowitz, A.J. Cost-effectiveness analysis in cardiac surgery: A review of its concepts and methodologies. J. Thorac. Cardiovasc. Surg. 2018, 155, 1671–1681. [Google Scholar] [CrossRef]
- Keizman, E.; Ram, E.; Kachel, E.; Sternik, L.; Raanani, E. The impact of COVID-19 pandemic on cardiac surgery in Israel. J. Cardiothorac. Surg. 2020, 15, 294. [Google Scholar] [CrossRef]
- Mohamed Abdel Shafi, A.; Hewage, S.; Harky, A. The impact of COVID-19 on the provision of cardiac surgical services. J. Card. Surg. 2020, 35, 1295–1297. [Google Scholar] [CrossRef]
- Salenger, R.; Etchill, E.W.; Ad, N.; Matthew, T.; Alejo, D.; Glenn Whitman, G.; Lawton, J.S.; Lau, C.L.; Gammie, C.F.; Gammie, J.S. The Surge After the Surge: Cardiac Surgery Post-COVID-19. Ann. Thorac. Surg. 2020, 110, 2020–2025. [Google Scholar] [CrossRef]
- Sobolev, B.G.; Fradet, G.; Hayden, R.; Kuramoto, L.; Levy, A.R.; FitzGerald, M.J. Delay in admission for elective coronary-artery bypass grafting is associated with increased in-hospital mortality. BMC Health Serv. Res. 2008, 8, 185. [Google Scholar] [CrossRef]
- Head, S.J.; da Costa, B.R.; Beumer, B.; Stefanini, G.G.; Alfonso, F.; Clemmensen, P.M.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; et al. Adverse events while awaiting myocardial revascularization: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2017, 52, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, N. Better patient flow in cardiac and thoracic surgery could save £45m a year in England. BMJ 2018, 361, k1624. [Google Scholar] [CrossRef] [PubMed]
- Richens, D. Cardiothoracic Surgery Getting It Right the First Time (GRITFT) Programme National Specialty Report. 2018. Available online: https://gettingitrightfirsttime.co.uk/wp-content/uploads/2018/04/GIRFT-Cardiothoracic-Report-1.pdf (accessed on 21 June 2022).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Tetzlaff, J.; Tricco, A.C.; Sampson, M.; Altman, D. Epidemiology and reporting characteristics of systematic reviews. PLoS Med. 2007, 4, e78. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality in Nonrandomized Studies in Meta-Analyses. Available online: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp (accessed on 24 June 2022).
- Barlow, R.E.; Irony, T.Z. Foundations of Statistical Quality Control. In Current Issues in Statistical Inference: Essays in Honor of D. Basu; Ghosh, M., Pathak, P.K., Eds.; Institute of Mathematical Statistics: Hayward, CA, USA, 1992; pp. 99–112. [Google Scholar]
- Geoffrion, T.R.; Lynch, I.P.; Hsu, W.; Phelps, E.; Minhajuddin, A.; Tsai, E.; Timmons, A.; Greilich, P.E. An Implementation Science Approach to Handoff Redesign in a Cardiac Surgery Intensive Care Unit. Ann. Thorac. Surg. 2020, 109, 1782–1788. [Google Scholar] [CrossRef]
- Culig, M.H.; Kunkle, R.F.; Frndak, D.C.; Grunden, N.; Maher, T.D., Jr.; Magovern, G.J., Jr. Improving patient care in cardiac surgery using Toyota Production System based methodology. Ann. Thorac. Surg. 2011, 91, 394–399. [Google Scholar] [CrossRef]
- Kles, C.L.; Murrah, C.P.; Smith, K.; Baugus-Wellmeier, E.; Hurry, T.; Morris, C.D. Achieving and Sustaining Zero: Preventing Surgical Site Infections After Isolated Coronary Artery Bypass with Saphenous Vein Harvest Site Through Implementation of a Staff-Driven Quality Improvement Process. Dimens. Crit. Care Nurs. 2015, 34, 265–272. [Google Scholar] [CrossRef]
- Gutsche, J.T.; Erickson, L.; Ghadimi, K.; Augoustides, J.G.; Dimartino, J.; Szeto, W.Y.; Ochroch, E.A. Advancing extubation time for cardiac surgery patients using Lean work design. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1490–1496. [Google Scholar] [CrossRef]
- Lytsy, B.; Lindblom, R.P.; Ransjö, U.; Leo-Swenne, C. Hygienic interventions to decrease deep sternal wound infections following coronary artery bypass grafting. J. Hosp. Infect. 2015, 91, 326–331. [Google Scholar] [CrossRef]
- Watling, A.; Doucet, J.; Zohrabi, M.; Fedirko, J.; Hassan, A.; Lutchmedial, S.; MacLeod, J.; Pozeg, Z.; Brown, C.; Légaré, J.F. Impact on cardiac surgery volume of a comprehensive partnership with Integrated Health Solutions. Can. J. Surg. 2020, 63, E374–E382. [Google Scholar] [CrossRef]
- Van Tiel, F.H.; Elenbaas, T.W.; Voskuilen, B.M.; Verheggen, F.W.; Mochtar, B.; Stobberingh, E.E. Plan-do-study-act cycles as an instrument for improvement of compliance with infection control measures in care of patients after cardiothoracic surgery. J. Hosp. Infect. 2006, 62, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.A.; Doll, M.C.; McKinley, K.E.; Casale, A.S.; Bothe, A. ProvenCare: Quality 1improvement model for designing highly reliable care in cardiac surgery. Qual. Saf. Health Care 2009, 18, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Hefner, J.L.; Tripathi, R.S.; Abel, E.E.; Farneman, M.; Galloway, J.; Moffatt-Bruce, S.D. Quality Improvement Intervention to Decrease Prolonged Mechanical Ventilation After Coronary Artery Bypass Surgery. Am. J. Crit. Care 2016, 25, 423–430. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez, E.A.; Chavez-Valdez, R.; Holt, N.F.; Grogan, K.L.; Khalifeh, K.W.; Slater, T.; Winner, L.E.; Moyer, J.; Lehmann, C.U. Successful implementation of a perioperative glycemic control protocol in cardiac surgery: Barrier analysis and intervention using Lean Six Sigma. Anesth. Res Pr. 2011, 2011, 565069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Derksen, J.W.G.; May, A.M.; Koopman, M. The era of alternative designs to connect randomized clinical trials and real-world data. Nat Rev Clin Oncol. 2019, 16, 589. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Ding, J.; Guzzo, T.J. Improving Operating Room Efficiency. Curr. Urol. Rep. 2019, 20, 28. [Google Scholar] [CrossRef]
- Smith Michael, P.; Sandberg Warren, S.; Foss, J.; Kanda, M.; Barsoum, W.; Schubert, A. High-throughput Operating Room System for Joint Arthroplasties Durably Outperforms Routine Processes. Anesthesiology 2008, 109, 25–35. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Ferrari-Light, D.; Ren-Fielding, C.; Fielding, G.; Perry, N.; Rabinovich, A.; Saraceni, M.; Fitzpatrick, M.; Jain, S.; Pachter, L. Improving Operating Room Turnover Time in a New York City Academic Hospital via Lean. Ann. Thorac. Surg. 2019, 107, 1011–1016. [Google Scholar] [CrossRef]
- Cerfolio, R.J. Lean, Efficient, and Profitable Operating Rooms: How I Teach It. Ann. Thorac. Surg. 2018, 105, 991–993. [Google Scholar] [CrossRef]
- Barnett, M.L.; Olenski, A.R.; Jena, A.B. Patient Mortality During Unannounced Accreditation Surveys at US Hospitals. JAMA Intern Med. 2017, 177, 693–700. [Google Scholar] [CrossRef]
- Ogrinc, G.; Davies, L.; Goodman, D.; Batalden, P.; Davidoff, F.; Stevens, D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised publication guidelines from a detailed consensus process. BMJ Qual. Saf. 2016, 25, 986–992. [Google Scholar] [CrossRef] [PubMed]
| Author, Year, Country | Duration | Patients/Procedures | Objective | Quality Improvement Methodology | Main Interventions | Outcomes |
|---|---|---|---|---|---|---|
| Geoffrion et al. (2020) USA [33] | 24 months (January 2015–March 2018) | Hand-offs pre-intervention (n = 64) and post-intervention (n = 62) Number of fidelity measurements (overall conformance score) (n = 57) Number of provider satisfaction measurements in redesign phase (n = 82), after 6 months (n = 98) and after 2.5 years (n = 81) | Reduce handoff (transfer of care) time | Twelve steps implementation process in four phases (planning, engaging, executing and evaluating) QIM activities: process mapping, PDSA cycles and multiple rapid-cycle process improvements | Redesign and implementation of the handoff process, implementation of handoff bundle and team training | Reduced total handoff time (in room to completion) from 12.6 ± 3.6 to 10.7 ± 2.2 min (p < 0.014) ‘ Improved fidelity from 18.5 ± 4.0 to 32.8 ± 9.5 (p < 0.001) Improved provider satisfaction after 6 months (84 vs. 80 of 100, p <0.02) and 2.5 years (84 vs. 87 of 100, p = 0.133) |
| Culig et al. (2011) USA [34] | 28 months (March 2008–June 2010) | CABG (n = 253) | Improve patient outcomes, reduce costs and improve patient satisfaction | Toyota Production System QIM activities: team training, value stream mapping, pull methodology, root cause analysis, visual management, Kanban, standardisation, one-by-one processing, 5S: sort, set in order, shine, standardise and sustain, stand-up meetings | Daily stand-up meetings Collaborative bedside rounds Pre-operative briefing Intra-operative implementation of checklist, ultrasonographic aortic imaging and cerebral oximetry, handoff standardisation Post-operative protocol for medication administration, extubation and glycaemic control | Lower risk-adjusted mortality/incidence of adverse events of 61%/57% than regional rate in Society of Thoracic Surgery database Costs savings of $884,000 for CABG ($3497 per CABG) |
| Kles et al. (2015) USA [35] | 32 months (May 2012–December 2014) | CABG (n = 262) | Reduce surgical site infection | Six Sigma (DMAIC) and Contextual model QIM activities: chart review, process mapping, direct observations of the process in real-time, flow-chart, standardisation, root cause analysis, contextual model | Infection prevention strategies: hair removal outside OR, routine use of mupirocin, glycaemic control, prophylactic antibiotic administration, antibiotic-impregnated sutures, soft silicone silver-impregnated dressing, dressing midsternal incision for 7 days | Reduction in incidence rate of surgical site infections from 3.74 to 0.7 per 100 procedures, and ultimately to 0 during 30 months and 590 procedures |
| Gutsche et al. (2014) USA [36] | 12 months (July 2011–July 2012) | Cardiac surgeries total (n = 404), pre-intervention (n = 195) and post-intervention (n = 171) | Improve rates of early extubation | Lean methodology QIM activities: spaghetti diagram, fishbone diagram, value stream mapping, root cause analysis, PDSA | Development of extubation guideline Countermeasures: usage of air warming blankets to prevent hypothermia, use of pain scale to titrate pain medication, treatment of hypertension with antihypertensive drugs (instead of opioids), improved weaning process and availability of equipment for extubation to prevent delays | Intervention predicted extubation in <6 h improved from 27% to 50% (p = 0.0001) Lower median length of intubation from 9.7 to 6.1 h (p = 0.0019) |
| Lytsy et al. (2015) Sweden [37] | 9 months (September 2009–July 2010) | CABG patients requiring surgical revision due to deep sternal wound infections pre-intervention (n = 80) and post-intervention (n = 13) | Illustrate that root cause analysis following by quality improvement can reduce DSWI after CABG | QIM activities: root cause analysis | Hygienic interventions in the pre-, intra- and post-operative care, e.g., hand gloves, disinfection, ultra cLean air, antibiotic prophylaxis, blood glucose control, wound dressing in place for three days | Deep sternal wound infection incidence per CABG operation decreased from 5.1% pre-intervention to 0.9% post-intervention |
| Watling et al. (2020) Canada [38] | 24 months (September 2016–2018) | Cardiac surgery (including TAVI) pre-intervention (n = 788) and post-intervention (n = 873) | Reduce waiting times | Lean methodology QIM activities: 5-day Kaizen (rapid improvement) workshop, impact-effort analysis, weekly dashboards | Fast-tracking from ICU to ward or bypassing the ICU Improved scheduling and listing Day of surgery admission Discharge protocol | Reduced wait time with 35% from median 52 to 35 days Increased annual number of surgical interventions from 788 to 873 (10.8%) An increase in cancellations of 7.5% due to limited ICU resources |
| Van Tiel et al. (2006) The Netherlands [39] | Not reported (Start–Autumn 2003) | CABG OR baseline (n = 116), follow-up (n = 248) and monitoring phase (n = 117) Ward baseline (n = 16), follow-up (n = 22) and monitoring phase (n = 18) | Improve compliance with infection control measures for the care of patients during and after cardiothoracic surgery | PDSA cycles | Instruction and training of correct hygienic procedures based on infection control in the OR and on the ward Feedback on the results of baseline measurement Use of posters in the OR Presence of QI team in the OR | Overall compliance score improved in the OR and surgical ward from baseline vs. follow-up phase vs. monitoring phase |
| Berry et al. (2009) USA [40] | 17 months (August 2005–February 2007) | CABG pre-intervention (n = 137) and post-intervention (n = 117) | To test whether process redesign by an integrated delivery system could implement evidence-based medical practices | ProvenCare programme QIM activities: multidisciplinary team meetings to review and validate best practice evidence, interview with patients, PDSA cycles | Implementation of 40 process elements (e.g., patient education materials, glycaemic control protocol, standard pre-operative anticoagulation protocol, diagnostics and medication, intra-operative time-out, documentation, antibiotic prophylaxis, and post-operative standardisation documentation, medical management, order sets) | Receiving all 40 elements in first month (59%) vs. post-intervention 100% (p = 0.001) Patient outcomes improved in 8 out of 9 measures (only discharge location to home significant) |
| Hefner et al. (2016) USA [41] | 12 months (January to June 2010–January to June 2011) | CABG surgeries pre-intervention (n = 68) and post-intervention (n = 58) | Reduce prolonged mechanical ventilation after CABG surgery | Lean methodology QIM activities: gap analysis, retrospective chart review, interviews with stakeholders and focus groups, root cause analysis, standardisation | Standardised extubating protocol Dry erase boards in patients’ room to facilitate team communication Edits of post-operative ICU order set to facilitate correct medication administration | Mechanical ventilation duration reduced from 11.4 h to 6.9 h (p <0.001) Number of patients reintubated reduced from 11.8% to 3.5% (p = 0.08) Rate of prolonged ventilation decreased from 29.4% to 8.6% (p = 0.004) |
| Martinez et al. (2011) USA [42] | 4 years (January 2003–March 2007) | Cardiac surgery patients admitted to CSICU total (n = 1892), baseline (n = 390) and final phase (n = 310) Glucose checks total (n = 81333), baseline (n = 3778) and final phase (n = 19043) | Generate a substantial and sustainable improvement in perioperative glucose control | Lean Six Sigma (DMAIC) QIM activities: baseline chart audit, baseline capability, process mapping, fishbone diagram, focus groups, standardisation | Perioperative insulin protocol Educational events | Admission glucose < 200 mg/dL at baseline 76% vs. final 94% (p <0.001) Glucose control > 6 h at baseline 0 vs. final phase 11% (p < 0.001) Glucose measurements increased from baseline 3 to final phase 12 per patient per day (p < 0.001) Hypoglycaemic events decreased from 1.7% at baseline to 0.9% at final phase (p < 0.001) |
| Study | Selection | Comparability | Outcome | Quality |
|---|---|---|---|---|
| Geoffrion et al. (2020) [33] | 3 | 1 | 3 | Good |
| Culig et al. (2011) [34] | 2 | 1 | 3 | Fair |
| Kles et al. (2015) [35] | 4 | 0 | 3 | Poor |
| Gutsche et al. (2014) [36] | 4 | 2 | 3 | Good |
| Lytsy et al. (2015) [37] | 4 | 1 | 3 | Good |
| Watling et al. (2020) [38] | 4 | 0 | 3 | Poor |
| Van Tiel et al. (2006) [39] | 2 | 0 | 2 | Poor |
| Berry et al. (2009) [40] | 4 | 2 | 2 | Good |
| Hefner et al. (2016) [41] | 4 | 0 | 3 | Poor |
| Martinez et al. (2011) [42] | 4 | 0 | 3 | Poor |
| Study | Relevant Disclosures and Conflict of Interest |
|---|---|
| Geoffrion et al. (2020) [33] | Not mentioned |
| Culig et al. (2011) [34] | Supported by grant from the Highmark Foundation of Western Pennsylvania |
| Kles et al. (2015) [35] | None |
| Gutsche et al. (2014) [36] | Not mentioned |
| Lytsy et al. (2015) [37] | None |
| Watling et al. (2020) [38] | Partnership with Integrated Health Solutions, Medtronic |
| Van Tiel et al. (2006) [39] | Not mentioned |
| Berry et al. (2009) [40] | None |
| Hefner et al. (2016) [41] | No financial disclosures |
| Martinez et al. (2011) [42] | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoefsmit, P.C.; Schretlen, S.; Burchell, G.; van den Heuvel, J.; Bonjer, J.; Dahele, M.; Zandbergen, R. Can Quality Improvement Methodologies Derived from Manufacturing Industry Improve Care in Cardiac Surgery? A Systematic Review. J. Clin. Med. 2022, 11, 5350. https://doi.org/10.3390/jcm11185350
Hoefsmit PC, Schretlen S, Burchell G, van den Heuvel J, Bonjer J, Dahele M, Zandbergen R. Can Quality Improvement Methodologies Derived from Manufacturing Industry Improve Care in Cardiac Surgery? A Systematic Review. Journal of Clinical Medicine. 2022; 11(18):5350. https://doi.org/10.3390/jcm11185350
Chicago/Turabian StyleHoefsmit, Paulien Christine, Stijn Schretlen, George Burchell, Jaap van den Heuvel, Jaap Bonjer, Max Dahele, and Reinier Zandbergen. 2022. "Can Quality Improvement Methodologies Derived from Manufacturing Industry Improve Care in Cardiac Surgery? A Systematic Review" Journal of Clinical Medicine 11, no. 18: 5350. https://doi.org/10.3390/jcm11185350
APA StyleHoefsmit, P. C., Schretlen, S., Burchell, G., van den Heuvel, J., Bonjer, J., Dahele, M., & Zandbergen, R. (2022). Can Quality Improvement Methodologies Derived from Manufacturing Industry Improve Care in Cardiac Surgery? A Systematic Review. Journal of Clinical Medicine, 11(18), 5350. https://doi.org/10.3390/jcm11185350






