Association of Fundus Autofluorescence Abnormalities and Pachydrusen in Central Serous Chorioretinopathy and Polypoidal Choroidal Vasculopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
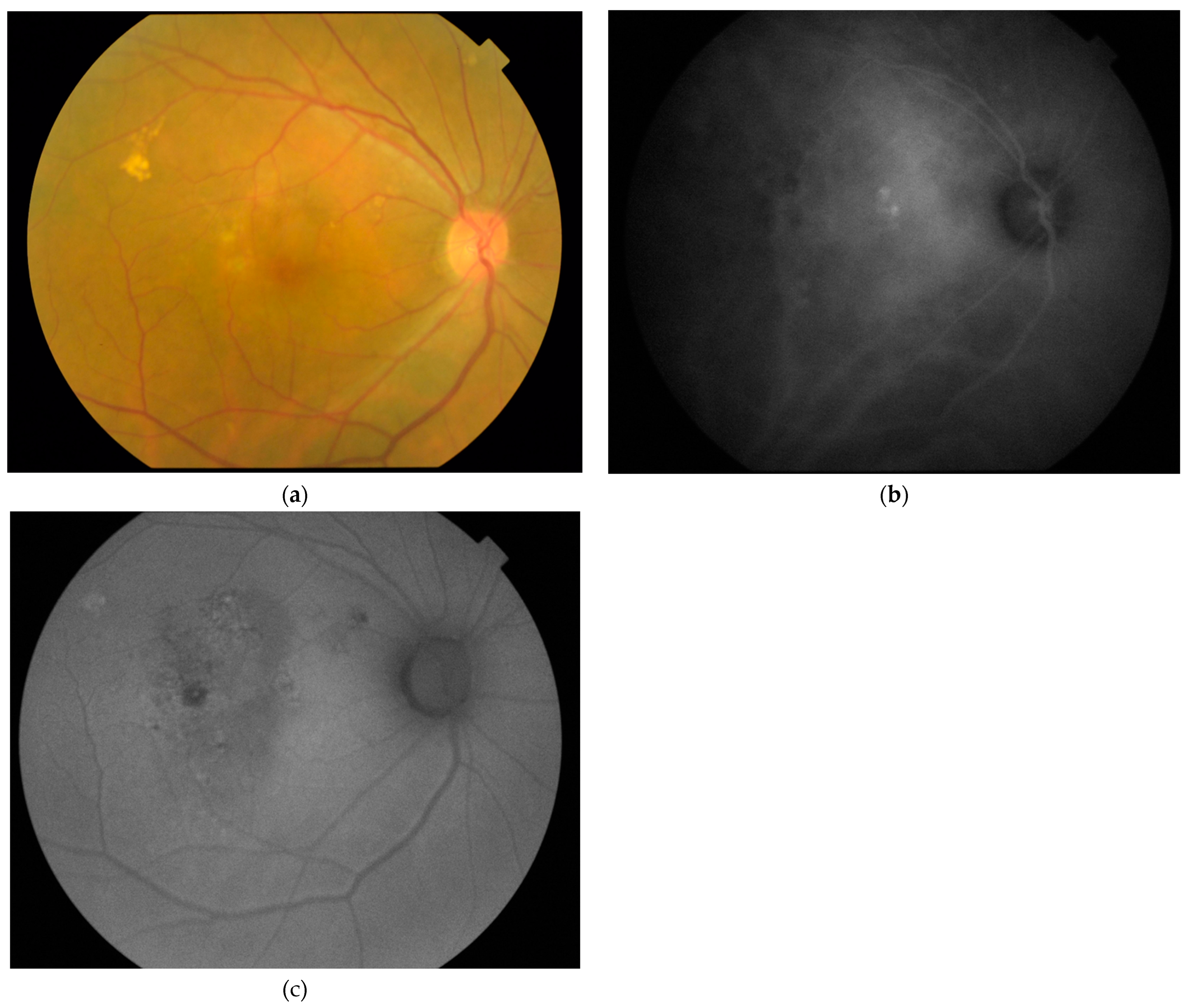
2.2. Imaging and Image Analyses
2.3. Statistical Analyses
3. Results
3.1. Patient Demongraphics
3.2. Eyes with Central Serous Chorioretinopathy
3.3. Eyes with Polypoidal Choroidal Vasculopathy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid disease. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Castro-Navarro, V.; Behar-Cohen, F.; Chang, W.; Joussen, A.M.; Lai, T.Y.Y.; Navarro, R.; Pearce, I.; Yanagi, Y.; Okada, A.A. Pachychoroid: Current concepts on clinical features and pathogenesis. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Disease expression in nonexudative age-related macular degeneration varies with choroidal thickness. Retina 2018, 38, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sivaprasad, S. Drusen and pachydrusen: The definition, pathogenesis, and clinical significance. Eye 2021, 35, 121–133. [Google Scholar] [CrossRef]
- Matsumoto, H.; Mukai, R.; Morimoto, M.; Tokui, S.; Kishi, S.; Akiyama, H. Clinical characteristics of pachydrusen in central serous chorioretinopathy. Graefes Arch. Clin. Exp. Ophthamlol. 2019, 257, 1127–1132. [Google Scholar] [CrossRef]
- Singh, S.R.; Chakurkar, R.; Goud, A.; Chhablani, J. Low incidence of pachydrusen in central serous chorioretinopathy in an Indian cohort. Indian J. Ophthalmol. 2020, 68, 118–122. [Google Scholar]
- Takahashi, A.; Hosoda, Y.; Miyake, M.; Miyata, M.; Oishi, A.; Tamura, H.; Ooto, S.; Yamashiro, K.; Tabara, Y.; Matsuda, F.; et al. Clinical and genetic characteristics of pachydrusen in eyes with central serous chorioretinopathy and general Japanese individuals. Ophthalmol. Retin. 2021, 5, 910–917. [Google Scholar] [CrossRef]
- Sheth, J.; Anantharaman, G.; Kumar, N.; Parachuri, N.; Bandello, F.; Kuppermann, B.D.; Loewenstein, A.; Sharma, A. Pachydrusen: The epidemiology of pachydrusen and its relevance to progression of pachychoroid disease spectrum. Eye 2020, 34, 1501–1503. [Google Scholar] [CrossRef]
- Singh, S.R.; Chakurkar, R.; Goud, A.; Rasheed, M.A.; Vupparaboina, K.K.; Chhablani, J. Pachydrusen in polypoidal choroidal vasculopathy in an Indian cohort. Indian J. Ophthalmol. 2019, 67, 1121–1126. [Google Scholar]
- Lee, J.; Byeon, S.H. Prevalence and clinical characteristics of pachydrusen in polypoidal choroidal vasculopathy: Multimodal image study. Retina 2019, 39, 670–678. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Lee, C.S.; Kim, S.S.; Koh, H.J.; Lee, S.C.; Byeon, S.H. Drusen subtypes and choroidal characteristics in Asian eyes with typical neovascular age-related macular degeneration. Retina 2020, 40, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Lee, J.H.; Chung, B.J.; Lee, K.; Lee, W.K. Choroidal morphology under pachydrusen. Clin. Exp. Ophthalmol. 2019, 47, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.; Cheong, K.X.; Ong, R.; Hamzah, H.; Yanagi, Y.; Wong, T.Y.; Chakravarthy, U.; Cheung, C. Macular neovascularization in eyes with pachydrusen. Sci. Rep. 2021, 11, 7495. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.L.; Joo, K.; Park, S.J.; Park, K.H.; Woo, S.J. Progression from intermediate to neovascular age-related macular degeneration according to drusen subtypes: Bundang AMD cohort study report 3. Acta Ophthalmol. 2022, 100, e710–e718. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Pfau, M.; Fleckenstein, M.; Staurenghi, G.; Sparrow, J.R.; Bindewald-Wittich, A.; Spaide, R.F.; Wolf, S.; Sadda, S.R.; Holz, F.G. Fundus autofluorescence imaging. Prog. Retin. Eye Res. 2021, 81, 100893. [Google Scholar] [CrossRef]
- Margolis, R.; Mukkamala, S.K.; Jampol, L.M.; Spaide, R.F.; Ober, M.D.; Sorenson, J.A.; Gentile, R.C.; Miller, J.A.; Sherman, J.; Freund, K.B. The expanded spectrum of focal choroidal excavation. Arch. Ophthalmol. 2011, 129, 1320–1325. [Google Scholar] [CrossRef]
- Warrow, D.J.; Hoang, Q.V.; Freund, K.B. Pachychoroid pigment epitheliopathy. Retina 2013, 33, 1659–1672. [Google Scholar] [CrossRef]
- Pang, C.E.; Freund, K.B. Pachychoroid neovasculopathy. Retina 2015, 35, 1–9. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, S.; Chen, Y. Characteristic appearances of fundus autofluorescence in treatment-naive and active polypoidal choroidal vasculopathy: A retrospective study of 170 patients. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1101–1110. [Google Scholar] [CrossRef]
- van Rijssen, T.J.; van Dijk, E.H.C.; Yzer, S.; Ohno-Matsui, K.; Keunen, J.E.E.; Schlingemann, R.O.; Sivaprasad, S.; Querques, G.; Downes, S.M.; Fauser, S.; et al. Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog. Retin. Eye Res. 2019, 73, 100770. [Google Scholar] [CrossRef]
- Han, J.; Cho, N.S.; Kim, K.; Kim, E.S.; Kim, D.G.; Kim, J.M.; Yu, S.Y. Fundus autofluorescence patterns in central serous chorioretinopathy. Retina 2020, 40, 1387–1394. [Google Scholar] [CrossRef]
- Kumar, V.; Azad, S.V.; Verma, S.; Surve, A.; Vohra, R.; Venkatesh, P. Peripapillary pachychoroid syndrome: New insights. Retina 2022, 42, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Gan, A.; Yanagi, Y.; Wong, T.Y.; Spaide, R. Association between choroidal thickness and drusen subtypes in age-related macular degeneration. Ophthalmol. Retin. 2018, 2, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Chung, Y.R.; Kim, C.; Lee, K.; Lee, W.K. The association of pachydrusen characteristics with choroidal thickness and patient’s age in polypoidal choroidal vasculopathy versus central serous chorioretinopathy. Int. J. Mol. Sci. 2022, 23, 8353. [Google Scholar] [CrossRef]
- Govindahari, V.; Singh, S.R.; Rajesh, B.; Gallego-Pinazo, R.; Marco, R.D.; Nair, D.V.; Nair, U.; Chhablani, J. Multicolor imaging in central serous chorioretinopathy—A quantitative and qualitative comparison with fundus autofluorescence. Sci. Rep. 2019, 9, 11728. [Google Scholar] [CrossRef]
- Shinojima, A.; Ozawa, Y.; Uchida, A.; Nagai, N.; Shinoda, H.; Kurihara, T.; Suzuki, M.; Minami, S.; Negishi, K.; Tsubota, K. Assessment of hypofluorescent foci on late-phase indocyanine green angiography in central serous chorioretinopathy. J. Clin. Med. 2021, 10, 2178. [Google Scholar] [CrossRef]
- Yamagishi, T.; Koizumi, H.; Yamazaki, T.; Kinoshita, S. Fundus autofluorescence in polypoidal choroidal vasculopathy. Ophthalmology 2012, 119, 1650–1657. [Google Scholar] [CrossRef]
- Notomi, S.; Shiose, S.; Ishikawa, K.; Fukuda, Y.; Kano, K.; Mori, K.; Wada, I.; Kaizu, Y.; Matsumoto, H.; Akiyama, M.; et al. Drusen and pigment abnormality predict the development of neovascular age-related macular degeneration in Japanese patients. PLoS ONE 2021, 16, e0255213. [Google Scholar] [CrossRef]
- Fukuda, Y.; Sakurada, Y.; Sugiyama, A.; Yoneyama, S.; Matsubara, M.; Kikushima, W.; Tanabe, N.; Parikh, R.; Kashiwagi, K. Pachydrusen in fellow eyes predict response to aflibercept monotherapy in patients with polypoidal choroidal vasculopathy. J. Clin. Med. 2020, 9, 2459. [Google Scholar] [CrossRef]

| Characteristics | All CSC Eyes (n = 72) | With Pachydrusen (n = 12) | Without Pachydrusen (n = 60) | p Value |
|---|---|---|---|---|
| Mean ± SD age (years) | 46.7 ± 8.8 | 56.3 ± 7.8 | 45.0 ± 7.8 | <0.001 1 |
| Mean ± SD SFCT (µm) | 359.2 ± 42.9 | 348.5 ± 33.8 | 361.8 ± 44.8 | 0.38 2 |
| FAF abnormality None <2 disc areas ≥2 disc areas | 1 (1.4%) 37 (51.4%) 34 (47.2%) | 0 (0.0%) 0 (0.0%) 12 (100.0%) | 1 (1.7%) 37 (61.7%) 32 (36.7%) | <0.001 3 |
| Characteristics | All PCV eyes (n = 34) | With Pachydrusen (n = 21) | Without Pachydrusen (n = 13) | p Value |
|---|---|---|---|---|
| Mean ± SD age (years) | 65.0 ± 8.2 | 68.8 ± 7.2 | 59.5 ± 6.6 | < 0.001 1 |
| Mean ± SD SFCT (µm) | 264.5 ± 68.0 | 262.8 ± 70.0 | 267.0 ± 68.7 | 0.88 2 |
| FAF abnormality <2 disc areas ≥2 disc areas | 5 (14.7%) 29 (85.3%) | 1 (4.8%) 20 (95.2%) | 4 (30.8%) 9 (69.2%) | 0.37 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, T.Y.Y.; Tang, Z.; Lai, A.C.W.; Szeto, S.K.H.; Lai, R.Y.K.; Cheung, C.Y. Association of Fundus Autofluorescence Abnormalities and Pachydrusen in Central Serous Chorioretinopathy and Polypoidal Choroidal Vasculopathy. J. Clin. Med. 2022, 11, 5340. https://doi.org/10.3390/jcm11185340
Lai TYY, Tang Z, Lai ACW, Szeto SKH, Lai RYK, Cheung CY. Association of Fundus Autofluorescence Abnormalities and Pachydrusen in Central Serous Chorioretinopathy and Polypoidal Choroidal Vasculopathy. Journal of Clinical Medicine. 2022; 11(18):5340. https://doi.org/10.3390/jcm11185340
Chicago/Turabian StyleLai, Timothy Y. Y., Ziqi Tang, Adrian C. W. Lai, Simon K. H. Szeto, Ricky Y. K. Lai, and Carol Y. Cheung. 2022. "Association of Fundus Autofluorescence Abnormalities and Pachydrusen in Central Serous Chorioretinopathy and Polypoidal Choroidal Vasculopathy" Journal of Clinical Medicine 11, no. 18: 5340. https://doi.org/10.3390/jcm11185340
APA StyleLai, T. Y. Y., Tang, Z., Lai, A. C. W., Szeto, S. K. H., Lai, R. Y. K., & Cheung, C. Y. (2022). Association of Fundus Autofluorescence Abnormalities and Pachydrusen in Central Serous Chorioretinopathy and Polypoidal Choroidal Vasculopathy. Journal of Clinical Medicine, 11(18), 5340. https://doi.org/10.3390/jcm11185340







