Therapeutic Efficacy and Safety of Intense Pulsed Light for Refractive Multiple Recurrent Chalazia
Abstract
:1. Introduction
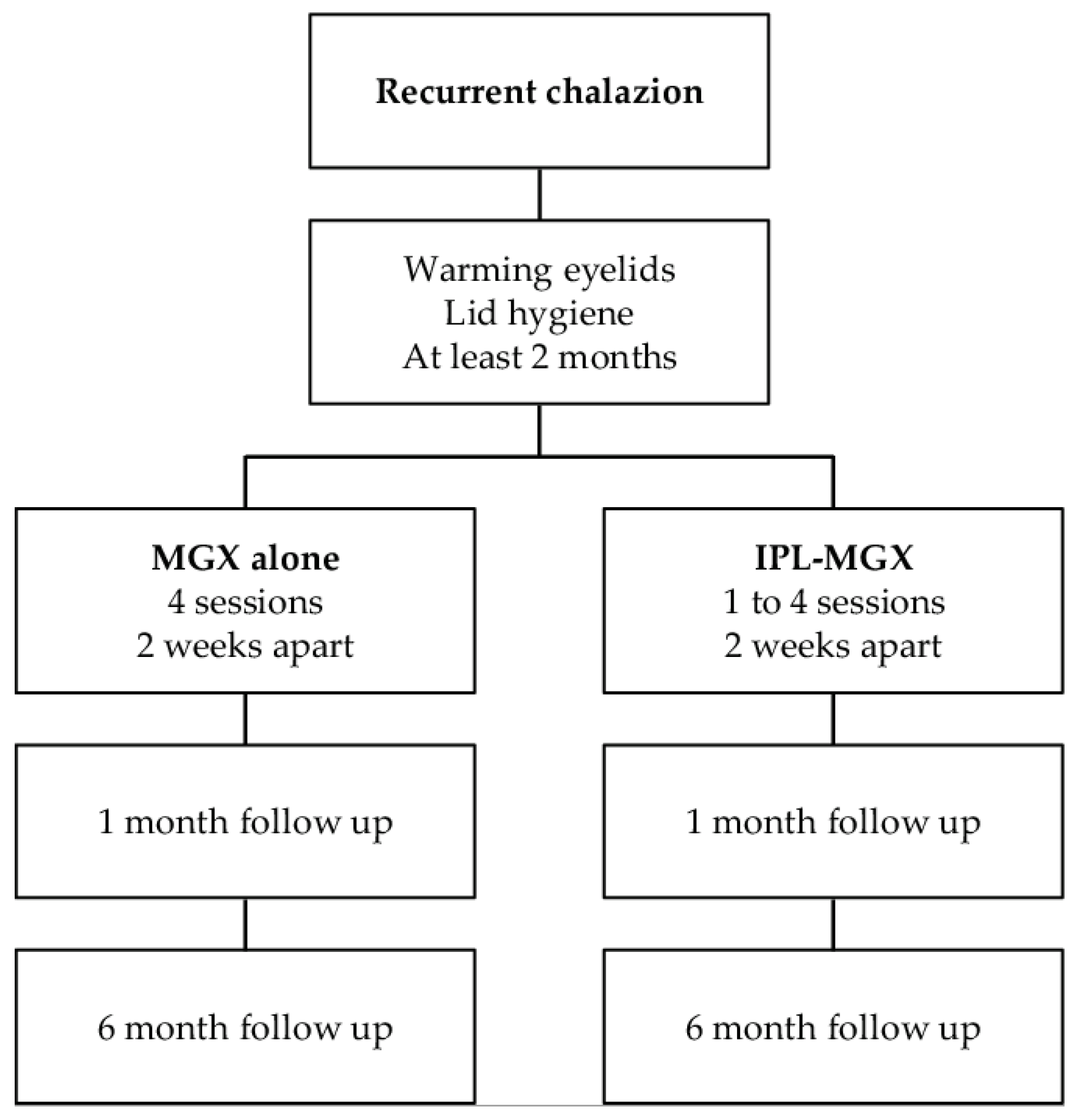
2. Materials and Methods
2.1. Patients
2.2. Experimental Design
2.3. Clinical Assessment
2.4. IPL-MGX Procedure
2.5. Statistical Analysis
3. Results
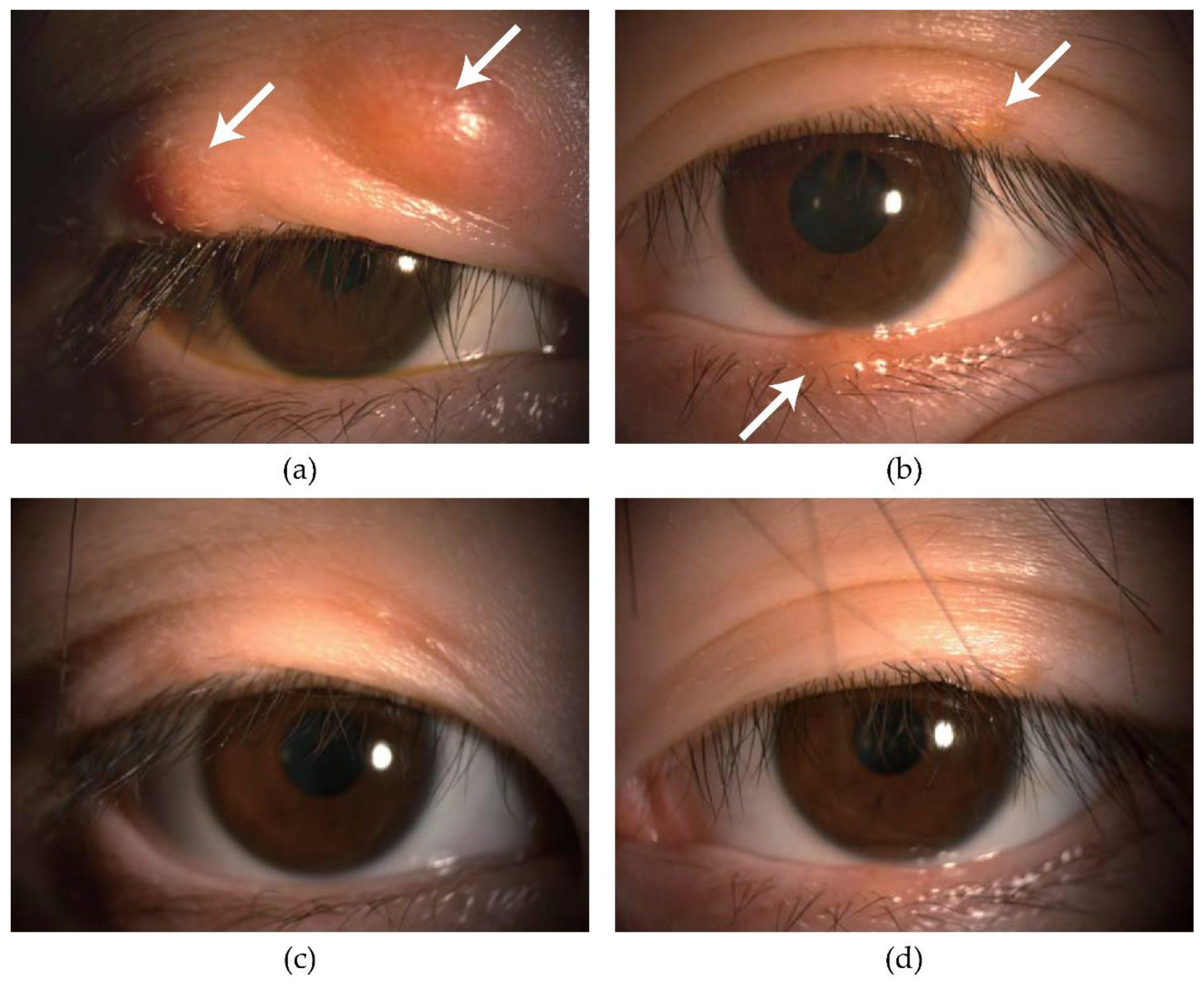
3.1. Efficacy of IPL-MGX
3.2. The Number of IPLs Required to Improve the Chalazion
3.3. Safety of IPL-MGX
4. Discussion
4.1. Risk Factors for Chalazion
4.2. Compared to the Previous Results
4.3. Compared to the Conventional Therapies
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perry, H.D.; Serniuk, R.A. Conservative treatment of chalazia. Ophthalmology 1980, 87, 218–221. [Google Scholar] [CrossRef]
- Gary, A.; Jordan, K.B. Chalazion; StatPearls Publishing: San Francisco, CA, USA, 2022. [Google Scholar]
- Nemet, A.Y.; Vinker, S.; Kaiserman, I. Associated morbidity of blepharitis. Ophthalmology 2011, 118, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Ding, X.; Tseng, S.C. High prevalence of demodex brevis infestation in chalazia. Am. J. Ophthalmol. 2014, 157, 342–348.e341. [Google Scholar] [CrossRef] [PubMed]
- Yam, J.C.; Tang, B.S.; Chan, T.M.; Cheng, A.C. Ocular demodicidosis as a risk factor of adult recurrent chalazion. Eur. J. Ophthalmol. 2014, 24, 159–163. [Google Scholar] [CrossRef]
- Evans, J.; Vo, K.B.H.; Schmitt, M. Chalazion: Racial risk factors for formation, recurrence, and surgical intervention. Can. J. Ophthalmol. 2021, 57, 242–246. [Google Scholar] [CrossRef]
- Duarte, A.F.; Moreira, E.; Nogueira, A.; Santos, P.; Azevedo, F. Chalazion surgery: Advantages of a subconjunctival approach. J. Cosmet. Laser Ther. 2009, 11, 154–156. [Google Scholar] [CrossRef]
- Wong, M.Y.; Yau, G.S.; Lee, J.W.; Yuen, C.Y. Intralesional triamcinolone acetonide injection for the treatment of primary chalazions. Int. Ophthalmol. 2014, 34, 1049–1053. [Google Scholar] [CrossRef]
- Lee, J.W.; Yau, G.S.; Wong, M.Y.; Yuen, C.Y. A comparison of intralesional triamcinolone acetonide injection for primary chalazion in children and adults. Sci. World J. 2014, 2014, 413729. [Google Scholar] [CrossRef]
- Wu, A.Y.; Gervasio, K.A.; Gergoudis, K.N.; Wei, C.; Oestreicher, J.H.; Harvey, J.T. Conservative therapy for chalazia: Is it really effective? Acta Ophthalmol. 2018, 96, e503–e509. [Google Scholar] [CrossRef]
- Nelson, J.D.; Shimazaki, J.; Benitez-del-Castillo, J.M.; Craig, J.P.; McCulley, J.P.; Den, S.; Foulks, G.N. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1930–1937. [Google Scholar] [CrossRef] [Green Version]
- Machalinska, A.; Zakrzewska, A.; Safranow, K.; Wiszniewska, B.; Machalinski, B. Risk Factors and Symptoms of Meibomian Gland Loss in a Healthy Population. J. Ophthalmol. 2016, 2016, 7526120. [Google Scholar] [CrossRef]
- Fukuoka, S.; Arita, R.; Shirakawa, R.; Morishige, N. Changes in meibomian gland morphology and ocular higher-order aberrations in eyes with chalazion. Clin. Ophthalmol. 2017, 11, 1031–1038. [Google Scholar] [CrossRef]
- Raulin, C.; Greve, B.; Grema, H. IPL technology: A review. Lasers Surg Med. 2003, 32, 78–87. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Tashbayev, B.; Yazdani, M.; Arita, R.; Fineide, F.; Utheim, T.P. Intense pulsed light treatment in meibomian gland dysfunction: A concise review. Ocul. Surf. 2020, 18, 583–594. [Google Scholar] [CrossRef]
- Wat, H.; Wu, D.C.; Rao, J.; Goldman, M.P. Application of intense pulsed light in the treatment of dermatologic disease: A systematic review. Dermatol. Surg. 2014, 40, 359–377. [Google Scholar] [CrossRef]
- Moreno-Arias, G.A.; Castelo-Branco, C.; Ferrando, J. Side-effects after IPL photodepilation. Dermatol. Surg. 2002, 28, 1131–1134. [Google Scholar]
- Lee, W.W.; Murdock, J.; Albini, T.A.; O’Brien, T.P.; Levine, M.L. Ocular damage secondary to intense pulse light therapy to the face. Ophthalmic Plast Reconstr. Surg. 2011, 27, 263–265. [Google Scholar] [CrossRef]
- Toyos, R.; McGill, W.; Briscoe, D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomed. Laser Surg. 2015, 33, 41–46. [Google Scholar] [CrossRef]
- Craig, J.P.; Chen, Y.H.; Turnbull, P.R. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1965–1970. [Google Scholar] [CrossRef]
- Vora, G.K.; Gupta, P.K. Intense pulsed light therapy for the treatment of evaporative dry eye disease. Curr. Opin. Ophthalmol. 2015, 26, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Vora, G.K.; Matossian, C.; Kim, M.; Stinnett, S. Outcomes of intense pulsed light therapy for treatment of evaporative dry eye disease. Can. J. Ophthalmol. 2016, 51, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Vegunta, S.; Patel, D.; Shen, J.F. Combination Therapy of Intense Pulsed Light Therapy and Meibomian Gland Expression (IPL/MGX) Can Improve Dry Eye Symptoms and Meibomian Gland Function in Patients with Refractory Dry Eye: A Retrospective Analysis. Cornea 2016, 35, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lv, H.; Song, H.; Zhang, M.; Liu, Y.; Hu, X.; Li, X.; Wang, W. Evaluation of the Safety and Effectiveness of Intense Pulsed Light in the Treatment of Meibomian Gland Dysfunction. J. Ophthalmol. 2016, 2016, 1910694. [Google Scholar] [CrossRef]
- Dell, S.J. Intense pulsed light for evaporative dry eye disease. Clin. Ophthalmol. 2017, 11, 1167–1173. [Google Scholar] [CrossRef]
- Dell, S.J.; Gaster, R.N.; Barbarino, S.C.; Cunningham, D.N. Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin. Ophthalmol. 2017, 11, 817–827. [Google Scholar] [CrossRef]
- Rong, B.; Tu, P.; Tang, Y.; Liu, R.X.; Song, W.J.; Yan, X.M. [Evaluation of short-term effect of intense pulsed light combined with meibomian gland expression in the treatment of meibomian gland dysfunction]. Zhonghua Yan Ke Za Zhi 2017, 53, 675–681. [Google Scholar]
- Liu, R.; Rong, B.; Tu, P.; Tang, Y.; Song, W.; Toyos, R.; Toyos, M.; Yan, X. Analysis of Cytokine Levels in Tears and Clinical Correlations After Intense Pulsed Light Treating Meibomian Gland Dysfunction. Am. J. Ophthalmol. 2017, 183, 81–90. [Google Scholar] [CrossRef]
- Guilloto Caballero, S.; Garcia Madrona, J.L.; Colmenero Reina, E. Effect of pulsed laser light in patients with dry eye syndrome. Arch. Soc. Esp. Oftalmol. 2017, 92, 509–515. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, N.; Gong, L.; Song, N. Changes in the Meibomian Gland After Exposure to Intense Pulsed Light in Meibomian Gland Dysfunction (MGD) Patients. Curr. Eye Res. 2017, 43, 308–313. [Google Scholar] [CrossRef]
- Albietz, J.M.; Schmid, K.L. Intense pulsed light treatment and meibomian gland expression for moderate to advanced meibomian gland dysfunction. Clin. Exp. Optom. 2018, 101, 23–33. [Google Scholar] [CrossRef]
- Arita, R.; Fukuoka, S.; Morishige, N. Therapeutic efficacy of intense pulsed light in patients with refractory meibomian gland dysfunction. Ocul. Surf. 2019, 17, 104–110. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, X.; Lin, L.; Di, M.; Chen, R.; Dong, J.; Jin, X. Efficacy of Intense Pulsed Light in the Treatment of Recurrent Chalaziosis. Front. Med. 2022, 9, 839908. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Ngo, W.; Situ, P.; Keir, N.; Korb, D.; Blackie, C.; Simpson, T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea 2013, 32, 1204–1210. [Google Scholar] [CrossRef]
- Arita, R.; Minoura, I.; Morishige, N.; Shirakawa, R.; Fukuoka, S.; Asai, K.; Goto, T.; Imanaka, T.; Nakamura, M. Development of Definitive and Reliable Grading Scales for Meibomian Gland Dysfunction. Am. J. Ophthalmol. 2016, 169, 125–137. [Google Scholar] [CrossRef]
- van Bijsterveld, O.P. Diagnostic tests in the Sicca syndrome. Arch. Ophthalmol. 1969, 82, 10–14. [Google Scholar] [CrossRef]
- Shimazaki, J.; Sakata, M.; Tsubota, K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch. Ophthalmol. 1995, 113, 1266–1270. [Google Scholar] [CrossRef]
- Arita, R.; Itoh, K.; Inoue, K.; Amano, S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008, 115, 911–915. [Google Scholar] [CrossRef]
- Shirmer, O. Studiun zur Physiologie und Pathologie der Tranenabsonderung und Tranenabfuhr. Albrecht von Graefes Arch. für Ophthalmol. 1903, 56, 197–291. [Google Scholar] [CrossRef]
- Korb, D.R.; Blackie, C.A.; McNally, E.N. Evidence suggesting that the keratinized portions of the upper and lower lid margins do not make complete contact during deliberate blinking. Cornea 2013, 32, 491–495. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Tohme, N.; Gorrin, E.; Kumar, N.; Goldhagen, B.; Galor, A. Prevalence and risk factors for chalazion in an older veteran population. Br. J. Ophthalmol. 2021, 106, 1200–1205. [Google Scholar] [CrossRef]
- Tarkowski, W.; Owczynska, M.; Blaszczyk-Tyszka, A.; Mlocicki, D. Demodex mites as potential etiological factor in chalazion—A study in Poland. Acta Parasitol. 2015, 60, 777–783. [Google Scholar] [CrossRef]
- Ben Simon, G.J.; Rosen, N.; Rosner, M.; Spierer, A. Intralesional triamcinolone acetonide injection versus incision and curettage for primary chalazia: A prospective, randomized study. Am. J. Ophthalmol. 2011, 151, 714–718 e711. [Google Scholar] [CrossRef]
- Goawalla, A.; Lee, V. A prospective randomized treatment study comparing three treatment options for chalazia: Triamcinolone acetonide injections, incision and curettage and treatment with hot compresses. Clin. Exp. Ophthalmol. 2007, 35, 706–712. [Google Scholar] [CrossRef]
- Aycinena, A.R.; Achiron, A.; Paul, M.; Burgansky-Eliash, Z. Incision and Curettage Versus Steroid Injection for the Treatment of Chalazia: A Meta-Analysis. Ophthalmic Plast Reconstr. Surg. 2016, 32, 220–224. [Google Scholar] [CrossRef]
| IPL-MGX | MGX Alone | p Value | |||
|---|---|---|---|---|---|
| Mean ± SD | (Range) | Mean ± SD | (Range) | ||
| Age (years) | 36.8 ± 12.1 | (19–51) | 37.7 ± 13.1 | (22–54) | 1.00 |
| Number of chalazia | 2.5 ± 0.8 | (2–4) | 2.3 ± 0.5 | (2–3) | 0.92 |
| Number of eyelids with chalazia | 2.3 ± 0.5 | (2–3) | 2.3 ± 0.5 | (2–3) | 1.00 |
| Duration of pre-lid-warming (months) | 30.9 ± 41.2 | (0.5–104) | 2.0 ± 2.1 | (0.5–6) | 0.29 |
| Size of the largest chalazion (mm) | 12.2 ± 5.6 | (5–18) | 10.5 ± 2.0 | (7–12) | 0.81 |
| Characteristics | Baseline | p Value for IPL-MGX vs. MGX Alone | Post-Treatment | p Value vs. Baseline | p Value for IPL-MGX vs. MGX Alone | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | (Range) | Mean ± SD | (Range) | |||||
| Number of IPL for improvement | MGX alone | |||||||
| IPL-MGX | 2.8 ± 1.3 | (1–4) | ||||||
| Plugging (0–3) | MGX alone | 2.7 ± 0.5 | (2–3) | 0.92 | 2.1 ± 0.9 | (1–3) | 0.063 | <0.001 ** |
| IPL-MGX | 2.6 ± 0.7 | (1–3) | 0.2 ± 0.4 | (0–1) | <0.001 ** | |||
| Vascularity (0–3) | MGX alone | 2.3 ± 0.8 | (1–3) | 0.77 | 2.3 ± 0.8 | (1–3) | 1.00 | <0.001 ** |
| IPL-MGX | 2.4 ± 0.8 | (1–3) | 0 ± 0 | (0–0) | <0.001 ** | |||
| Irregularity (0–2) | MGX alone | 0.9 ± 0.9 | (0–2) | 0.83 | 0.9 ± 0.9 | (0–2) | 1.00 | 0.27 |
| IPL-MGX | 1.0 ± 0.9 | (0–2) | 0.5 ± 0.5 | (0–1) | 0.031 * | |||
| CFS (0–9) | MGX alone | 2.0 ± 0.7 | (1–3) | 0.54 | 1.7 ± 0.7 | (1–3) | 0.125 | <0.001 ** |
| IPL-MGX | 2.1 ± 1.5 | (1–5) | 0.2 ± 0.4 | (0–1) | <0.001 ** | |||
| Meibum grade (0–3) | MGX alone | 2.5 ± 0.5 | (2–3) | 0.57 | 1.9 ± 0.8 | (1–3) | 0.016 * | <0.001 ** |
| IPL-MGX | 2.6 ± 0.7 | (1–3) | 0.3 ± 0.5 | (0–1) | <0.001 ** | |||
| Meiboscore (0–6) | MGX alone | 3.2 ± 0.9 | (2–4) | 0.88 | 3.2 ± 0.9 | (2–4) | 1.00 | 0.88 |
| IPL-MGX | 3.4 ± 1.5 | (2–6) | 3.4 ± 1.5 | (2–6) | 1.00 | |||
| Number of Demodex | MGX alone | 3.1 ± 0.9 | (2–4) | 0.52 | 3.3 ± 0.8 | (2–4) | 0.50 | <0.001 ** |
| IPL-MGX | 2.8 ± 0.9 | (2–4) | 0 ± 0 | (0–0) | <0.001 ** | |||
| Schirmer test value (mm) | MGX alone | 11.7 ± 4.7 | (5–20) | 0.50 | 11.0 ± 4.2 | (6–20) | 0.30 | 0.75 |
| IPL-MGX | 11.5 ± 7.5 | (4–26) | 11.5 ± 5.5 | (6–20) | 0.76 | |||
| Size (diameter) of chalazion (mm) | MGX alone | 8.8 ± 2.4 | (6–12) | 0.47 | 9.2 ± 2.6 | (6–12) | 0.38 | <0.001 ** |
| IPL-MGX | 9.0 ± 5.2 | (3–18) | 0 ± 0 | (0–0) | <0.001 ** | |||
| VAS score (0–100) | MGX alone | 61.2 ± 22 | (23–90) | 1.00 | 67.3 ± 21.2 | (30–90) | 0.063 | <0.001 ** |
| IPL-MGX | 61.7 ± 23.8 | (23–90) | 0 ± 0 | (0–0) | <0.001 ** | |||
| Baseline | p Value for IPL-MGX vs. MGX Alone | Post-Treatment | p Value vs. Baseline | p Value for IPL-MGX vs. MGX Alone | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | (Range) | Mean ± SD | (Range) | ||||
| MGX alone | 11.8 ± 2.0 | (9–15) | 0.94 | 12.3 ± 2.2 | (9–15) | 1.00 | 0.003 * |
| IPL-MGX | 11.2 ± 4.3 | (4–15) | 0 ± 0 | (0–0) | 0.031 * | ||
| Characteristics | ρ | p Value |
|---|---|---|
| Age | 0.12 | 0.82 |
| Number of chalazia | 0.66 | 0.16 |
| Number of eyelids with chalazia | 0.67 | 0.15 |
| Duration of pre-lid-warming | 0.62 | 0.19 |
| Size of the largest chalazion | 0.94 | 0.005 * |
| SPEED score at baseline | −0.03 | 0.95 |
| Baseline Parameters | ρ | p Value |
|---|---|---|
| Plugging | 0.74 | 0.006 * |
| Vascularity | 0.56 | 0.059 |
| Irregularity | 0.52 | 0.086 |
| CFS | 0.89 | <0.001 ** |
| Meibum grade | 0.74 | 0.006 * |
| Meiboscore | 0.57 | 0.051 |
| Number of Demodex | 0.73 | 0.007 * |
| Schirmer test value | 0.61 | 0.034 * |
| VAS score | −0.24 | 0.45 |
| Characteristics | Baseline | p Value for IPL-MGX vs. MGX Alone | Post-Treatment | p Value for IPL-MGX vs. MGX Alone | p Value vs. Baseline | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | (Range) | Mean ± SD | (Range) | |||||
| LogMAR visual acuity | MGX alone | −0.06 ± 0.07 | (−0.18–0.00) | 0.37 | −0.07 ± 0.06 | (−0.18–0.00) | 0.38 | 0.50 |
| IPL-MGX | −0.07 ± 0.03 | (−0.08–0.00) | −0.08 ± 0 | (−0.08–0.08) | 0.50 | |||
| IOP (mmHg) | MGX alone | 16.7 ± 1.4 | (15–19) | 0.93 | 16.6 ± 1.1 | (14–19) | 0.98 | 0.77 |
| IPL-MGX | 16.8 ± 1.5 | (15–19) | 16.6 ± 1.7 | (15–18) | 1.00 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arita, R.; Fukuoka, S. Therapeutic Efficacy and Safety of Intense Pulsed Light for Refractive Multiple Recurrent Chalazia. J. Clin. Med. 2022, 11, 5338. https://doi.org/10.3390/jcm11185338
Arita R, Fukuoka S. Therapeutic Efficacy and Safety of Intense Pulsed Light for Refractive Multiple Recurrent Chalazia. Journal of Clinical Medicine. 2022; 11(18):5338. https://doi.org/10.3390/jcm11185338
Chicago/Turabian StyleArita, Reiko, and Shima Fukuoka. 2022. "Therapeutic Efficacy and Safety of Intense Pulsed Light for Refractive Multiple Recurrent Chalazia" Journal of Clinical Medicine 11, no. 18: 5338. https://doi.org/10.3390/jcm11185338
APA StyleArita, R., & Fukuoka, S. (2022). Therapeutic Efficacy and Safety of Intense Pulsed Light for Refractive Multiple Recurrent Chalazia. Journal of Clinical Medicine, 11(18), 5338. https://doi.org/10.3390/jcm11185338






