Transdermal Maltose-Based Microneedle Patch as Adjunct to Enhance Topical Anesthetic before Intravenous Cannulation of Pediatric Thalassemic Patients Receiving Blood Transfusion: A Randomized Controlled Trial Protocol
Abstract
1. Introduction
1.1. Research Background
1.2. Objectives
- (1)
- To compare VAS pain scores from an individual receiving the following four treatment interventions (a) using a microneedle with 1 Finger Tip Unit (FTU) EMLA® cream applied for 30 min, (b) a microneedle with 0.5 FTU EMLA® applied for 30 min, (c) a microneedle with 1 FTU EMLA® applied for 15 min, and (d) 1 FTU EMLA® cream only (without MN) applied for 30 min (control) in a randomized fashion over four consecutive visits.
- (2)
- To compare skin conductance algesimeter indices from individuals receiving the above-allocated interventions.
- (3)
- To determine the agreement between the VAS pain scores and the skin conductance algesimeter index.
2. Materials and Methods
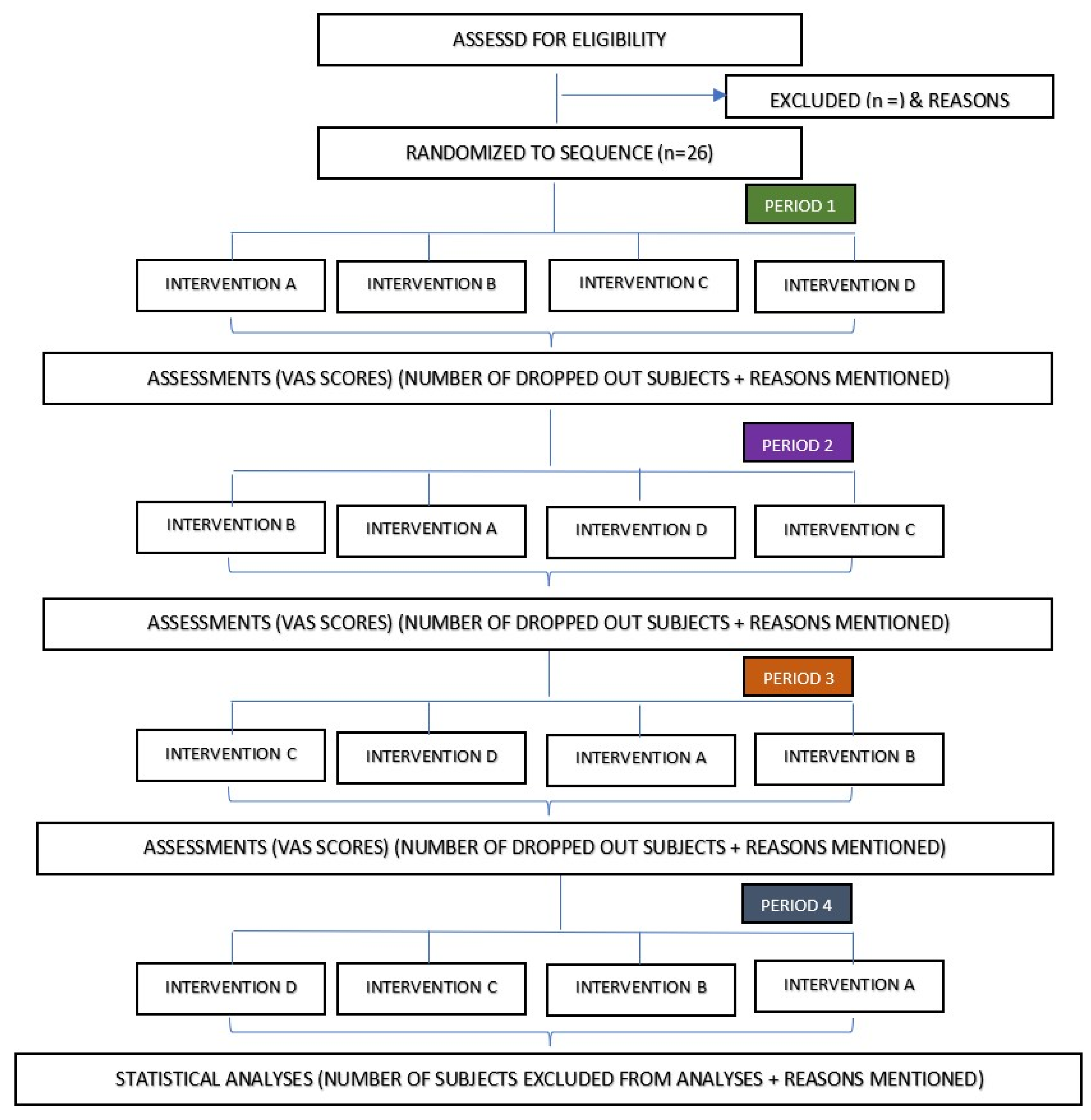
2.1. Study Design
2.2. Study Setting
2.3. Sample Size Calculation
2.4. Recruitment
2.5. Study Population and Sampling Method
2.6. Inclusion Criteria
2.7. Exclusion Criteria
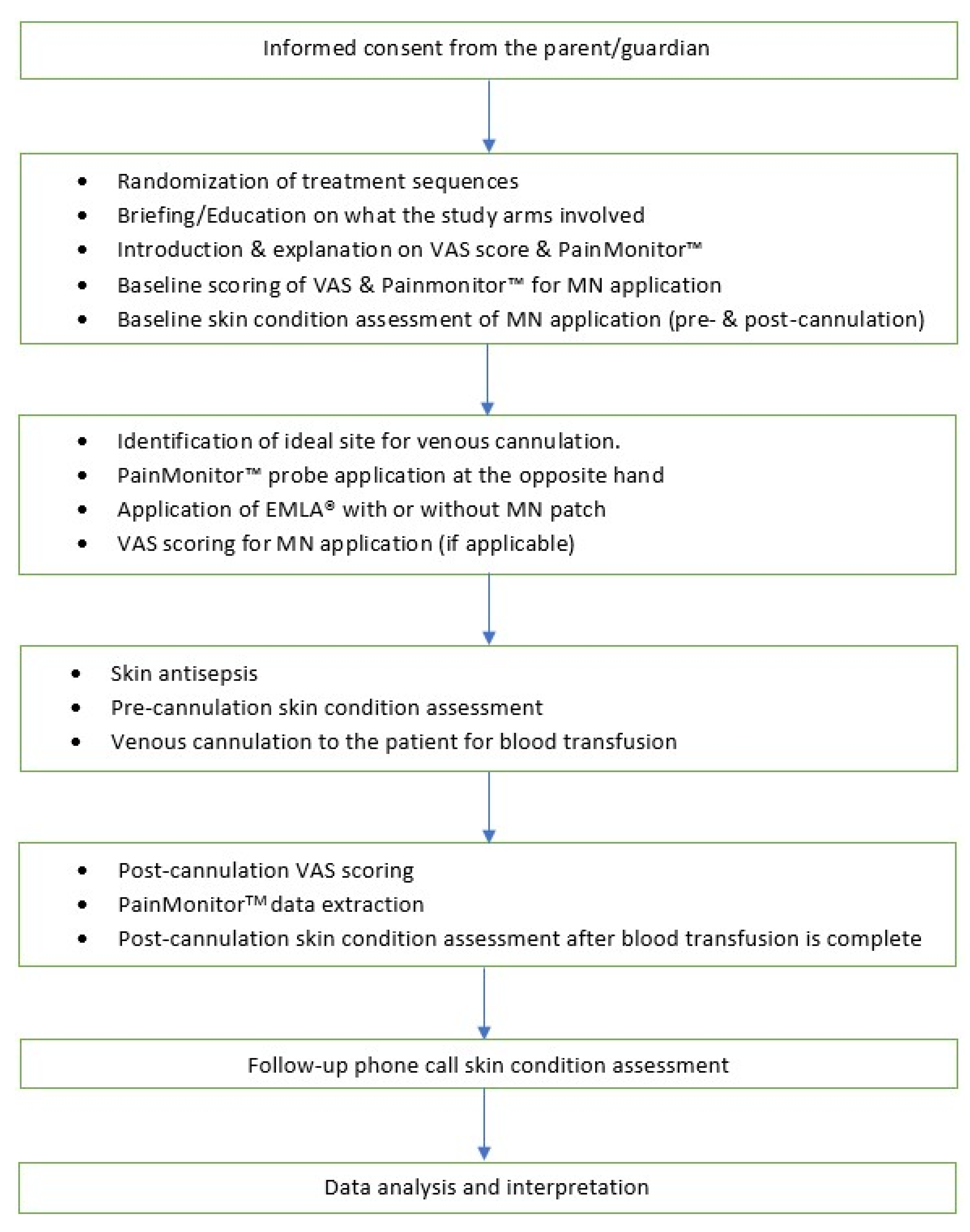
2.8. Randomization
2.9. Allocation Concealment Mechanisms
2.10. Consent and Confidentiality
2.11. Research Materials
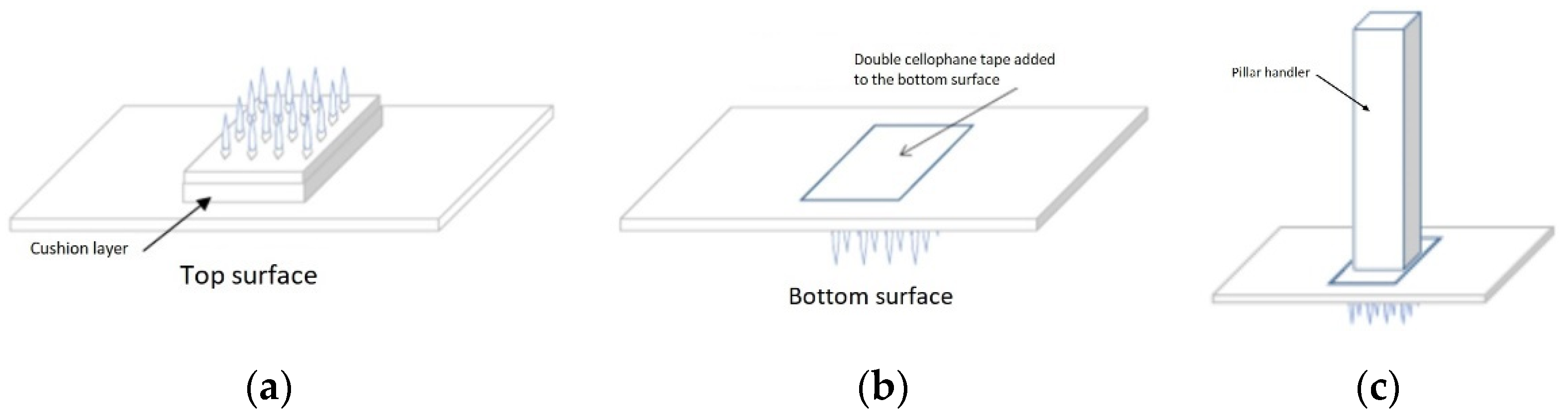
2.12. Fabrication of Solid Maltose Microneedles
2.13. Administration of Interventions/Controls
2.14. Pain Assessment
2.15. Strategies to Improve Adherence to Interventions
2.16. Relevant Concomitant Care Permitted or Prohibited during the Trial
2.17. Provision of Post-Trial Care
2.18. Research Outcomes
2.19. Data Collection
2.20. Data Management
2.21. Statistical Analysis
2.22. Ethical Issues
2.23. Trial Oversight and Monitoring
2.23.1. Data Monitoring Committee
2.23.2. Safety Assessment
2.23.3. Plans for Communicating Important Protocol Modifications
2.23.4. Dissemination Policy
3. Discussion
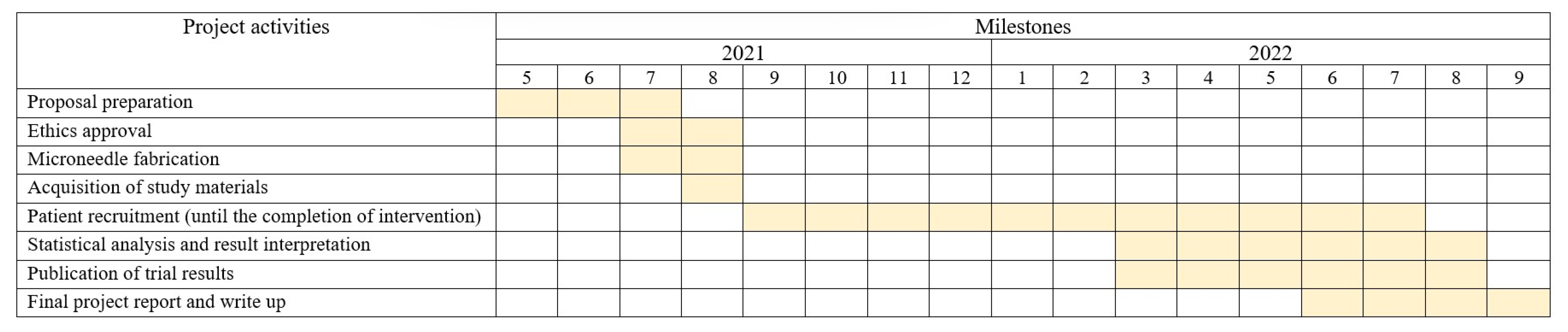
4. Trial Status
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katende, G.; Mugabi, B. Comforting strategies and perceived barriers to pediatric pain management during IV line insertion procedure in Uganda’s national referral hospital: A descriptive study. BMC Pediatrics 2015, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Dahlquist, L.M.; Busby, S.M.; Slifer, K.J.; Tucker, C.L.; Eischen, S.; Hilley, L.; Sulc, W. Distraction for Children of Different Ages Who Undergo Repeated Needle Sticks. J. Pediatric Oncol. Nurs. 2002, 19, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Tucak, A.; Sirbubalo, M.; Hindija, L.; Rahić, O.; Hadžiabdić, J.; Muhamedagić, K.; Čekić, A.; Vranić, E. Microneedles: Characteristics, Materials, Production Methods and Commercial Development. Micromachines 2020, 11, 961. [Google Scholar] [CrossRef]
- de Waard-van der Spek, F.B.; Van Den Berg, G.M.; Oranje, A.P. EMLA Cream: An Improved Local Anesthetic. Review of Current Literature. Pediatric Dermatol. 1992, 9, 126–131. [Google Scholar] [CrossRef]
- Houck, C.S.; Sethna, N.F. Transdermal analgesia with local anesthetics in children: Review, update and future directions. Expert Rev. Neurother. 2005, 5, 625–634. [Google Scholar] [CrossRef]
- Mooney, K.; McElnay, J.C.; Donnelly, R.F. Children’s views on microneedle use as an alternative to blood sampling for patient monitoring. Int. J. Pharm. Pract. 2013, 22, 335–344. [Google Scholar] [CrossRef]
- Pires, L.R.; Vinayakumar, K.; Turos, M.; Miguel, V.; Gaspar, J. A Perspective on Microneedle-Based Drug Delivery and Diagnostics in Paediatrics. J. Pers. Med. 2019, 9, 49. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Jung, D.-I.; Kim, Y.-I.; Yang, J.-H.; Shin, S.-C. Anesthetic effects of lidocaine hydrochloride gel using low frequency ultrasound of 0.5 MHz. J. Pharm. Pharm. Sci. 2007, 10, 1–8. [Google Scholar]
- Murthy, S.N.; Sammeta, S.M.; Bowers, C. Magnetophoresis for enhancing transdermal drug delivery: Mechanistic studies and patch design. J. Control. Release 2010, 148, 197–203. [Google Scholar] [CrossRef]
- Zempsky, W.T.; Sullivan, J.; Paulson, D.M.; Hoath, S.B. Evaluation of a low-dose lidocaine lontophoresis system for topical anesthesia in adults and children: A randomized, controlled trial. Clin. Ther. 2004, 26, 1110–1119. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Mehta, P.; Arshad, M.S.; Kucuk, I.; Chang, M.W.; Ahmad, Z. Transdermal Microneedles—A Materials Perspective. AAPS PharmSciTech 2019, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Caffarel-Salvador, E.; Tuan-Mahmood, T.M.; McElnay, J.C.; McCarthy, H.O.; Mooney, K.; Woolfson, A.D.; Donnelly, R.F. Potential of hydrogel-forming and dissolving microneedles for use in paediatric populations. Int. J. Pharm. 2015, 489, 158–169. [Google Scholar] [CrossRef]
- Nguyen, H. Safety of Microneedles for Transdermal Drug Delivery. J. Pharmacovigil. 2018, 6, e172. [Google Scholar] [CrossRef]
- Gowda, A.; Healey, B.; Ezaldein, H.; Merati, M. A Systematic Review Examining the Potential Adverse Effects of Microneedling. J. Clin. Aesthet. Dermatol. 2021, 14, 45–54. [Google Scholar]
- He, X.; Sun, J.; Zhuang, J.; Xu, H.; Liu, Y.; Wu, D. Microneedle System for Transdermal Drug and Vaccine Delivery: Devices, Safety, and Prospects. Dose Response 2019, 17, 1559325819878585. [Google Scholar] [CrossRef]
- Palodkar, K.K.; Malleswara Rao, N.N.; Iyer, S.; Puttalingaiah, R.T.; Sadhu, V.; Aminabhavi, T.M.; Reddy, K.R.; Sainath, A.V.S. Maltose-based methacrylated polymer architectures and their biocompatibility. Mater. Today Chem. 2022, 23, 100669. [Google Scholar] [CrossRef]
- Gupta, J.; Felner, E.I.; Prausnitz, M.R. Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol. Ther. 2011, 13, 451–456. [Google Scholar] [CrossRef]
- Norman, J.J.; Brown, M.R.; Raviele, N.A.; Prausnitz, M.R.; Felner, E.I. Faster pharmacokinetics and increased patient acceptance of intradermal insulin delivery using a single hollow microneedle in children and adolescents with type 1 diabetes. Pediatric Diabetes 2013, 14, 459–465. [Google Scholar] [CrossRef]
- Samant, P.P.; Niedzwiecki, M.M.; Raviele, N.; Tran, V.; Mena-Lapaix, J.; Walker, D.I.; Felner, E.I.; Jones, D.P.; Miller, G.W.; Prausnitz, M.R. Sampling interstitial fluid from human skin using a microneedle patch. Sci. Transl. Med. 2020, 12, eaaw0285. [Google Scholar] [CrossRef] [PubMed]
- Emory University. Microneedle Patch Study in Healthy Infants/Young Children. Clinicaltrials.gov Identifier: NCT03207763. Updated: 10 November 2020 . Available online: https://clinicaltrials.gov/ct2/show/study/NCT03207763 (accessed on 14 July 2021).
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Srouji, R.; Ratnapalan, S.; Schneeweiss, S. Pain in children: Assessment and nonpharmacological management. Int. J. Pediatr. 2010, 2010, 474838. [Google Scholar] [CrossRef]
- Beltramini, A.; Milojevic, K.; Pateron, D. Pain Assessment in Newborns, Infants, and Children. Pediatr. Ann. 2017, 46, e387–e395. [Google Scholar] [CrossRef] [PubMed]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15, S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Storm, H. Changes in skin conductance as a tool to monitor nociceptive stimulation and pain. Curr. Opin. Anaesthesiol. 2008, 21, 796–804. [Google Scholar] [CrossRef]
- van der Lee, R.; Jebbink, L.J.M.G.; van Herpen, T.H.M.; d’Haens, E.J.; Bierhuizen, J.; van Lingen, R.A. Feasibility of monitoring stress using skin conduction measurements during intubation of newborns. Eur. J. Pediatrics 2016, 175, 237–243. [Google Scholar] [CrossRef][Green Version]
- Chan, A.-W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. Br. Med. J. 2013, 346, e7586. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988; p. 567. [Google Scholar]
- Daly, S.; Claydon, N.C.A.; Newcombe, R.G.; Seong, J.; Addy, M.; West, N.X. Randomised controlled trial of a microneedle patch with a topical anaesthetic for relieving the pain of dental injections. J. Dent. 2021, 107, 103617. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi. Med. J. 2012, 24, 69–71. [Google Scholar]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Vitor-Costa, M.; Okuno, N.M.; Bortolotti, H.; Bertollo, M.; Boggio, P.S.; Fregni, F.; Altimari, L.R. Improving Cycling Performance: Transcranial Direct Current Stimulation Increases Time to Exhaustion in Cycling. PLoS ONE 2015, 10, e0144916. [Google Scholar] [CrossRef]
- Cavalera, C.; Pagnini, F.; Rovaris, M.; Mendozzi, L.; Pugnetti, L.; Garegnani, M.; Molinari, E. A telemedicine meditation intervention for people with multiple sclerosis and their caregivers: Study protocol for a randomized controlled trial. Trials 2016, 17, 1–6. [Google Scholar] [CrossRef]
- Chang, H.A.; Fang, W.H.; Wan, F.J.; Tzeng, N.S.; Liu, Y.P.; Shyu, J.F.; Chang, T.C.; Huang, S.Y.; Chang, C.C. Attenuated vagally-mediated heart rate variability at rest and in response to postural maneuvers in patients with generalized anxiety disorder. Psychol. Med. 2020, 50, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Oberfeld, D.; Franke, T. Evaluating the robustness of repeated measures analyses: The case of small sample sizes and nonnormal data. Behav. Res. Methods 2013, 45, 792–812. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Uschner, D.; Schindler, D.; Hilgers, R.-D.; Heussen, N. randomizeR: An R Package for the Assessment and Implementation of Randomization in Clinical Trials. J. Stat. Softw. 2018, 85, 22. [Google Scholar] [CrossRef]
- World Health Organization. Growth Reference 5–19 Years. Available online: http://www.who.int/growthref/who2007_bmi_for_age/en/ (accessed on 6 May 2021).
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- National Pharmaceutical Regulatory Agency (NPRA) MoHM. Malaysian Guideline for Good Clinical Practice. 4th ed. Malaysia: National Committee for Clinical Research (NCCR) 2018. Available online: https://www.crc.gov.my/wp-content/uploads/2018/03/Malaysian_gcp_4th_Edition28Final_29.pdf (accessed on 15 March 2021).
- US Department of Health and Human Services. Common Terminlogy Criteria for Adverse Events (CTCAE) Version 5. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 31 August 2021).
- Center for Biologics Evaluation and Research, US Food and Drug Administration (US FDA). Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Clinical Trials. Available online: https://www.fda.gov/media/73679/download (accessed on 31 August 2021).
| ABCD | BACD | CADB | DABC |
| ACBD | BADC | CABD | DACB |
| ABDC | BDAC | CBAD | DBAC |
| ACDB | BDCA | CBDA | DBCA |
| ADBC | BCAD | CDAB | DCAB |
| ADCB | BCDA | CDBA | DCBA |
| Materials | Manufacture |
|---|---|
| PainMonitor™ | Medstorm, Norway |
| EMLA® Cream | Aspen, Malaysia |
| Microneedle patch | Alnair Photonics, Japan |
| Sham patch | Alnair Photonics, Japan |
| Visual Analogue Scale ruler | Schlenker Enterprises, Illinois, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Jalal, M.I.; Ooi, K.S.; Foo, K.C.; Hamzah, A.A.; Goh, C.S.; Dee, C.F.; Ooi, P.C.; Buyong, M.R.; Low, T.Y.; Chua, X.Y.; et al. Transdermal Maltose-Based Microneedle Patch as Adjunct to Enhance Topical Anesthetic before Intravenous Cannulation of Pediatric Thalassemic Patients Receiving Blood Transfusion: A Randomized Controlled Trial Protocol. J. Clin. Med. 2022, 11, 5291. https://doi.org/10.3390/jcm11185291
Abdul Jalal MI, Ooi KS, Foo KC, Hamzah AA, Goh CS, Dee CF, Ooi PC, Buyong MR, Low TY, Chua XY, et al. Transdermal Maltose-Based Microneedle Patch as Adjunct to Enhance Topical Anesthetic before Intravenous Cannulation of Pediatric Thalassemic Patients Receiving Blood Transfusion: A Randomized Controlled Trial Protocol. Journal of Clinical Medicine. 2022; 11(18):5291. https://doi.org/10.3390/jcm11185291
Chicago/Turabian StyleAbdul Jalal, Muhammad Irfan, Kai Shen Ooi, Kai Cheong Foo, Azrul Azlan Hamzah, Chee Seong Goh, Chang Fu Dee, Poh Choon Ooi, Muhamad Ramdzan Buyong, Teck Yew Low, Xin Yun Chua, and et al. 2022. "Transdermal Maltose-Based Microneedle Patch as Adjunct to Enhance Topical Anesthetic before Intravenous Cannulation of Pediatric Thalassemic Patients Receiving Blood Transfusion: A Randomized Controlled Trial Protocol" Journal of Clinical Medicine 11, no. 18: 5291. https://doi.org/10.3390/jcm11185291
APA StyleAbdul Jalal, M. I., Ooi, K. S., Foo, K. C., Hamzah, A. A., Goh, C. S., Dee, C. F., Ooi, P. C., Buyong, M. R., Low, T. Y., Chua, X. Y., Lau, D. S. C., Abdul Latiff, Z., & Cheah, F. C. (2022). Transdermal Maltose-Based Microneedle Patch as Adjunct to Enhance Topical Anesthetic before Intravenous Cannulation of Pediatric Thalassemic Patients Receiving Blood Transfusion: A Randomized Controlled Trial Protocol. Journal of Clinical Medicine, 11(18), 5291. https://doi.org/10.3390/jcm11185291








