DSLchild-Algorithm-Based Hearing Aid Fitting Can Improve Speech Comprehension in Mildly Distressed Patients with Chronic Tinnitus and Mild-to-Moderate Hearing Loss
Abstract
:1. Introduction
2. Materials and Methods
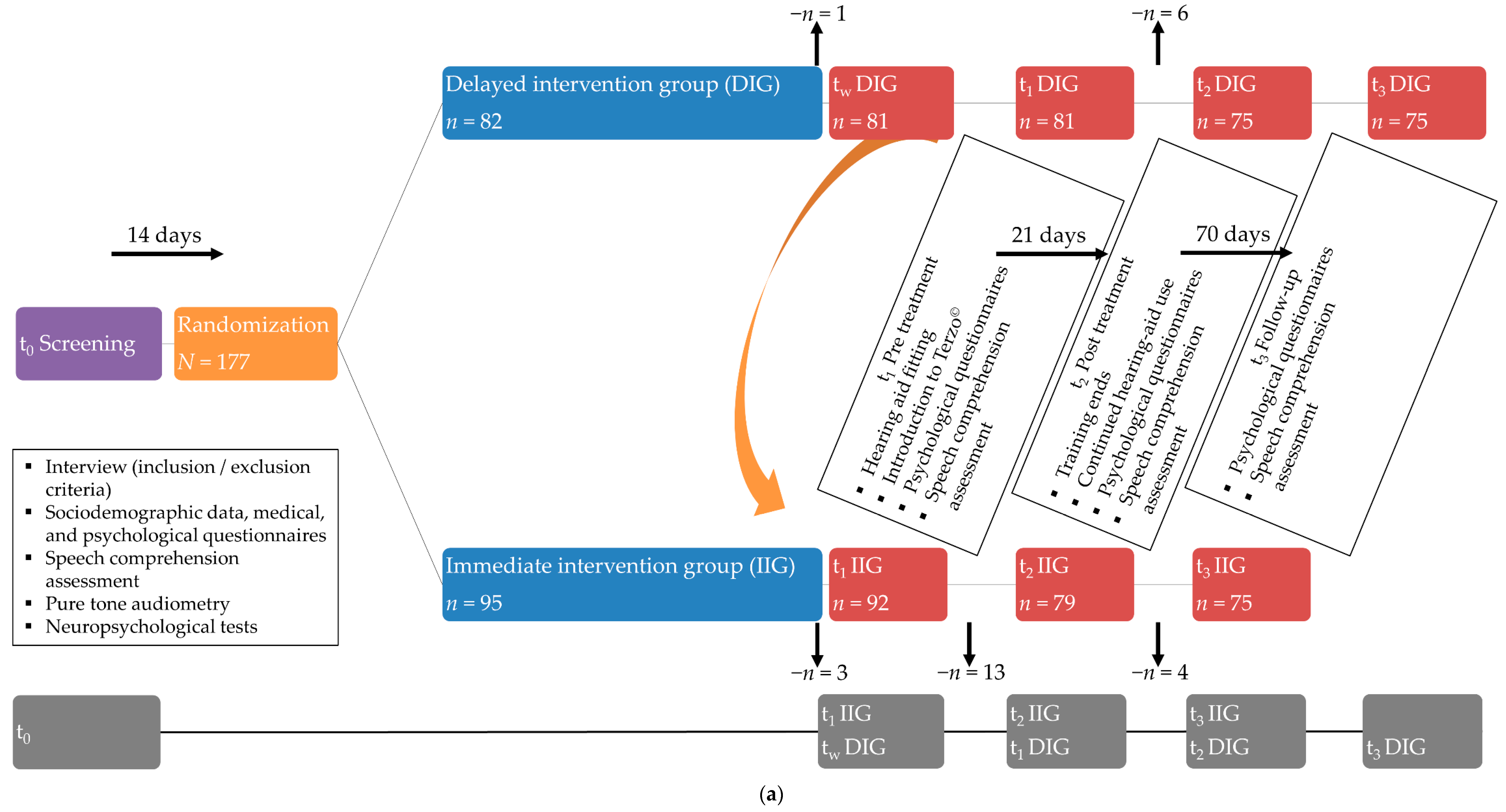
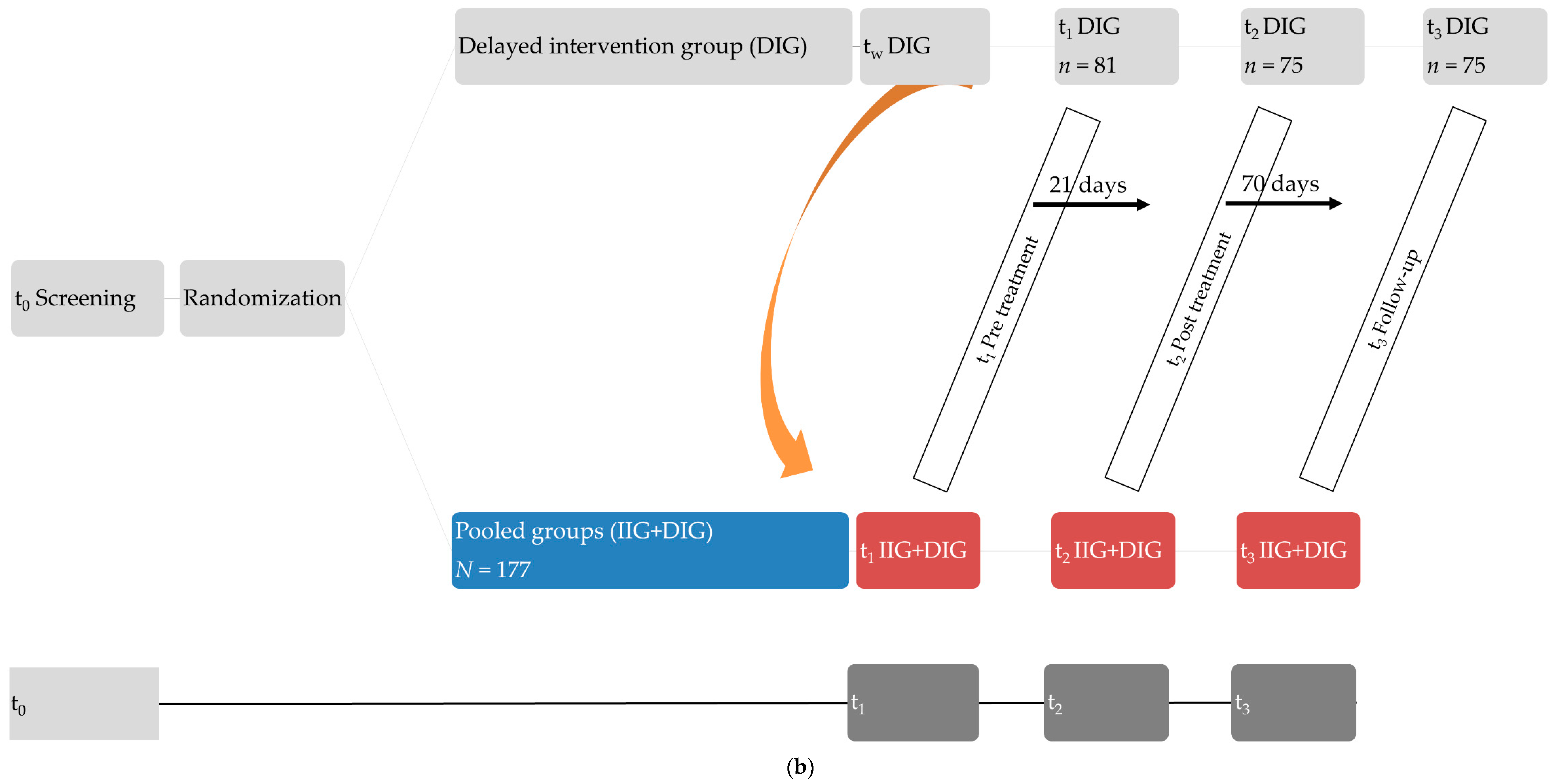
2.1. Participants and Design
Hearing Therapy
2.2. Measures
2.2.1. Speech Comprehension
2.2.2. Hearing Ability
2.2.3. Tinnitus-Related Distress
2.2.4. Psychological Epiphenomena
2.3. Statistical Analyses
3. Results
3.1. Descriptive Values for Patients with Mild or Moderate Hearing Loss
3.2. Correlational Analyses of Baseline Values with SC per Noise Interference Condition for Patients with Mild or Moderate Hearing Loss
3.3. Correlational Analyses of Baseline Values with Change in SC per Noise Interference Condition for Patients with Mild or Moderate Hearing Loss
3.4. Terzo© Hearing Therapy and Change in SC across Timepoints and Noise Interference Conditions
Follow-Up Analyses Investigating the Influence of Age on the Effects of Terzo© Hearing Therapy on SC at 0 dB Noise Interference
4. Discussion
4.1. Patients with Mild Hearing Loss
4.1.1. Speech Comprehension at Baseline
4.1.2. Change in Speech Comprehension with Treatment
4.1.3. Effects of Terzo© Hearing Therapy
4.2. Patients with Moderate Hearing Loss
4.2.1. Speech Comprehension at Baseline
4.2.2. Change in Speech Comprehension with Treatment
4.2.3. Effects of Terzo© Hearing Therapy
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Ridder, D.; Schlee, W.; Vanneste, S.; Londero, A.; Weisz, N.; Kleinjung, T.; Shekhawat, G.S.; Elgoyhen, A.B.; Song, J.-J.; Andersson, G. Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal). Prog. Brain Res. 2021, 260, 1–25. [Google Scholar]
- Biswas, R.; Hall, D.A. Prevalence, incidence, and risk factors for tinnitus. In The Behavioral Neuroscience of Tinnitus; Springer: Cham, Switzerland, 2020; pp. 3–28. [Google Scholar]
- McCormack, A.; Edmondson-Jones, M.; Somerset, S.; Hall, D. A systematic review of the reporting of tinnitus prevalence and severity. Hear. Res. 2016, 337, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Langguth, B.; Kreuzer, P.M.; Kleinjung, T.; De Ridder, D. Tinnitus: Causes and clinical management. Lancet Neurol. 2013, 12, 920–930. [Google Scholar] [CrossRef]
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef]
- Shargorodsky, J.; Curhan, G.C.; Farwell, W.R. Prevalence and characteristics of tinnitus among US adults. Am. J. Med. 2010, 123, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Ivansic, D.; Guntinas-Lichius, O.; Müller, B.; Volk, G.F.; Schneider, G.; Dobel, C. Impairments of speech comprehension in patients with tinnitus—A review. Front. Aging Neurosci. 2017, 9, 224. [Google Scholar] [CrossRef]
- Zeng, F.-G.; Richardson, M.; Turner, K. Tinnitus does not interfere with auditory and speech perception. J. Neurosci. 2020, 40, 6007–6017. [Google Scholar] [CrossRef]
- Hoare, D.J.; Edmondson-Jones, M.; Sereda, M.; Akeroyd, M.A.; Hall, D. Amplification with hearing aids for patients with tinnitus and co-existing hearing loss. Cochrane Database Syst. Rev. 2014, 1, CD010151. [Google Scholar] [CrossRef]
- Cima, R.F.F.; Mazurek, B.; Haider, H.; Kikidis, D.; Lapira, A.; Noreña, A.; Hoare, D.J. A multidisciplinary European guideline for tinnitus: Diagnostics, assessment, and treatment. Hno 2019, 67, 10–42. [Google Scholar] [CrossRef]
- Ferguson, M.A.; Kitterick, P.T.; Chong, L.Y.; Edmondson-Jones, M.; Barker, F.; Hoare, D.J. Hearing aids for mild to moderate hearing loss in adults. Cochrane Database Syst. Rev. 2017, 9, CD012023. [Google Scholar] [CrossRef]
- Hoppe, U.; Hast, A.; Hocke, T. Speech perception with hearing aids in comparison to pure-tone hearing loss. HNO 2014, 62, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.; Clark, T.M.; Johnson, J.S. Patient experiences with multiband full dynamic range compression. Ear Hear. 1992, 13, 320–330. [Google Scholar] [PubMed]
- Mondelli, M.F.C.G.; Santos, M.d.M.d.; José, M.R. Speech perception in noise in unilateral hearing loss. Braz. J. Otorhinolaryngol. 2016, 82, 427–432. [Google Scholar] [CrossRef]
- Helfer, K.S.; Wilber, L.A. Hearing loss, aging, and speech perception in reverberation and noise. J. Speech Lang. Hear. Res. 1990, 33, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Rudner, M.; Lunner, T. Cognitive spare capacity as a window on hearing aid benefit. In Proceedings of the Seminars in Hearing; Thieme Medical Publishers: New York, NY, USA, 2013; Volume 34, pp. 298–307. [Google Scholar]
- Fitzhugh, M.C.; LaCroix, A.N.; Rogalsky, C. Distinct Contributions of Working Memory and Attentional Control to Sentence Comprehension in Noise in Persons With Stroke. J. Speech Lang. Hear. Res. 2021, 64, 3230–3241. [Google Scholar] [CrossRef]
- Xie, Z.; Zinszer, B.D.; Riggs, M.; Beevers, C.G.; Chandrasekaran, B. Impact of depression on speech perception in noise. PLoS ONE 2019, 14, e0220928. [Google Scholar] [CrossRef]
- Christopher, G.; MacDonald, J. The impact of clinical depression on working memory. Cognit. Neuropsychiatry 2005, 10, 379–399. [Google Scholar] [CrossRef]
- Mattys, S.L.; Seymour, F.; Attwood, A.S.; Munafò, M.R. Effects of acute anxiety induction on speech perception: Are anxious listeners distracted listeners? Psychol. Sci. 2013, 24, 1606–1608. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.P. Anxiety and working memory capacity: A meta-analysis and narrative review. Psychol. Bull. 2016, 142, 831–864. [Google Scholar] [CrossRef]
- Lawrence, B.J.; Jayakody, D.M.; Henshaw, H.; Ferguson, M.A.; Eikelboom, R.H.; Loftus, A.M.; Friedland, P.L. Auditory and cognitive training for cognition in adults with hearing loss: A systematic review and meta-analysis. Trends Hear. 2018, 22, 2331216518792096. [Google Scholar] [CrossRef]
- Henshaw, H.; Ferguson, M.A. Efficacy of individual computer-based auditory training for people with hearing loss: A systematic review of the evidence. PLoS ONE 2013, 8, e62836. [Google Scholar]
- Gaeta, L.; Stark, R.K.; Ofili, E. Methodological Considerations for Auditory Training Interventions for Adults With Hearing Loss: A Rapid Review. Am. J. Audiol. 2021, 30, 211–225. [Google Scholar] [CrossRef]
- Roberts, L.E.; Bosnyak, D.J. Auditory training in tinnitus. In Textbook of Tinnitus; Springer: Berlin, Germany, 2011; pp. 563–573. [Google Scholar]
- Hoare, D.J.; Kowalkowski, V.L.; Kang, S.; Hall, D.A. Systematic review and meta-analyses of randomized controlled trials examining tinnitus management. Laryngoscope 2011, 121, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Boecking, B.; Rausch, L.; Psatha, S.; Nyamaa, A.; Dettling-Papargyris, J.; Funk, C.; Brueggemann, P.; Rose, M.; Mazurek, B. Hearing Therapy Improves Tinnitus-Related Distress in Mildly Distressed Patients with Chronic Tinnitus and Mild-to-Moderate Hearing Loss: A Randomized-Controlled Cross-Over Design. J. Clin. Med. 2022, 11, 1764. [Google Scholar] [CrossRef] [PubMed]
- Chun, M.M.; Golomb, J.D.; Turk-Browne, N.B. A taxonomy of external and internal attention. Annu. Rev. Psychol. 2011, 62, 73–101. [Google Scholar] [CrossRef] [PubMed]
- Ingram, R.E. Self-focused attention in clinical disorders: Review and a conceptual model. Psychol. Bull. 1990, 107, 156–176. [Google Scholar] [CrossRef]
- Neff, P.; Simões, J.; Psatha, S.; Nyamaa, A.; Boecking, B.; Rausch, L.; Dettling-Papargyris, J.; Funk, C.; Brueggemann, P.; Mazurek, B. The impact of tinnitus distress on cognition. Sci. Rep. 2021, 11, 2243. [Google Scholar] [CrossRef]
- Funk, C.; Wohlfeil, J.; Jauch, E.; Sorg, R. Manual der terzo Gehörtherapie 2008.
- Moodie, S.T.; Scollie, S.D.; Bagatto, M.P.; Keene, K.; The Network of Pediatric Audiologists of Canada. Fit-to-targets for the desired sensation level version 5.0 a hearing aid prescription method for children. Am. J. Audiol. 2017, 26, 251–258. [Google Scholar] [CrossRef]
- Scollie, S.; Seewald, R.; Cornelisse, L.; Moodie, S.; Bagatto, M.; Laurnagaray, D.; Beaulac, S.; Pumford, J. The desired sensation level multistage input/output algorithm. Trends Amplif. 2005, 9, 159–197. [Google Scholar] [CrossRef]
- Hoth, S. Der Freiburger Sprachtest: Eine Säule der Sprachaudiometrie im deutschsprachigen Raum (Leitthema). HNO 2016, 64, 540–548. [Google Scholar] [CrossRef]
- Organization, W.H. Report of the Informal Working Group on Prevention of Deafness and Hearing loss Programme Planning, Geneva, 18–21 June 1991; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Goebel, G.; Hiller, W. Tinnitus-Fragebogen:(TF); ein Instrument zur Erfassung von Belastung und Schweregrad bei Tinnitus; Handanweisung; Hogrefe, Verlag für Psychologie: Göttingen, Germany, 1998. [Google Scholar]
- Kleinjung, T.; Fischer, B.; Langguth, B.; Sand, P.G.; Hajak, G.; Dvorakova, J.; Eichhammer, P. Validierung einer deutschsprachigen Version des “Tinnitus Handicap Inventory”. Psychiatr. Prax. 2007, 34, S140–S142. [Google Scholar] [CrossRef]
- Brueggemann, P.; Szczepek, A.; Kleinjung, T.; Ojo, M.; Mazurek, B. Validierung der deutschen Version des Tinnitus Functional Index (TFI). Laryngo-Rhino-Otol 2017, 96, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Fliege, H.; Rose, M.; Arck, P.; Walter, O.B.; Kocalevent, R.-D.; Weber, C.; Klapp, B.F. The Perceived Stress Questionnaire (PSQ) reconsidered: Validation and reference values from different clinical and healthy adult samples. Psychosom. Med. 2005, 67, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Buss, U.; Snaith, R.P. HADS-D: Hospital Anxiety and Depression Scale (German Version); Hans Huber: Bern, Switzerland, 1995; Volume 1, p. 995. [Google Scholar]
- Tritt, K.; von Heymann, F.; Zaudig, M.; Zacharias, I.; Söllner, W.; Loew, T. Entwicklung des Fragebogens» ICD-10-Symptom-Rating «(ISR). Z. Psychosom. Med. Psychother. 2008, 54, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.F.; Schirmer, N.; Tritt, K.; Klapp, B.F.; Fliege, H. Retest-Reliabilität und Änderungssensitivität des ICD-10-Symptom-Rating (ISR) in verschiedenen Stichproben. PPmP-Psychother. Psychosom. Med. Psychol. 2011, 61, 162–169. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: Abingdon-on-Thames, UK, 1988. [Google Scholar]
- Stevens, G.; Flaxman, S.; Brunskill, E.; Mascarenhas, M.; Mathers, C.D.; Finucane, M. Global and regional hearing loss prevalence: An analysis of 42 studies in 29 countries. Eur. J. Public Health 2013, 23, 146–152. [Google Scholar] [CrossRef]
- Roth, T.N.; Hanebuth, D.; Probst, R. Prevalence of age-related hearing loss in Europe: A review. Eur. Arch. Otorhinolaryngol. 2011, 268, 1101–1107. [Google Scholar] [CrossRef]
- Seidman, M.D.; Ahmad, N.; Bai, U. Molecular mechanisms of age-related hearing loss. Ageing Res. Rev. 2002, 1, 331–343. [Google Scholar] [CrossRef]
- Frisina, R.D. Age-related hearing loss: Ear and brain mechanisms. Ann. N. Y. Acad. Sci. 2009, 1170, 708–717. [Google Scholar] [CrossRef]
- Rousset, F.; Nacher-Soler, G.; Coelho, M.; Ilmjarv, S.; Kokje, V.B.C.; Marteyn, A.; Cambet, Y.; Perny, M.; Roccio, M.; Jaquet, V. Redox activation of excitatory pathways in auditory neurons as mechanism of age-related hearing loss. Redox Biol. 2020, 30, 101434. [Google Scholar] [CrossRef]
- Bowl, M.R.; Dawson, S.J. Age-related hearing loss. Cold Spring Harb. Perspect. Med. 2019, 9, a033217. [Google Scholar] [CrossRef] [PubMed]
- Isaacowitz, D.M.; Charles, S.T.; Carstensen, L.L. Emotion and cognition. In The Handbook of Aging and Cognition; Lawrence Erlbaum Associates Inc.: Mahwah, NJ, USA, 2000. [Google Scholar]
- Gray, J.R. Integration of emotion and cognitive control. Curr. Dir. Psychol. Sci. 2004, 13, 46–48. [Google Scholar] [CrossRef]
- Gray, J.A. Brain systems that mediate both emotion and cognition. Cogn. Emot. 1990, 4, 269–288. [Google Scholar] [CrossRef]
- Bell, M.A.; Wolfe, C.D. Emotion and cognition: An intricately bound developmental process. Child Dev. 2004, 75, 366–370. [Google Scholar] [CrossRef]
- Shoham, N.; Lewis, G.; Favarato, G.; Cooper, C. Prevalence of anxiety disorders and symptoms in people with hearing loss: A systematic review. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 649–660. [Google Scholar] [CrossRef]
- Blazer, D.G.; Tucci, D.L. Hearing loss and psychiatric disorders: A review. Psychol. Med. 2019, 49, 891–897. [Google Scholar] [CrossRef]
- Hickson, L.; Meyer, C.; Lovelock, K.; Lampert, M.; Khan, A. Factors associated with success with hearing aids in older adults. Int. J. Audiol. 2014, 53, S18–S27. [Google Scholar] [CrossRef]
- Boecking, B.; Brueggemann, P.; Kleinjung, T.; Mazurek, B. All for One and One for All?–Examining Convergent Validity and Responsiveness of the German Versions of the Tinnitus Questionnaire (TQ), Tinnitus Handicap Inventory (THI), and Tinnitus Functional Index (TFI). Front. Psychol. 2021, 12, 596037. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.; Fortnum, H. Why do people fitted with hearing aids not wear them? Int. J. Audiol. 2013, 52, 360–368. [Google Scholar] [CrossRef]
- Jagoda, L.; Giroud, N.; Neff, P.; Kegel, A.; Kleinjung, T.; Meyer, M. Speech perception in tinnitus is related to individual distress level-A neurophysiological study. Hear. Res. 2018, 367, 48–58. [Google Scholar] [CrossRef]
- Acar, B.; Yurekli, M.F.; Babademez, M.A.; Karabulut, H.; Karasen, R.M. Effects of hearing aids on cognitive functions and depressive signs in elderly people. Arch. Gerontol. Geriatr. 2011, 52, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Brewster, K.K.; Pavlicova, M.; Stein, A.; Chen, M.; Chen, C.; Brown, P.J.; Roose, S.P.; Kim, A.H.; Golub, J.S.; Brickman, A. A pilot randomized controlled trial of hearing aids to improve mood and cognition in older adults. Int. J. Geriatr. Psychiatry 2020, 35, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Shoham, N.; Lewis, G.; McManus, S.; Cooper, C. Common mental illness in people with sensory impairment: Results from the 2014 adult psychiatric morbidity survey. BJPsych Open 2019, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, R.T.; Reed, N.S.; Brewster, K.K.; Huang, A.; Rebok, G.; Rutherford, B.R.; Lin, F.R. Association of hearing loss with psychological distress and utilization of mental health services among adults in the United States. JAMA Netw. Open 2020, 3, e2010986. [Google Scholar] [CrossRef] [PubMed]
- Pichora-Fuller, M.K.; Singh, G. Effects of age on auditory and cognitive processing: Implications for hearing aid fitting and audiologic rehabilitation. Trends Amplif. 2006, 10, 29–59. [Google Scholar] [CrossRef]
- Pessoa, L. On the relationship between emotion and cognition. Nat. Rev. Neurosci. 2008, 9, 148–158. [Google Scholar] [CrossRef]
- Pessoa, L. How do emotion and motivation direct executive control? Trends Cogn. Sci. 2009, 13, 160–166. [Google Scholar] [CrossRef]
- Blair, K.S.; Smith, B.W.; Mitchell, D.G.; Morton, J.; Vythilingam, M.; Pessoa, L.; Fridberg, D.; Zametkin, A.; Nelson, E.E.; Drevets, W.C. Modulation of emotion by cognition and cognition by emotion. Neuroimage 2007, 35, 430–440. [Google Scholar] [CrossRef]
- Brewster, K.K.; Zilcha-Mano, S.; Wallace, M.L.; Kim, A.H.; Brown, P.J.; Roose, S.P.; Golub, J.S.; Galatioto, J.; Kuhlmey, M.; Rutherford, B.R. A Precision Medicine Tool to Understand Who Responds Best to Hearing Aids in Late-Life Depression. Int. J. Geriatr. Psychiatry 2022, 37. [Google Scholar] [CrossRef]
- Houmøller, S.S.; Wolff, A.; Möller, S.; Narne, V.K.; Narayanan, S.K.; Godballe, C.; Hougaard, D.D.; Loquet, G.; Gaihede, M.; Hammershøi, D. Prediction of successful hearing aid treatment in first-time and experienced hearing aid users: Using the international outcome inventory for hearing aids. Int. J. Audiol. 2022, 61, 119–129. [Google Scholar] [CrossRef]
- Jayakody, D.M.; Almeida, O.P.; Speelman, C.P.; Bennett, R.J.; Moyle, T.C.; Yiannos, J.M.; Friedland, P.L. Association between speech and high-frequency hearing loss and depression, anxiety and stress in older adults. Maturitas 2018, 110, 86–91. [Google Scholar] [CrossRef] [PubMed]

| n | % | ||
|---|---|---|---|
| Education | |||
| Completed junior apprenticeship | 72 | 40.7 | |
| Completed senior apprenticeship | 40 | 22.6 | |
| University degree | 60 | 33.9 | |
| Other | 4 | 2.3 | |
| Employment ‘yes’ | 105 | 59.3 | |
| Relationship status | |||
| Single | 25 | 14.1 | |
| Married | 114 | 64.4 | |
| Divorced | 27 | 15.3 | |
| Widowed | 10 | 5.6 | |
| Duration of tinnitus | |||
| <0.5 year | 5 | 2.8 | |
| 0.5–1 year | 9 | 5.1 | |
| 1–2 years | 23 | 13.0 | |
| 2–5 years | 24 | 13.6 | |
| >5 years | 107 | 60.5 | |
| Tinnitus onset | |||
| Gradual | 92 | 52.0 | |
| Sudden | 73 | 41.2 | |
| Frequency | |||
| Very High | 37 | 20.9 | |
| High | 104 | 58.8 | |
| Middle | 32 | 18.1 | |
| Low | 3 | 1.7 | |
| Past psychotherapy ‘yes’ | 53 | 29.9 | |
| Use of hearing aid ‘yes’ | 53 | 31.5 | |
| Mild (n = 124) | Moderate (n = 53) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | M | SD | M | SD | Difference | t(df) | d |
| Age | 58.46 | 7.17 | 62.35 | 7.51 | −3.89 | t(174) = −3.24 ** | −0.54 |
| Hearing ability | 33.47 | 5.08 | 45.62 | 3.88 | −12.15 | t(126.99) = −17.31 *** | −2.56 |
| SC 0 dB | 92.89 | 9.55 | 83.73 | 13.78 | 9.17 | t(71.071) = 4.33 *** | 0.84 |
| SC 55 dB | 61.41 | 15.06 | 47.25 | 13.83 | 14.15 | t(170) = 5.76 *** | 0.96 |
| SC 65 dB | 24.30 | 16.81 | 15.39 | 14.03 | 8.91 | t(170) = 3.33 ** | 0.56 |
| TQ | 33.32 | 15.76 | 33.23 | 17.03 | 0.10 | ||
| THI | 33.39 | 21.96 | 32.04 | 22.49 | 1.35 | ||
| TFI | 39.58 | 20.35 | 40.48 | 20.77 | −0.90 | ||
| PSQ | 30.05 | 16.65 | 32.86 | 23.89 | −2.82 | ||
| HADS_a | 6.35 | 4.07 | 7.43 | 4.75 | −1.09 | ||
| HADS_d | 4.98 | 4.38 | 6.15 | 5.25 | −1.17 | ||
| ISR | 0.61 | 0.50 | 0.74 | 0.59 | −0.13 | ||
| Mild (n = 124) | Moderate (n = 53) | Mild (n = 124) | Moderate (n = 53) | Mild (n = 124) | Moderate (n = 53) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Noise Interference | SC 0 dB | SC 0 dB | z | SC 55 dB | SC 55 dB | z | SC 65 dB | SC 65 dB | z |
| Age | −0.25 ** | ||||||||
| Hearing ability | −0.37 ** | −0.35 * | −0.29 ** | −0.42 ** | −0.25 ** | ||||
| TQ | −0.04 | 0.37 ** | 2.45 * | −0.20 * | 0.21 | −2.39 * | |||
| THI | −0.21 * | 0.23 | −2.62 ** | ||||||
| TFI | −0.20 ** | 0.23 | −2.57 * | −0.31 ** | 0.19 | −2.99 ** | −0.20 * | 0.23 | −2.56 * |
| PSQ | |||||||||
| HADS_a | −0.22 * | ||||||||
| HADS_d | |||||||||
| ISR | −0.28 ** |
| Change in SC | t0–t1 | t1–t2 | t2–t3 | t0–t1 | t1–t2 | t2–t3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effects | Hearing Aids | Auditory Training | Stability | Hearing Aids | Auditory Training | Stability | ||||||||||||
| Noise Interference | 0 dB | 0 dB | 0 dB | 55 dB | 55 dB | 55 dB | ||||||||||||
| Hearing loss | Mild n = 124 | Mod n = 53 | z | Mild n = 124 | Mod n = 53 | z | Mild n = 124 | Mod n = 53 | z | Mild n = 124 | Mod n = 53 | z | Mild n = 124 | Mod n = 53 | z | Mild n = 124 | Mod n = 53 | z |
| Age | −0.21 * | −0.30 * | ||||||||||||||||
| Hearing ability | −0.37 ** | |||||||||||||||||
| TQ | 0.36 * | −0.26 ** | 0.22 | −2.71 ** | 0.34 ** | 0.05 | ||||||||||||
| THI | −0.27 ** | 0.10 | −2.08 * | 0.31 ** | 0.02 | −0.09 | 0.29 * | −2.23 * | ||||||||||
| TFI | −0.30 ** | 0.16 | −2.52 * | 0.31 ** | 0.08 | −0.19 * | 0.27 | −2.68 ** | ||||||||||
| PSQ | 0.23 * | −0.28 ** | 0.06 | 0.20 * | 0.18 | |||||||||||||
| HADS_a | 0.21 * | 0.20 | ||||||||||||||||
| HADS_d | 0.19 * | 0.21 | ||||||||||||||||
| ISR | 0.25 * | 0.16 | ||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boecking, B.; Rausch, L.; Psatha, S.; Nyamaa, A.; Dettling-Papargyris, J.; Funk, C.; Oppel, K.; Brueggemann, P.; Rose, M.; Mazurek, B. DSLchild-Algorithm-Based Hearing Aid Fitting Can Improve Speech Comprehension in Mildly Distressed Patients with Chronic Tinnitus and Mild-to-Moderate Hearing Loss. J. Clin. Med. 2022, 11, 5244. https://doi.org/10.3390/jcm11175244
Boecking B, Rausch L, Psatha S, Nyamaa A, Dettling-Papargyris J, Funk C, Oppel K, Brueggemann P, Rose M, Mazurek B. DSLchild-Algorithm-Based Hearing Aid Fitting Can Improve Speech Comprehension in Mildly Distressed Patients with Chronic Tinnitus and Mild-to-Moderate Hearing Loss. Journal of Clinical Medicine. 2022; 11(17):5244. https://doi.org/10.3390/jcm11175244
Chicago/Turabian StyleBoecking, Benjamin, Leonie Rausch, Stamatina Psatha, Amarjargal Nyamaa, Juliane Dettling-Papargyris, Christine Funk, Kevin Oppel, Petra Brueggemann, Matthias Rose, and Birgit Mazurek. 2022. "DSLchild-Algorithm-Based Hearing Aid Fitting Can Improve Speech Comprehension in Mildly Distressed Patients with Chronic Tinnitus and Mild-to-Moderate Hearing Loss" Journal of Clinical Medicine 11, no. 17: 5244. https://doi.org/10.3390/jcm11175244
APA StyleBoecking, B., Rausch, L., Psatha, S., Nyamaa, A., Dettling-Papargyris, J., Funk, C., Oppel, K., Brueggemann, P., Rose, M., & Mazurek, B. (2022). DSLchild-Algorithm-Based Hearing Aid Fitting Can Improve Speech Comprehension in Mildly Distressed Patients with Chronic Tinnitus and Mild-to-Moderate Hearing Loss. Journal of Clinical Medicine, 11(17), 5244. https://doi.org/10.3390/jcm11175244









