Efficacy of Combination Therapy with Epinephrine Local Injection and Hemostatic Clips on Active Diverticular Bleeding
Abstract
1. Introduction
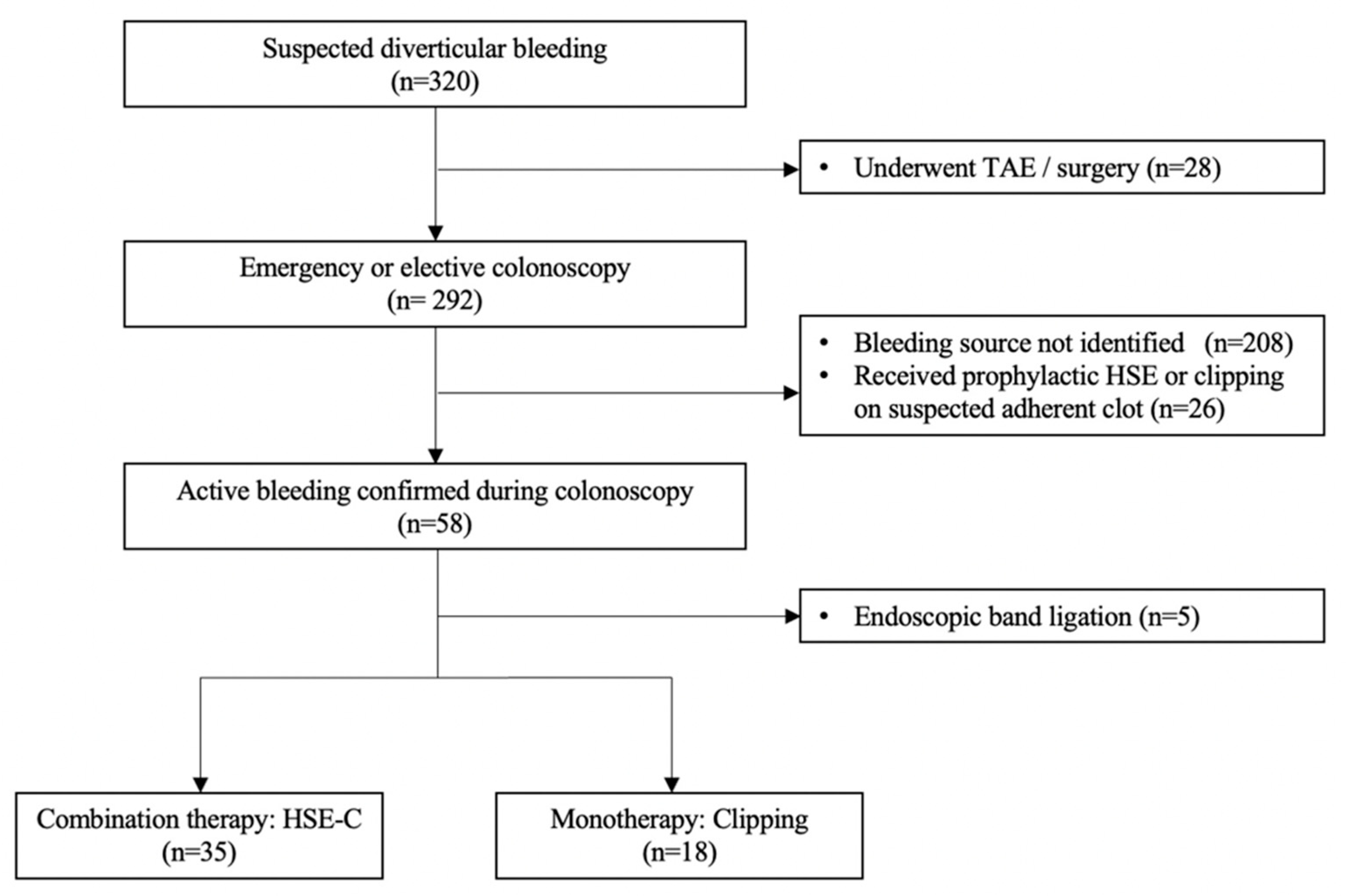
2. Materials and Methods
2.1. Study Setting
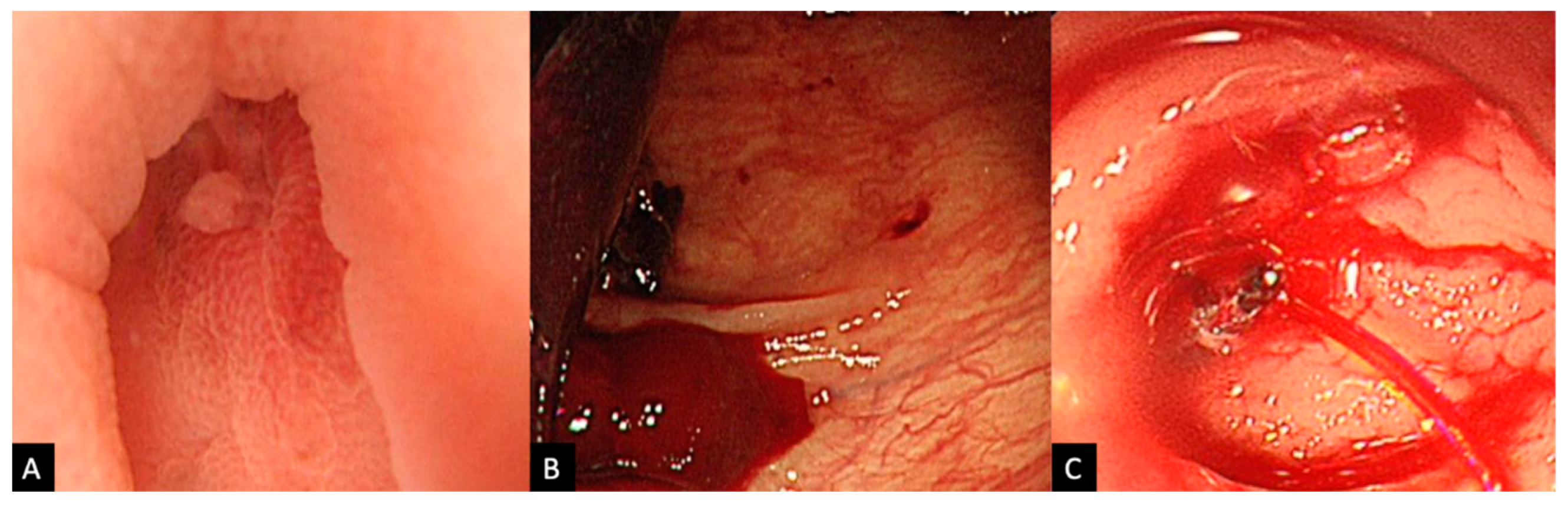
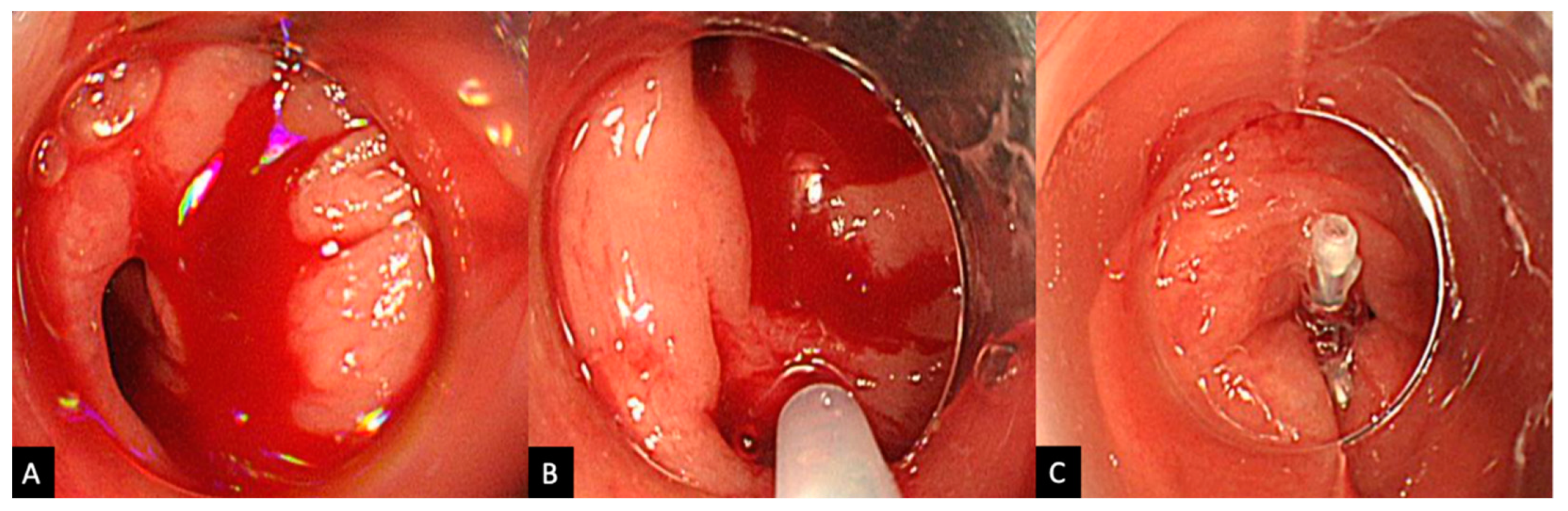
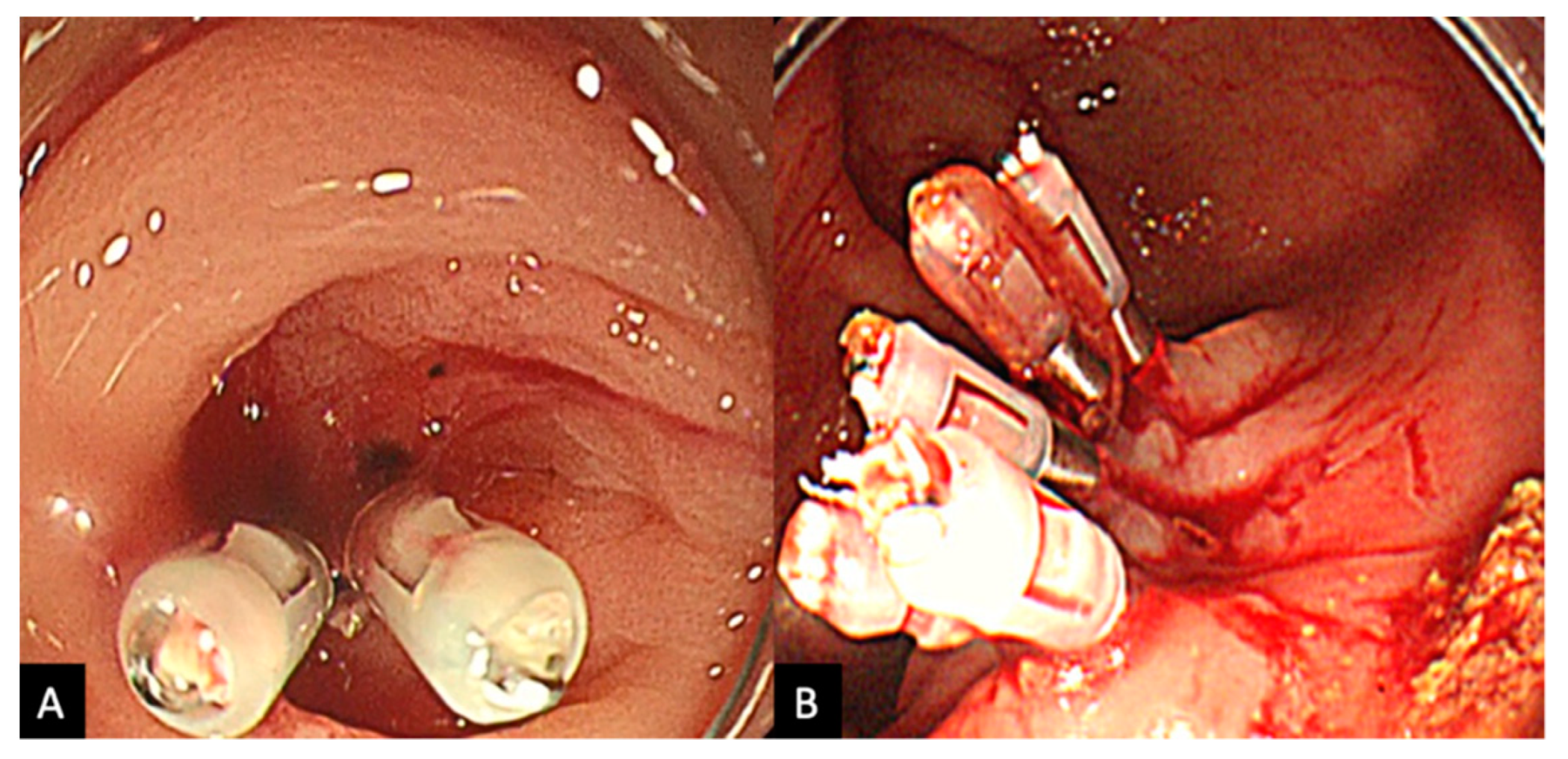
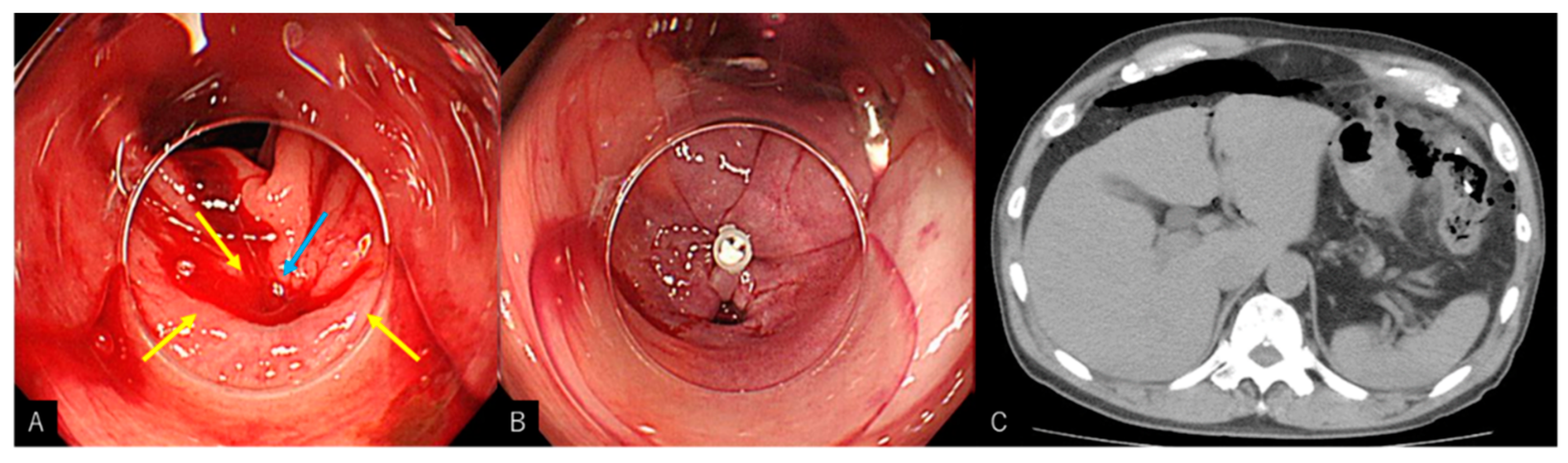
2.2. Procedure
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Outcomes
3.3. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laine, L.; Yang, H.; Chang, S.C.; Datto, C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am. J. Gastroenterol. 2012, 107, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Hreinsson, J.P.; Gumundsson, S.; Kalaitzakis, E.; Björnsson, E.S. Lower gastrointestinal bleeding: Incidence, etiology, and outcomes in a population-based setting. Eur. J. Gastroenterol. Hepatol. 2013, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: A population-based study. Am. J. Gastroenterol. 1997, 92, 419–424. [Google Scholar] [PubMed]
- Ron-Tal Fisher, O.; Gralnek, I.M.; Eisen, G.M.; Williams, J.L.; Holub, J.L. Endoscopic hemostasis is rarely used for hematochezia: A population-based study from the Clinical Outcomes Research Initiative National Endoscopic Database. Gastrointest. Endosc. 2014, 79, 317–325. [Google Scholar] [CrossRef][Green Version]
- Oakland, K.; Guy, R.; Uberoi, R.; Hogg, R.; Mortensen, N.; Murphy, M.F.; Jairath, V. Acute lower GI bleeding in the UK: Patient characteristics, interventions and outcomes in the first nationwide audit. Gut 2018, 67, 654–662. [Google Scholar] [CrossRef]
- McGuire, H.H., Jr. Bleeding colonic diverticula. A reappraisal of natural history and management. Ann. Surg. 1994, 220, 653–656. [Google Scholar] [CrossRef]
- Jensen, D.M.; Machicado, G.A. Diagnosis and treatment of severe hematochezia. The role of urgent colonoscopy after purge. Gastroenterology 1988, 95, 1569–1574. [Google Scholar] [CrossRef]
- Kato, M. Endoscopic Therapy for Acute Diverticular Bleeding. Clin. Endosc. 2019, 52, 419–425. [Google Scholar] [CrossRef]
- Kaltenbach, T.; Watson, R.; Shah, J.; Friedland, S.; Sato, T.; Shergill, A.; McQuaid, K.; Soetikno, R. Colonoscopy with clipping is useful in the diagnosis and treatment of diverticular bleeding. Clin. Gastroenterol. Hepatol. 2012, 10, 131–137. [Google Scholar] [CrossRef]
- Bloomfeld, R.S.; Rockey, D.C.; Shetzline, M.A. Endoscopic therapy of acute diverticular hemorrhage. Am. J. Gastroenterol. 2001, 96, 2367–2372. [Google Scholar] [CrossRef]
- Setoyama, T.; Ishii, N.; Fujita, Y. Enodoscopic band ligation (EBL) is superior to endoscopic clipping for the treatment of colonic diverticular hemorrhage. Surg. Endosc. 2011, 25, 3574–3578. [Google Scholar] [CrossRef] [PubMed]
- Wedi, E.; von Renteln, D.; Jung, C.; Tchoumak, I.; Roth, V.; Gonzales, S.; Leroy, J.; Hochberger, J. Treatment of acute colonic diverticular bleeding in high risk patients, using an over-the-scope clip: A case series. Endoscopy 2016, 48, E383–E385. [Google Scholar] [CrossRef] [PubMed]
- Green, B.T.; Rockey, D.C.; Portwood, G.; Tarnasky, P.R.; Guarisco, S.; Branch, M.S.; Leung, J.; Jowell, P. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: A randomized controlled trial. Am. J. Gastroenterol. 2005, 100, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Kawano, K.; Takenaka, M.; Kawano, R.; Kagoshige, D.; Kawase, Y.; Moriguchi, T.; Tanabe, H.; Katoh, T.; Nishi, K.; Kudo, M. Efficacy of Over-The-Scope Clip Method as a Novel Hemostatic Therapy for Colonic Diverticular Bleeding. J. Clin. Med. 2021, 10, 2891. [Google Scholar] [CrossRef]
- Ishii, N.; Omata, F.; Nagata, N.; Kaise, M. Effectiveness of endoscopic treatments for colonic diverticular bleeding. Gastrointest. Endosc. 2018, 87, 58–66. [Google Scholar] [CrossRef]
- Tsuruoka, N.; Takedomi, H.; Sakata, Y.; Shimoda, R. Recent Trends in Treatment for Colonic Diverticular Bleeding in Japan. Digestion 2020, 101, 12–17. [Google Scholar] [CrossRef]
- Kim, Y.I.; Marcon, N.E. Injection therapy for colonic diverticular bleeding. A case study. J. Clin. Gastroenterol. 1993, 17, 46–48. [Google Scholar] [CrossRef]
- Jensen, D.M.; Machicado, G.A.; Jutabha, R.; Kovacs, T.O. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N. Engl. J. Med. 2000, 342, 78–82. [Google Scholar] [CrossRef]
- Hirao, M.; Kobayashi, T.; Masuda, K.; Yamaguchi, S.; Noda, K.; Matsuura, K.; Naka, H.; Kawauchi, H.; Namiki, M. Endoscopic local injection of hypertonic saline-epinephrine solution to arrest hemorrhage from the upper gastrointestinal tract. Gastrointest. Endosc. 1985, 31, 313–317. [Google Scholar] [CrossRef]
- Kaise, M.; Nagata, N.; Ishii, N.; Omori, J.; Goto, O.; Iwakiri, K. Epidemiology of colonic diverticula and recent advances in the management of colonic diverticular bleeding. Dig. Endosc. 2020, 32, 240–250. [Google Scholar] [CrossRef]
- Yen, E.F.; Ladabaum, U.; Muthusamy, V.R.; Cello, J.P.; McQuaid, K.R.; Shah, J.N. Colonoscopic treatment of acute diverticular hemorrhage using endoclips. Dig. Dis. Sci. 2008, 53, 2480–2485. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Artifon, E.; Chu, A.; Halwan, B. Effectiveness of endoclips for the treatment of stigmata of recent hemorrhage in the colon of patients with acute lower gastrointestinal tract bleeding. Dig. Dis. Sci. 2011, 56, 2978–2986. [Google Scholar] [CrossRef]
- Pasha, S.F.; Shergill, A.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Early, D.; Evans, J.A.; Fisher, D.; Fonkalsrud, L.; Hwang, J.H.; et al. The role of endoscopy in the patient with lower GI bleeding. Gastrointest. Endosc. 2014, 79, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Niikura, R.; Yoshida, S.; Hirata, Y.; Koike, K. Endoscopic management of colonic diverticular bleeding. Dig. Endosc. 2015, 27, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Goelder, S.K.; Brueckner, J.; Messmann, H. Endoscopic hemostasis state of the art—Nonvariceal bleeding. World J. Gastrointest. Endosc. 2016, 8, 205–211. [Google Scholar] [CrossRef]
- Lewis, S.B.; Khayat, M.; Srinivasa, R.; Chick, J.F.B.; Gemmete, J.J.; Srinivasa, R.N. Bleeding diverticulum of the colon treated with CT-guided percutaneous injection of epinephrine and cyanoacrylate. Radiol. Case Rep. 2018, 13, 596–598. [Google Scholar] [CrossRef]
- Gagliardi, M.; Oliviero, G.; Fusco, M.; Napoli, M.; Sica, A.; Maurano, A.; Sica, M.; Zulli, C. Novel Hemostatic Gel as Rescue Therapy for Postsphincterotomy Bleeding Refractory to Self-Expanding Metallic Stent Placement. ACG Case Rep. J. 2022, 9, e00744. [Google Scholar] [CrossRef]
- Ramirez, F.C.; Johnson, D.A.; Zierer, S.T.; Walker, G.J.; Sanowski, R.A. Successful endoscopic hemostasis of bleeding colonic diverticula with epinephrine injection. Gastrointest. Endosc. 1996, 43, 167–170. [Google Scholar] [CrossRef]
- Kishino, T.; Kanemasa, K.; Kitamura, Y.; Fukumoto, K.; Okamoto, N.; Shimokobe, H. Usefulness of direct clipping for the bleeding source of colonic diverticular hemorrhage (with videos). Endosc. Int. Open 2020, 8, E377–E385. [Google Scholar] [CrossRef]
- Nagata, N.; Ishii, N.; Kaise, M.; Shimbo, T.; Sakurai, T.; Akiyama, J.; Uemura, N. Long-term recurrent bleeding risk after endoscopic therapy for definitive colonic diverticular bleeding: Band ligation versus clipping. Gastrointest. Endosc. 2018, 88, 841–853.e844. [Google Scholar] [CrossRef]
- Akutsu, D.; Narasaka, T.; Wakayama, M.; Terasaki, M.; Kaneko, T.; Matsui, H.; Suzuki, H.; Hyodo, I.; Mizokami, Y. Endoscopic detachable snare ligation: A new treatment method for colonic diverticular hemorrhage. Endoscopy 2015, 47, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Kishino, T.; Nagata, N.; Kobayashi, K.; Yamauchi, A.; Yamada, A.; Omori, J.; Ikeya, T.; Aoyama, T.; Tominaga, N.; Sato, Y.; et al. Endoscopic direct clipping versus indirect clipping for colonic diverticular bleeding: A large multicenter cohort study. United Eur. Gastroenterol. J. 2022, 10, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Hirata, N.; Omata, F.; Itoh, T.; Uemura, M.; Matsuda, M.; Suzuki, S.; Iizuka, Y.; Fukuda, K.; Fujita, Y. Location in the ascending colon is a predictor of refractory colonic diverticular hemorrhage after endoscopic clipping. Gastrointest. Endosc. 2012, 76, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, Y.; Ikeya, T.; Ishii, N.; Takasu, A.; Honda, H.; Nakamura, K.; Fukuda, K. Endoscopic Band Ligation for Acute Lower Gastrointestinal Bleeding. Intern. Med. 2019, 58, 3505–3508. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Ishii, N.; Ikeya, T.; Ego, M.; Shimamura, Y.; Takagi, K.; Nakamura, K.; Fukuda, K.; Fujita, Y. Comparison of long-term outcomes between endoscopic band ligation and endoscopic clipping for colonic diverticular hemorrhage. Endosc. Int. Open 2015, 3, E529–E533. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yasuda, H.; Fukuoka, A.; Kiyokawa, H.; Kato, M.; Yamashita, M.; Matsuo, Y.; Yamamoto, H.; Otsubo, T.; Itoh, F. Delayed perforation after endoscopic band ligation for colonic diverticular hemorrhage. Clin. J. Gastroenterol. 2020, 13, 6–10. [Google Scholar] [CrossRef]
- Takahashi, S.; Inaba, T.; Tanaka, N. Delayed perforation after endoscopic band ligation for treatment of colonic diverticular bleeding. Dig. Endosc. 2016, 28, 484. [Google Scholar] [CrossRef]
- Niikura, R.; Nagata, N.; Aoki, T.; Shimbo, T.; Tanaka, S.; Sekine, K.; Kishida, Y.; Watanabe, K.; Sakurai, T.; Yokoi, C.; et al. Predictors for identification of stigmata of recent hemorrhage on colonic diverticula in lower gastrointestinal bleeding. J. Clin. Gastroenterol. 2015, 49, e24–e30. [Google Scholar] [CrossRef]
| HSE-C (n = 35) | Clipping (n = 18) | p Value | |
|---|---|---|---|
| Age ± SD (range) | 76.7 ± 9.3 (61–93) | 69.4 ± 9.7 (50–83) | 0.011 * |
| Male % (n) | 62.9 (22) | 77.8 (14) | 0.358 |
| Concurrent Disease | |||
| Hypertension % (n) | 88.6 (31) | 66.7 (12) | 0.071 |
| Diabetes mellitus % (n) | 8.60 (3) | 22.2 (4) | 0.211 |
| Coronary artery disease % (n) | 22.9 (8) | 33.3 (6) | 0.515 |
| Antithrombotic agents % (n) | 65.7 (23) | 38.9 (7) | 0.083 |
| Aspirin | 40 (14) | 38.9 (7) | 1.000 |
| Clopidogrel | 11.4 (4) | 22.2 (4) | 0.421 |
| Cilostazol | 8.6 (3) | 5.5 (1) | 1.000 |
| Warfarin | 22.9 (8) | 11.1 (2) | 0.464 |
| Extravasation on CT scan % (n) | 70.5 (24) | 44.4 (8) | 0.155 |
| Right-sided bleeding % (n) | 57.2 (20) | 88.9 (16) | 0.029 * |
| HSE-C (n = 35) | Clipping (n = 18) | p Value | |
|---|---|---|---|
| Successful primary hemostasis % (n) | 91.4 (32/35) | 66.7 (12/18) | 0.048 * |
| Early recurrent bleeding (within 30 days) % (n) | 18.8 (6/32) | 8.3 (1/12) | 0.653 |
| Requirement for surgery during hospitalization % (n) | 11.4 (4/35) | 5.5 (1/18) | 0.651 |
| Persistent bleeding % (n) | 75 (3/4) | 100 (1/1) | |
| Delayed perforation % (n) | 25 (1/4) | 0 | |
| HSE local injection | |||
| Total amount (mL) ± SD | 2.1 ± 1.5 | N/A | |
| Number of injections ± SD | 2.5 ± 1.25 | N/A | |
| Transfusion % (n) | 45.7 (16/35) | 72.2 (13/18) | 0.085 |
| Adverse Events % (n) | 2.9 (1/35) | 0 (0/18) | 1.000 |
| Follow-up lost within 30 days of discharge | 11.4 (4/35) | 5.5 (1/18) | 0.651 |
| HSE-C (n = 35) | Clipping (n = 18) | p Value | |
|---|---|---|---|
| Direct clipping: Indirect clipping (direct %) | 21:14 (60) | 3:15 (16.7) | 0.004 * |
| Rate of successful hemostasis | |||
| Direct clipping % (n) | 85.7 (18/21) | 33.3 (1/3) | 0.099 |
| Indirect clipping % (n) | 100 (14/14) | 73.3 (11/15) | 0.996 |
| Rate of recurrent bleeding | |||
| Direct clipping % (n) | 16.7 (3/18) | 0 (0/1) | 1.000 |
| Indirect clipping % (n) | 21.4 (3/14) | 9.1 (1/11) | 0.604 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamada, S.; Teramoto, A.; Zukeyama, R.; Matsukawa, S.; Fukuhara, T.; Takaki, R.; Utsumi, T.; Nakamura, M.; Kobashikawa, K.; Uchima, N.; et al. Efficacy of Combination Therapy with Epinephrine Local Injection and Hemostatic Clips on Active Diverticular Bleeding. J. Clin. Med. 2022, 11, 5195. https://doi.org/10.3390/jcm11175195
Hamada S, Teramoto A, Zukeyama R, Matsukawa S, Fukuhara T, Takaki R, Utsumi T, Nakamura M, Kobashikawa K, Uchima N, et al. Efficacy of Combination Therapy with Epinephrine Local Injection and Hemostatic Clips on Active Diverticular Bleeding. Journal of Clinical Medicine. 2022; 11(17):5195. https://doi.org/10.3390/jcm11175195
Chicago/Turabian StyleHamada, Seiji, Akira Teramoto, Ryuta Zukeyama, Shinobu Matsukawa, Tomofumi Fukuhara, Ryo Takaki, Takahiro Utsumi, Masamoto Nakamura, Kasen Kobashikawa, Nobufumi Uchima, and et al. 2022. "Efficacy of Combination Therapy with Epinephrine Local Injection and Hemostatic Clips on Active Diverticular Bleeding" Journal of Clinical Medicine 11, no. 17: 5195. https://doi.org/10.3390/jcm11175195
APA StyleHamada, S., Teramoto, A., Zukeyama, R., Matsukawa, S., Fukuhara, T., Takaki, R., Utsumi, T., Nakamura, M., Kobashikawa, K., Uchima, N., Nakayoshi, T., & Kinjo, F. (2022). Efficacy of Combination Therapy with Epinephrine Local Injection and Hemostatic Clips on Active Diverticular Bleeding. Journal of Clinical Medicine, 11(17), 5195. https://doi.org/10.3390/jcm11175195





