Triglyceride–Glucose Index May Predict Renal Survival in Patients with IgA Nephropathy
Abstract
:1. Background
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Pathological Data Collection
2.3. Treatment and Definitions
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants
3.2. Correlation of the TyG Index with IgAN
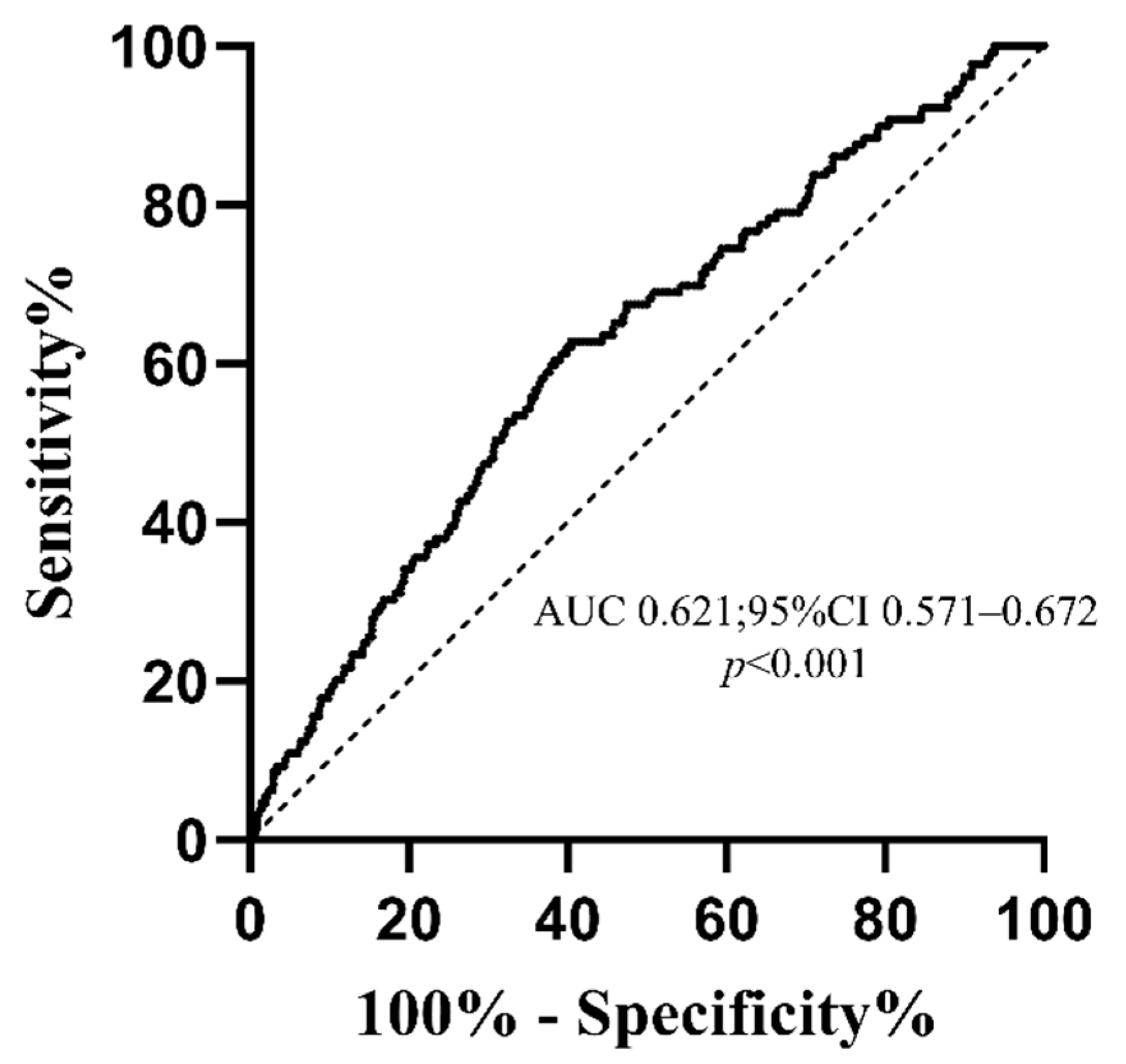
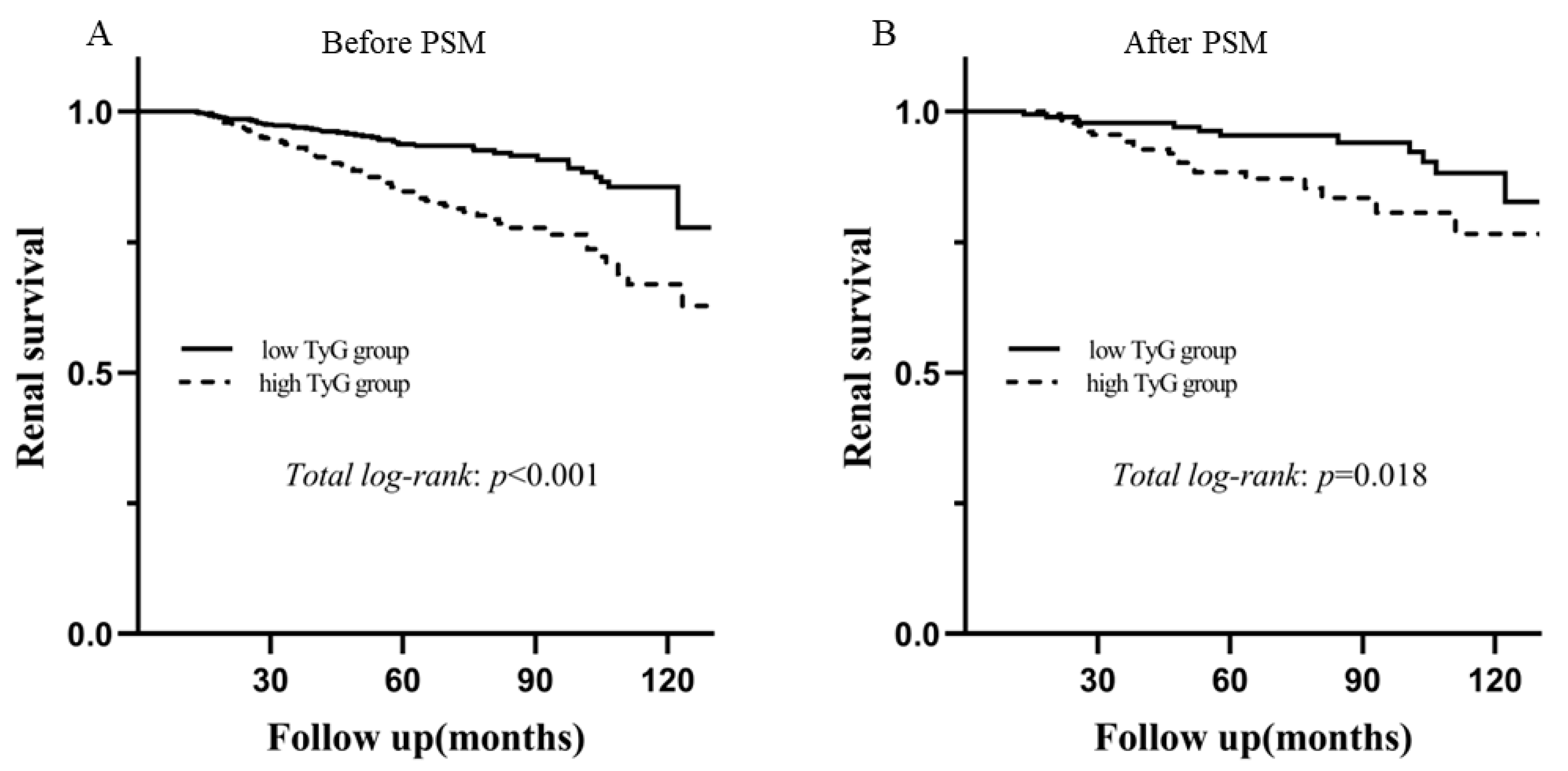
3.3. The TyG Index for Predicting Renal Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pattrapornpisut, P.; Avila-Casado, C.; Reich, H.N. IgA Nephropathy: Core Curriculum 2021. Am. J. Kidney Dis. 2021, 78, 429–441. [Google Scholar] [CrossRef]
- Rodas, L.; Barnadas, E.; Pereira, A.; Castrejon, N.; Saurina, A.; Calls, J.; Calzada, Y.; Madrid, Á.; Blasco, M.; Poch, E.; et al. The Density of Renal Lymphatics Correlates With Clinical Outcomes in IgA Nephropathy. Kidney Int. Rep. 2022, 7, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, A.; DeFronzo, R.A. Insulin Resistance and Atherosclerosis: Implications for Insulin-Sensitizing Agents. Endocr. Rev. 2019, 40, 1447–1467. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am. J. Physiol. Metab. 2008, 294, E15–E26. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, L.; Li, M.; Zhou, W.; Wang, T.; Zhu, L.; Bao, H.; Cheng, X.; Li, P. Association between the Surrogate Markers of Insulin Resistance and Chronic Kidney Disease in Chinese Hypertensive Patients. Front. Med. 2022, 9, 831648. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Qin, Z.; Zhao, J.; Geng, J.; Chang, K.; Liao, R.; Su, B. Higher Triglyceride-Glucose Index Is Associated With Increased Likelihood of Kidney Stones. Front. Endocrinol. 2021, 12, 774567. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Martínez-Rodríguez, J.; González-Lucán, M.; Fernández-Fernández, C.; Castro-Quintela, E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1449–1455. [Google Scholar] [CrossRef]
- Deger, S.M.; Hewlett, J.R.; Gamboa, J.; Ellis, C.D.; Hung, A.M.; Siew, E.D.; Mamnungu, C.; Sha, F.; Bian, A.; Stewart, T.G.; et al. Insulin resistance is a significant determinant of sarcopenia in advanced kidney disease. Am. J. Physiol. Metab. 2018, 315, E1108–E1120. [Google Scholar] [CrossRef]
- Spoto, B.; Pisano, A.; Zoccali, C. Insulin resistance in chronic kidney disease: A systematic review. Am. J. Physiol. Physiol. 2016, 311, F1087–F1108. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, L.; Qin, Y.; Luo, E.; Wang, D.; Qiao, Y.; Tang, C.; Yan, G. Elevated TyG Index Predicts Incidence of Contrast-Induced Nephropathy: A Retrospective Cohort Study in NSTE-ACS Patients Implanted With DESs. Front. Endocrinol. 2022, 13, 817176. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Brozek, W.; Concin, H.; Nagel, G.; Kerschbaum, J.; Lhotta, K.; Ulmer, H.; Zitt, E. The Triglyceride-Glucose Index and Obesity-Related Risk of End-Stage Kidney Disease in Austrian Adults. JAMA Netw. Open 2021, 4, e212612. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Qin, A.; Dong, L.; Wang, S.; Liu, X.; Yang, D.; Tan, J.; Zhou, X.; Tang, Y.; Qin, W. Prognostic Value of Triglyceride to High-Density Lipoprotein Cholesterol Ratio (TG/HDL-C) in IgA Nephropathy Patients. Front. Endocrinol. 2022, 13, 877794. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, R.B.; Wang, Y.D.; Zhang, X.G.; Rong, N.; Tang, L.; Chen, X.M. Higher HOMA-IR index and correlated factors of insulin resistance in patients with IgA nephropathy. Clin. Nephrol. 2012, 78, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Lassenius, M.I.; Pietiläinen, K.H.; Kaartinen, K.; Pussinen, P.J.; Syrjänen, J.; Forsblom, C.; Pörsti, I.; Rissanen, A.; Kaprio, J.; Mustonen, J.; et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 2011, 34, 1809–1815. [Google Scholar] [CrossRef]
- Trimarchi, H.; Barratt, J.; Cattran, D.C.; Cook, H.T.; Coppo, R.; Haas, M.; Liu, Z.H.; Roberts, I.S.; Yuzawa, Y.; Zhang, H.; et al. Oxford Classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017, 91, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, J.; Cattran, D.C. The KDIGO practice guideline on glomerulonephritis: Reading between the (guide)lines--application to the individual patient. Kidney Int. 2012, 82, 840–856. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef]
- Wang, J.; Xie, P.; Huang, J.M.; Qu, Y.; Zhang, F.; Wei, L.G.; Fu, P.; Huang, X.J. The new Asian modified CKD-EPI equation leads to more accurate GFR estimation in Chinese patients with CKD. Int. Urol. Nephrol. 2016, 48, 2077–2081. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, J.; Zhang, P.; Zhong, F.; Cai, J.; Ma, A. Association of dietary fiber intake with hyperuricemia in U.S. adults. Food Funct. 2019, 10, 4932–4940. [Google Scholar] [CrossRef]
- Tan, J.; Song, G.; Wang, S.; Dong, L.; Liu, X.; Jiang, Z.; Qin, A.; Tang, Y.; Qin, W. Platelet-to-Albumin Ratio: A Novel IgA Nephropathy Prognosis Predictor. Front. Immunol. 2022, 13, 842362. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, X.; Wang, X.; Chen, H.; Xiao, H.; Tang, H.; Feng, L.; Xiang, Z.; Zou, H.; Shao, X. Comparison of Novel Metabolic Indices in Estimation of Chronic Kidney Diseases in a Southern Chinese Population. Diabetes Metab. Syndr. Obesity Targets Ther. 2020, 13, 4919–4927. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, S.; Jing, L.; Tian, Y.; Xing, L. Estimate of reduced glomerular filtration rate by triglyceride-glucose index: Insights from a general Chinese population. Postgrad. Med. 2019, 131, 287–294. [Google Scholar] [CrossRef]
- Shang, J.; Yu, D.; Cai, Y.; Wang, Z.; Zhao, B.; Zhao, Z.; Simmons, D. The triglyceride glucose index can predict newly diagnosed biopsy-proven diabetic nephropathy in type 2 diabetes: A nested case control study. Medicine 2019, 98, e17995. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.C.; Xu, J.N.; Wang, T.T.; Hua, F.; Li, J.J. Triglyceride-glucose index as a marker in cardiovascular diseases: Landscape and limitations. Cardiovasc. Diabetol. 2022, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Hong, Y.A.; Min, J.W.; Koh, E.S.; Kim, H.D.; Ban, T.H.; Kim, Y.S.; Kim, Y.K.; Shin, S.J.; Kim, S.Y.; et al. Hypertriglyceridemia Is Associated with More Severe Histological Glomerulosclerosis in IgA Nephropathy. J. Clin. Med. 2021, 10, 4236. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.N.; Ferder, L.; Manucha, W. Emerging Role of Nitric Oxide and Heat Shock Proteins in Insulin Resistance. Curr. Hypertens. Rep. 2016, 18, 1. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, Y.J.; Park, K.; Lee, H.S.; Park, H.K.; Han, J.H.; Ahn, S.B. Metabolic Score for Insulin Resistance Is Inversely Related to Incident Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 3039. [Google Scholar] [CrossRef]
- Xiong, S.; Chen, Q.; Chen, X.; Hou, J.; Chen, Y.; Long, Y.; Yang, S.; Qi, L.; Su, H.; Huang, W.; et al. Adjustment of the GRACE score by the triglyceride glucose index improves the prediction of clinical outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc. Diabetol. 2022, 21, 145. [Google Scholar] [CrossRef]

| Variable | Unmatched Cohort | Matched Cohort | ||||
|---|---|---|---|---|---|---|
| Low TyG | High TyG | p Value | Low TyG | High TyG | p Value | |
| Numbers (%) | 690 (57.0) | 520 (43.0) | 183 | 183 | ||
| Age (year) | 30 (24–39) | 35 (27–43) | 34.0 (27.0–42.0) | 34.0 (26.0–42.0) | 0.836 | |
| Gender (male, %) | 279 (40.4) | 258 (49.6) | 0.002 | 95 (51.9) | 90 (49.2) | 0.676 |
| Hypertension (%) | 145 (21.0) | 180 (34.6) | <0.001 | 52 (28.4) | 45 (24.6) | 0.477 |
| SBP (mmHg) | 123 (113–135) | 128 (119–140) | <0.001 | 125.0 (116.0–138.0) | 124.0 (117.0–136.0) | 0.953 |
| DBP (mmHg) | 80 (73–89) | 84.5 (77.0–93.0) | <0.001 | 81.0 (75.0–92.0) | 82.0 (75.0–89.0) | 0.915 |
| BMI (kg/m2) | 21.5 (19.6–24.0) | 23.7 (21.1–26.6) | <0.001 | 22.2 (20.3–25.0) | 20.5 (23.1–25.7) | 0.249 |
| Smoking (%) | 97 (14.1) | 108 (20.8) | 0.002 | 35 (19.1) | 33 (18.0) | 0.893 |
| CKD stages (%) | <0.001 | 0.613 | ||||
| Stage 1 | 437 (63.3) | 223 (42.9) | 86 (47.0) | 96 (52.5) | ||
| Stage 2 | 150 (21.7) | 156 (30.0) | 58 (31.7) | 50 (27.3) | ||
| Stage 3 | 92 (13.3) | 116 (22.3) | 35 (19.1) | 35 (19.1) | ||
| Stage 4 | 11 (1.6) | 25 (4.8) | 4 (2.2) | 2 (1.1) | ||
| Pathologic | ||||||
| M1 (%) | 522 (75.7) | 401 (76.3) | 0.585 | 142 (77.6) | 136 (74.3) | 0.541 |
| E1 (%) | 27 (3.9) | 30 (5.8) | 0.134 | 7 (3.8) | 4 (2.2) | 0.543 |
| S1 (%) | 398 (57.7) | 335 (64.4) | 0.018 | 109 (59.6) | 113 (61.7) | 0.748 |
| T1-2/T0 (%) | 106 (15.4) | 131 (25.2) | <0.001 | 33 (18.0) | 31 (16.9) | 0.891 |
| C1-2/C0 (%) | 146 (21.2) | 133 (25.6) | 0.073 | 48 (26.2) | 40 (21.9) | 0.392 |
| Clinical | ||||||
| Cr (umol/L) | 76.3 (61.6–98.9) | 91.4 (71.0–121.0) | <0.001 | 87.0 (70.0–110.0) | 84.4 (68.0–109.0) | 0.441 |
| eGFR (mL/min/1.73 m2) | 103.1 (75.9–121.1) | 82.4 (57.9–107.6) | <0.001 | 86.5 (66.3–112.4) | 92.7 (66.3–114.4) | 0.484 |
| ALB (g/L) | 40.4 (36.8–43.6) | 39.9 (35.7–43.3) | 0.071 | 40.0 (35.3–44.0) | 41.0 (37.1–44.0) | 0.318 |
| TG (mmol/L) | 1.08 (0.84–1.30) | 2.19 (1.80–3.00) | <0.001 | 1.1 (0.9–1.3) | 2.0 (1.7–2.9) | <0.001 |
| FPG (mmol/L) | 4.7 (4.4–5.1) | 5.1 (4.7–5.6) | <0.001 | 4.7 (4.4–5.0) | 5.1 (4.7–5.6) | <0.001 |
| Proteinuria (g/d) | 1.01 (0.57–2.04) | 2.00 (1.00–3.36) | <0.001 | 1.4 (0.7–2.8) | 1.4 (0.9–2.9) | 0.544 |
| URBC (/HP) | 22.0 (8.0–76.3) | 15.0 (5.0–54.0) | 0.002 | 18.0 (6.0–61.0) | 15.0 (5.0–54.0) | 0.436 |
| Anemia (%) | 83 (12.0) | 85 (16.3) | 0.036 | 26 (14.2) | 25 (13.7) | 1.000 |
| Hyperuricemia (%) | 211 (30.6) | 245 (47.1) | <0.001 | 70 (38.3) | 74 (40.4) | 0.748 |
| Treatment | <0.001 | 0.376 | ||||
| supportive care | 316 (45.8) | 182 (35.0) | 64 (35.0) | 75 (41.0) | ||
| steroids only | 230 (33.3) | 192 (36.9) | 73 (39.9) | 61 (33.3) | ||
| Immunosuppression and/or steroids | 144 (20.9) | 146 (28.1) | 46 (55.4) | 47 (25.7) | ||
| Variables | Correlation Coefficient (r) | p Value |
|---|---|---|
| Proteinuria | 0.331 | <0.001 |
| Hb | 0.033 | 0.253 |
| Alb | −0.095 | 0.001 |
| BMI | 0.350 | <0.001 |
| UA | 0.244 | <0.001 |
| eGFR | −0.262 | <0.001 |
| Variables | OR | 95%CI | p Value |
|---|---|---|---|
| M | 1.085 | 0.829–1.419 | 0.554 |
| E | 1.503 | 0.882–2.562 | 0.134 |
| S | 1.329 | 1.051–1.680 | 0.018 |
| T1-2/T0 | 1.855 | 1.393–2.471 | <0.001 |
| C1-2/T0 | 1.281 | 0.979–1.675 | 0.071 |
| Hypertension | 1.990 | 1.538–2.574 | <0.001 |
| Smoking | 1.603 | 1.185–2.167 | <0.002 |
| eGFR < 60 mL/min.1.73 m2 | 2.120 | 1.594–2.819 | <0.001 |
| TyG Index | Univariant | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| Before PSM | 2.483 (1.736–3.551) | <0.001 | 1.882 (1.312–2.700) | 0.001 | 2.510 (1.398–4.508) | 0.002 | 2.509 (1.396–4.511) | 0.002 |
| After PSM | 2.295 (1.131–4.657 | 0.021 | 2.391 (1.175–4.864 | 0.016 | 2.545 (1.250–5.182) | 0.010 | 2.654 (1.299–5.423) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, A.; Tan, J.; Wang, S.; Dong, L.; Jiang, Z.; Yang, D.; Zhou, H.; Zhou, X.; Tang, Y.; Qin, W. Triglyceride–Glucose Index May Predict Renal Survival in Patients with IgA Nephropathy. J. Clin. Med. 2022, 11, 5176. https://doi.org/10.3390/jcm11175176
Qin A, Tan J, Wang S, Dong L, Jiang Z, Yang D, Zhou H, Zhou X, Tang Y, Qin W. Triglyceride–Glucose Index May Predict Renal Survival in Patients with IgA Nephropathy. Journal of Clinical Medicine. 2022; 11(17):5176. https://doi.org/10.3390/jcm11175176
Chicago/Turabian StyleQin, Aiya, Jiaxing Tan, Siqing Wang, Lingqiu Dong, Zheng Jiang, Dandan Yang, Huan Zhou, Xiaoyuan Zhou, Yi Tang, and Wei Qin. 2022. "Triglyceride–Glucose Index May Predict Renal Survival in Patients with IgA Nephropathy" Journal of Clinical Medicine 11, no. 17: 5176. https://doi.org/10.3390/jcm11175176
APA StyleQin, A., Tan, J., Wang, S., Dong, L., Jiang, Z., Yang, D., Zhou, H., Zhou, X., Tang, Y., & Qin, W. (2022). Triglyceride–Glucose Index May Predict Renal Survival in Patients with IgA Nephropathy. Journal of Clinical Medicine, 11(17), 5176. https://doi.org/10.3390/jcm11175176








