Radiomics in Urolithiasis: Systematic Review of Current Applications, Limitations, and Future Directions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
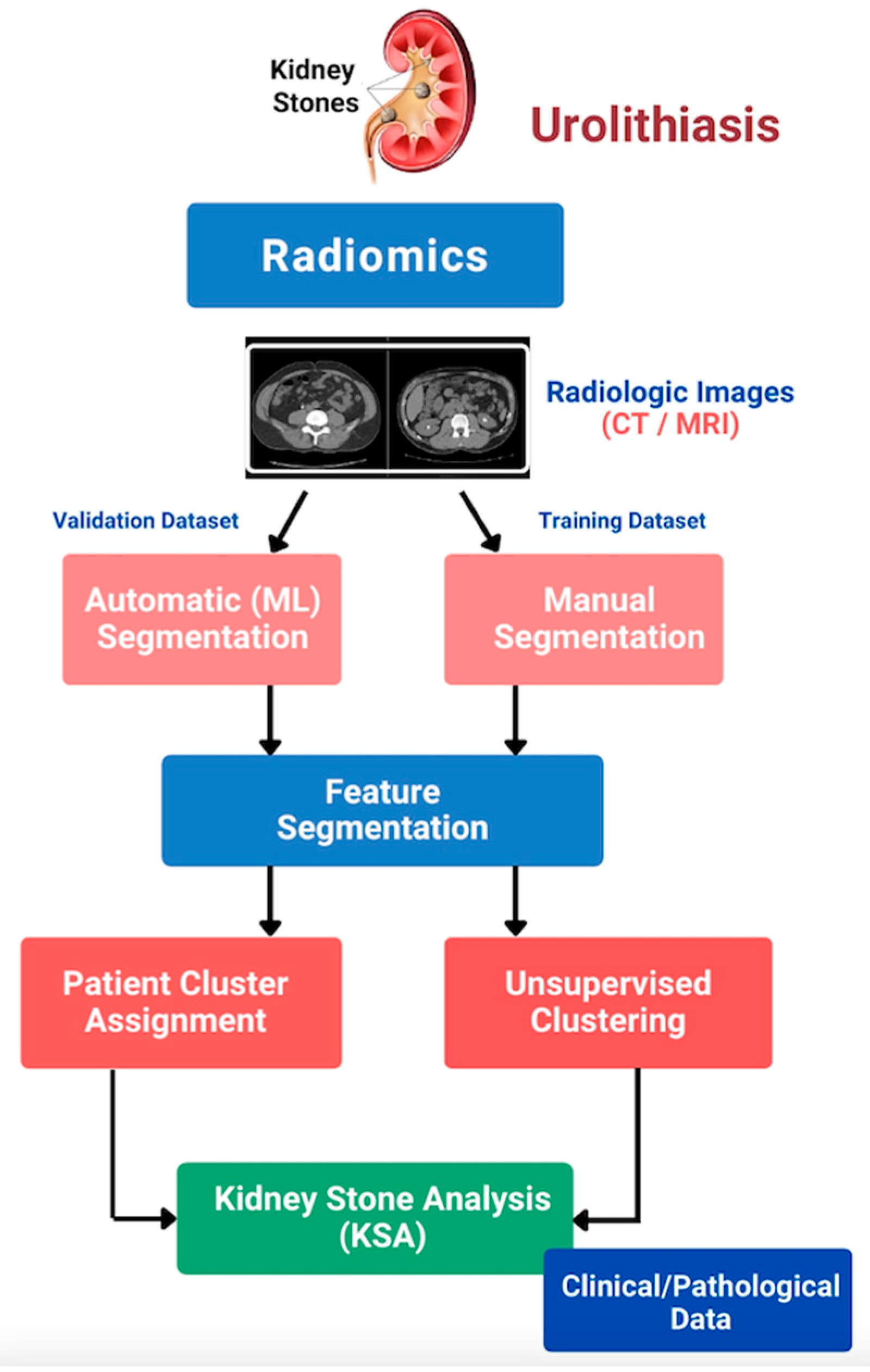
3. Results
4. Discussion
4.1. Diagnostics
4.1.1. Differentiating Ureteric Calculi and Phleboliths
4.1.2. Pre-Operative Identification of Stone Type
4.2. Evaluating Treatment Outcomes
4.2.1. Prediction of Spontaneous Stone Passage
4.2.2. Therapeutic Utility in ESWL
4.2.3. Predicting Stone Burden Affecting RIRS/PCNL Stone-Free Rates Outcomes
4.3. Current Limitations and Future Directions
4.4. Take Home Messages
- Predicting success of spontaneous stone passage with medical expulsion therapy.
- Differentiating between calculi and phleboliths.
- Pre-operative accurate identification of stone type.
- Predicting stone burden affecting RIRS/PCNL stone-free rate outcomes.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B
| PubMed | 405 Articles |
|---|---|
| ((stone * AND (renal OR kidney OR ureter OR ureteric OR bladder)) OR (‘Urolithiasis’ [MeSH]) OR (‘Calculi’ [MeSH]) OR (‘Kidney Calculi’ [MeSH]) OR nephrolithiasis OR ureterolithiasis OR cystolithiasis) AND (“artificial intelligence” [MeSH] OR “AI” OR “radiomic *” OR “machine learning” OR “deep learning”) | |
| EMBASE | 713 articles |
| ((stone OR stones) AND (renal OR kidney OR ureter OR ureteric OR bladder) OR ‘urolithiasis’/exp OR ‘calculi’/exp OR ‘nephrolithiasis’/exp OR ‘ureterolithiasis’/exp OR cystolithiasis) AND (‘artificial intelligence’/exp OR ‘ai’ OR ‘radiomic’ OR ‘radiomics’/exp OR ‘machine learning’/exp OR ‘deep learning’) | |
| Scopus | 214 articles |
| TITLE-ABS-KEY (((stone OR stones OR calculi OR calculus AND (renal OR kidney OR ureter OR ureteric OR bladder)) OR urolithiasis OR nephrolithiasis OR ureterolithiasis OR cystolithiasis) AND (“artificial intelligence” OR “AI” OR “radiomic” OR “radiomics” OR “machine learning” OR “deep learning”)) | |
References
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef]
- Vailati-Riboni, M.; Palombo, V.; Loor, J.J. What are Omics Sciences? Periparturient Diseases of Dairy Cows; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–7. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“How-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Irakliotis, L.; Carlson, T.; Lucas, S.; Perlowitz, B. Demystifying Big Data: A Practical Guide to Transforming the Business of Government. Available online: https://bigdatawg.nist.gov/_uploadfiles/M0068_v1_3903747095.pdf (accessed on 13 August 2022).
- Castellano, G.; Bonilha, L.; Li, L.M.; Cendes, F. Texture analysis of medical images. Clin. Radiol. 2004, 59, 1061–1069. [Google Scholar] [CrossRef]
- Tourassi, G.D. Journey toward computer-aided diagnosis: Role of image texture analysis. Radiology 1999, 213, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.W.; Namdar, K.; Biswas, A.; Monah, S.; Khalvati, F.; Ertl-Wagner, B.B. Radiomics, machine learning, and artificial intelligence—What the neuroradiologist needs to know. Neuroradiology 2021, 63, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef]
- Cho, H.; Lee, H.Y.; Kim, E.; Lee, G.; Kim, J.; Kwon, J.; Park, H. Radiomics-guided deep neural networks stratify lung adenocarcinoma prognosis from CT scans. Commun. Biol. 2021, 4, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhang, G.; Qiu, X.; Tan, W.; Yin, X.; Liao, L. Deep Learning with Radiomics for Disease Diagnosis and Treatment: Challenges and Potential. Front. Oncol. 2022, 12, 773840. [Google Scholar] [CrossRef]
- Scrivener, M.; de Jong, E.E.C.; van Timmeren, J.; Pieters, T.; Ghaye, B.; Geets, X. Radiomics applied to lung cancer: A review. Transl. Cancer Res. 2016, 5, 398–409. [Google Scholar] [CrossRef]
- Avanzo, M.; Stancanello, J.; El Naqa, I. Beyond imaging: The promise of radiomics. Phys. Med. 2017, 38, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLOS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- De Perrot, T.; Hofmeister, J.; Burgermeister, S.; Martin, S.P.; Feutry, G.; Klein, J.; Montet, X. Differentiating kidney stones from phleboliths in unenhanced low-dose computed tomography using radiomics and machine learning. Eur. Radiol. 2019, 29, 4776–4782. [Google Scholar] [CrossRef]
- Homayounieh, F.; Doda Khera, R.; Bizzo, B.C.; Ebrahimian, S.; Primak, A.; Schmidt, B.; Saini, S.; Kalra, M.K. Prediction of burden and management of renal calculi from whole kidney radiomics: A multicenter study. Abdom. Radiol. 2021, 46, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Xun, Y.; Chen, M.; Liang, P.; Tripathi, P.; Deng, H.; Zhou, Z.; Xie, Q.; Li, C.; Wang, S.; Li, Z.; et al. A Novel Clinical-Radiomics Model Pre-operatively Predicted the Stone-Free Rate of Flexible Ureteroscopy Strategy in Kidney Stone Patients. Front. Med. 2020, 7, 576925. [Google Scholar] [CrossRef]
- Luk, A.C.O.; Cleaveland, P.; Olson, L.; Neilson, D.; Srirangam, S.J. Pelvic Phlebolith: A Trivial Pursuit for the Urologist? J. Endourol. 2017, 31, 342–347. [Google Scholar] [CrossRef]
- Carius, B.M.; Long, B. Is This Your Stone? Distinguishing Phleboliths and Nephroliths on Imaging in the Emergency Department Setting. J. Emerg. Med. 2022, 62, 316–323. [Google Scholar] [CrossRef]
- Cui, X.; Che, F.; Wang, N.; Liu, X.; Zhu, Y.; Zhao, Y.; Bi, J.; Li, Z.; Zhang, G. Preoperative Prediction of Infection Stones Using Radiomics Features from Computed Tomography. IEEE Access 2019, 7, 122675–122683. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, H.; Batur, J.; Shi, Z.; Tuerxun, A.; Abulajiang, A.; Lu, S.; Kong, J.; Huang, L.; Wu, S.; et al. A multicenter study to develop a non-invasive radiomic model to identify urinary infection stone in vivo using machine-learning. Kidney Int. 2021, 100, 870–880. [Google Scholar] [CrossRef]
- Tang, L.; Li, W.; Zeng, X.; Wang, R.; Yang, X.; Luo, G.; Chen, Q.; Wang, L.; Song, B. Value of artificial intelligence model based on unenhanced computed tomography of urinary tract for preoperative prediction of calcium oxalate monohydrate stones in vivo. Ann. Transl. Med. 2021, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.M.Z.; Somani, B.; Naik, N.; Talasila, A.; Shah, M.; Reddy, S.; Sachdev, G.; Hussein Beary, R.; Hegde, P. Application of deep learning convolutional neural network in prediction of stone location, skin to stone distance and composition in renal lithiasis: A single center pilot study. Eur. Urol. 2021, 79, S336. [Google Scholar] [CrossRef]
- Yang, B.; Veneziano, D.; Somani, B.K. Artificial intelligence in the diagnosis, treatment and prevention of urinary stones. Curr. Opin. Urol. 2020, 30, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, P.; Ferrero, A.; Bartlett, D.J.; Khandelwal, A.; Marcus, R.; Lieske, J.C.; Moen, T.R.; Mara, K.C.; Enders, F.T.; McCollough, C.H.; et al. Automated radiomic analysis of CT images to predict likelihood of spontaneous passage of symptomatic renal stones. Emerg. Radiol. 2021, 28, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.D.; Wang, Y.-N.; Kreider, W.; Cunitz, B.W.; Starr, F.; Lee, D.; Nazari, Y.; Williams, J.C., Jr.; Bailey, M.R.; Sorensen, M.D. Evaluation of Renal Stone Comminution and Injury by Burst Wave Lithotripsy in a Pig Model. J. Endourol. 2019, 33, 787–792. [Google Scholar] [CrossRef]
- Bhanot, R.; Jones, P.; Somani, B. Minimally Invasive Surgery for the Treatment of Ureteric Stones—State-of-the-Art Review. Res. Rep. Urol. 2021, 13, 227–236. [Google Scholar] [CrossRef]
- Karim, S.S.; Hanna, L.; Geraghty, R.; Somani, B.K. Role of pelvicalyceal anatomy in the outcomes of retrograde intrarenal surgery (RIRS) for lower pole stones: Outcomes with a systematic review of literature. Urolithiasis 2020, 48, 263–270. [Google Scholar] [CrossRef]
- Reddy, T.G.; Assimos, D.G. Optimizing Stone-free Rates with Ureteroscopy. Rev. Urol. 2015, 17, 160–164. [Google Scholar]
- Ermis, O.; Somani, B.; Reeves, T.; Guven, S.; Pes, P.L.; Chawla, A.; Hegde, P.; de la Rosette, J. Definition, treatment and outcome of residual fragments in staghorn stones. Asian J. Urol. 2020, 7, 116–121. [Google Scholar] [CrossRef]
- Lim, E.J.; Teoh, J.Y.; Fong, K.Y.; Emiliani, E.; Gadzhiev, N.; Gorelov, D.; Tanidir, Y.; Sepulveda, F.; Al-Terki, A.; Khadgi, S.; et al. Propensity score-matched analysis comparing retrograde intrarenal surgery with percutaneous nephrolithotomy in anomalous kidneys. Minerva Urol. Nephrol. 2022. [Google Scholar] [CrossRef]
- García Rojo, E.; Teoh, J.Y.-C.; Castellani, D.; Brime Menéndez, R.; Tanidir, Y.; Benedetto Galosi, A.; Bhatia, T.P.; Soebhali, B.; Sridharan, V.; Corrales, M.; et al. Real-world Global Outcomes of Retrograde Intrarenal Surgery in Anomalous Kidneys: A High Volume International Multicenter Study. Urology 2022, 159, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Gok, A.; Polat, H.; Cift, A.; Yucel, M.O.; Gok, B.; Sirik, M.; Benlioglu, C.; Kalyenci, B. The hounsfield unit value calculated with the aid of non-contrast computed tomography and its effect on the outcome of percutaneous nephrolithotomy. Urolithiasis 2015, 43, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.; Johnson, L.; Ganesh, H.; Davenport, D.; Smelser, W.; Crispen, P.; Venkatesh, R. Stone Size Limits the Use of Hounsfield Units for Prediction of Calcium Oxalate Stone Composition. Urology 2015, 85, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Selby, M.G.; Vrtiska, T.J.; Krambeck, A.E.; McCollough, C.H.; Elsherbiny, H.E.; Bergstralh, E.J.; Lieske, J.C.; Rule, A.D. Quantification of asymptomatic kidney stone burden by computed tomography for predicting future symptomatic stone events. Urology 2015, 85, 45–50. [Google Scholar] [CrossRef]
- Wisenbaugh, E.S.; Paden, R.G.; Silva, A.C.; Humphreys, M.R. Dual-energy vs conventional computed tomography in determining stone composition. Urology 2014, 83, 1243–1247. [Google Scholar] [CrossRef]
- Jones, P.; Pietropaolo, A.; Chew, B.H.; Somani, B.K. Atlas of Scoring Systems, Grading Tools, and Nomograms in Endourology: A Comprehensive Overview from the TOWER Endourological Society Research Group. J. Endourol. 2021, 35, 1863–1882. [Google Scholar] [CrossRef]
- Lohmann, P.; Bousabarah, K.; Hoevels, M.; Treuer, H. Radiomics in radiation oncology—Basics, methods, and limitations. Strahlenther Onkol. 2020, 196, 848–855. [Google Scholar] [CrossRef]
- Traverso, A.; Wee, L.; Dekker, A.; Gillies, R. Repeatability and Reproducibility of Radiomic Features: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1143–1158. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [Green Version]
| No. | Author (Year) | Type of Study | Objective | Number of Patients and Breakdown | Number of Radiomics Features | Utility | Conclusion |
|---|---|---|---|---|---|---|---|
| 1 | Perrot et al., (2022) | In-vivo | Identification of Urolithiasis | Training set: 369 patients (211 kidney stones, 201 phleboliths) Testing set: 43 patients (24 kidney stones, 23 phleboliths) | NR | Accuracy: 85.1% Sensitivity: 91.7% Specificity: 78.3% Positive predictive value: 81.5% Negative predictive value: 90.0% AUC: 0.902 | Machine learning reinforced with machine learning enable accurate discernment between renal calculi and phleboliths on low-dose CT in patients with acute flank pain. |
| 2 | Cui et al., (2022) | In-vivo | Prediction of Stone Type | 157 patients (98 infection kidney stones, 59 non-infection kidney stones) | 54 radiomics features (16 morphological, 38 textural) → reduced to 27 key features (16 morphological, 11 textural) by the LASSO algorithm | Accuracy: 90.7% Sensitivity: 85.81% Specificity: 93.96% Positive predictive value: 91% Negative predictive value: 91% AUC: 0.97 | Quantitative nomogram with radiomics method is useful for pre-operative prediction of infection versus non-infection kidney stones. |
| 3 | Zheng et al., (2022) | In-vivo | Prediction of Stone Type | Training set: 314 patients (41 infection stones, 273 non-infection stones) Internal validation set: 134 patients (22 infection stones, 112 non-infection stones) External validation set 1: 594 patients (111 infection stones, 483 non-infection stones) External validation set 2: 156 patients (18 infection stones, 138 non-infection stones) | 1316 radiomics features → 24 key features with non-zero coefficients selected by the LASSO algorithm | Training set: AUC: 0.864 (95% CI 0.802–0.926) Internal validation set: 0.832 (95% CI 0.742–0.923) External validation set 1: 0.825 (95% CI 0.783–0.866) External validation set 2: 0.812 (95% CI 0.710–0.914) | Radiomics model developed can be a non-invasive method to detect urinary infection stones in vivo, benefitting subsequent management and patient prognosis. |
| 4 | Tang et al., (2022) | In-vivo | Prediction of Stone Composition | 543 patients (373 calcium oxalate monohydrate stones, 170 non-COM stones) | 1218 radiomics features extracted → 8 features with non-zero coefficients were selected for by the LASSO algorithm | Accuracy: 88.5% Sensitivity: 90.5% Specificity: 84.3% Training set AUC: 0.935 (95% CI 0.907–0.962) Testing set AUC: 0.933 (95% CI 0.893–0.973) | Artificial intelligence models incorporated with radiomics can predict COM and non-COM stones in vivo pre-operatively with robust accuracy, sensitivity, and specificity values. |
| 5 | Hameed et al., (2022) | In-vitro | Prediction of Stone Composition | NR | NR | Average accuracy: 87% Calcium oxalate monohydrate stone accuracy: 89% Calcium oxalate dihydrate stone accuracy: 85% Struvite stone accuracy: 86% Uric acid stone accuracy: 93% Calcium hydrogen phosphate stone accuracy: 89% | The artificial intelligence (deep learning-convolutional neural network DL-CNN) model reinforced with radiomics is successful in predicting various types of stone composition with high accuracy values. |
| 6. | Xun et al., (2020) | In-vivo | PCNL: To develop and validate a novel clinical–radiomics nomogram model for pre-operatively predicting the stone-free rate of flexible ureteroscopy in patients with a single kidney stone | Training set: 99 patients Testing set (internal validation): 43 patients | Radiomics feature selection and signature building were conducted by using the least absolute shrinkage and selection operator (LASSO) method. With penalty parameter tuning conducted by 10-fold cross-validation, LASSO was performed to select robust and non-redundant features from the primary cohort. A radiomics signature was created by a linear combination of selected features weighted by their respective coefficients, and the relevant radiomics score was calculated for each patient. | AUC test group: 0.949 (95% CI, 0.910–0.989) AUC validation group: 0.947 (95% CI, 0.883–1) | Radiomics score, stone volume, hydronephrosis level, and operator experience were crucial for RIRS strategy |
| 7. | Homayounieh et al., (2020) | In-vivo | RIRS: To assess if auto segmentation-assisted radiomics can predict disease burden, hydronephrosis, and treatment strategies in patients with renal calculi. | 202 patients who underwent clinically indicated, non-contrast abdomen-pelvis CT for suspected or known renal calculi. | Deidentified CT images were processed with the radi- omics prototype (Radiomics, Frontier, Siemens Healthineers), which automatically segmented each kidney to obtain 1690 first-, shape-, and higher-order radiomics. | AUC: 0.91 (95% CI 0.85–0.92) | Automated segmentation and radiomics of entire kidneys can assess hydronephrosis presence, stone burden, and treatment strategies for renal calculi |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, E.J.; Castellani, D.; So, W.Z.; Fong, K.Y.; Li, J.Q.; Tiong, H.Y.; Gadzhiev, N.; Heng, C.T.; Teoh, J.Y.-C.; Naik, N.; et al. Radiomics in Urolithiasis: Systematic Review of Current Applications, Limitations, and Future Directions. J. Clin. Med. 2022, 11, 5151. https://doi.org/10.3390/jcm11175151
Lim EJ, Castellani D, So WZ, Fong KY, Li JQ, Tiong HY, Gadzhiev N, Heng CT, Teoh JY-C, Naik N, et al. Radiomics in Urolithiasis: Systematic Review of Current Applications, Limitations, and Future Directions. Journal of Clinical Medicine. 2022; 11(17):5151. https://doi.org/10.3390/jcm11175151
Chicago/Turabian StyleLim, Ee Jean, Daniele Castellani, Wei Zheng So, Khi Yung Fong, Jing Qiu Li, Ho Yee Tiong, Nariman Gadzhiev, Chin Tiong Heng, Jeremy Yuen-Chun Teoh, Nithesh Naik, and et al. 2022. "Radiomics in Urolithiasis: Systematic Review of Current Applications, Limitations, and Future Directions" Journal of Clinical Medicine 11, no. 17: 5151. https://doi.org/10.3390/jcm11175151
APA StyleLim, E. J., Castellani, D., So, W. Z., Fong, K. Y., Li, J. Q., Tiong, H. Y., Gadzhiev, N., Heng, C. T., Teoh, J. Y.-C., Naik, N., Ghani, K., Sarica, K., De La Rosette, J., Somani, B., & Gauhar, V. (2022). Radiomics in Urolithiasis: Systematic Review of Current Applications, Limitations, and Future Directions. Journal of Clinical Medicine, 11(17), 5151. https://doi.org/10.3390/jcm11175151









