Manganese Superoxide Dismutase as a Novel Oxidative Stress Biomarker for Predicting Paroxysmal Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Baseline Characteristics
2.3. Assessment of Oxidative Stress Biomarkers
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Multivariate Predictors of AF
3.3. Correlation between MnSOD and Other Parameters
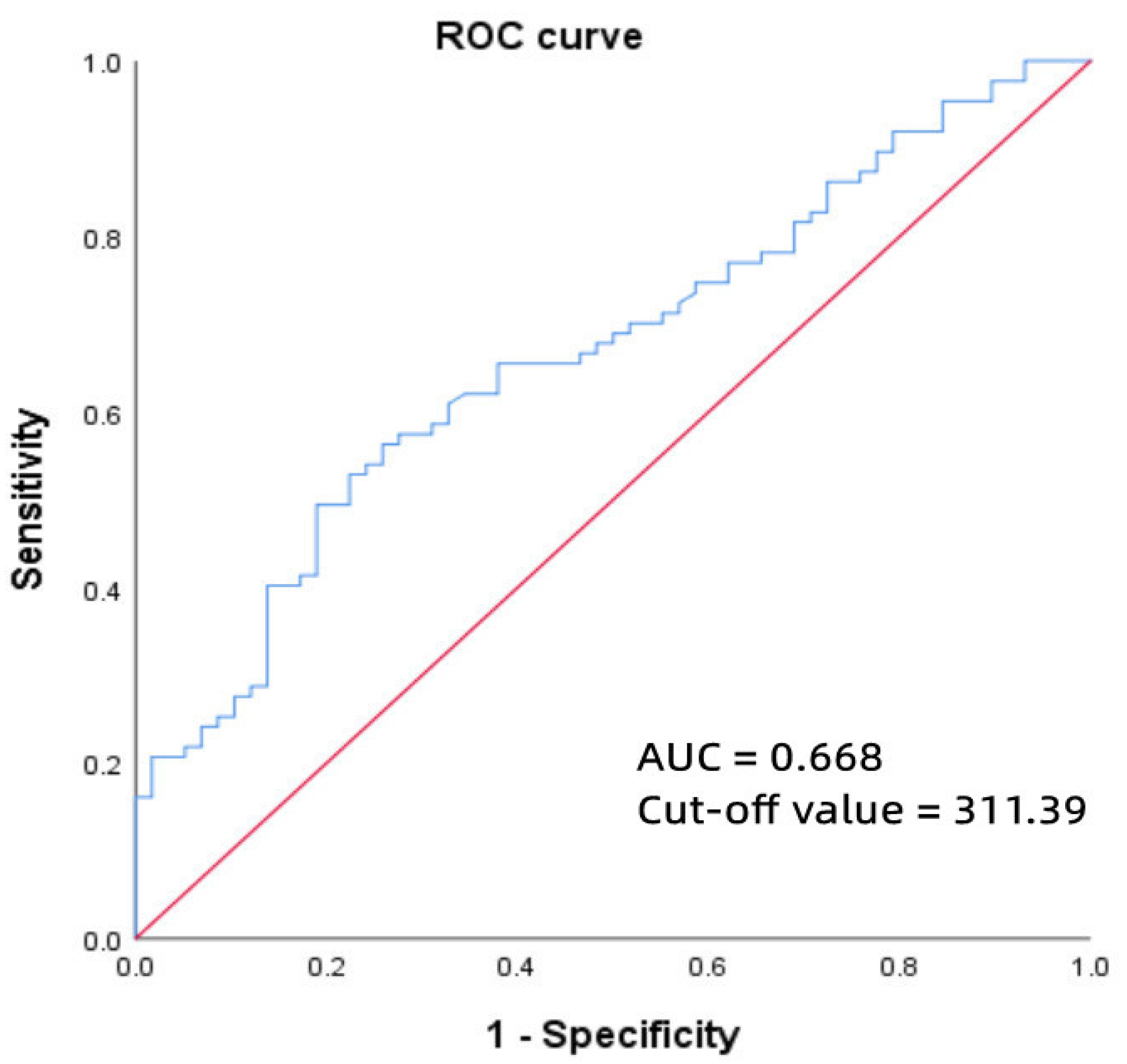
3.4. ROC Curve of MnSOD and Paroxysmal AF
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Hijazi, Z.; Oldgren, J.; Siegbahn, A.; Granger, C.B.; Wallentin, L. Biomarkers in atrial fibrillation: A clinical review. Eur. Heart J. 2013, 34, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lip, G.Y.; Apostolakis, S. Inflammation in atrial fibrillation. J. Am. Coll. Cardiol. 2012, 60, 2263–2270. [Google Scholar] [CrossRef]
- Vílchez, J.A.; Roldán, V.; Hernández-Romero, D.; Valdés, M.; Lip, G.Y.; Marín, F. Biomarkers in atrial fibrillation: An overview. Int. J. Clin. Pract. 2014, 68, 434–443. [Google Scholar] [CrossRef]
- Korantzopoulos, P.; Kolettis, T.M.; Galaris, D.; Goudevenos, J.A. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int. J. Cardiol. 2007, 115, 135–143. [Google Scholar] [CrossRef]
- Li, C.; Zhou, H.M. The role of manganese superoxide dismutase in inflammation defense. Enzym. Res. 2011, 2011, 387176. [Google Scholar] [CrossRef]
- Kim, N.Y.; Chun, D.H.; Kim, S.Y.; Kim, N.K.; Baik, S.H.; Hong, J.H.; Kim, K.S.; Shin, C.S. Prognostic Value of Systemic Inflammatory Indices, NLR, PLR, and MPV, for Predicting 1-Year Survival of Patients Undergoing Cytoreductive Surgery with HIPEC. J. Clin. Med. 2019, 8, 589. [Google Scholar] [CrossRef]
- Kurtul, A.; Ornek, E. Platelet to Lymphocyte Ratio in Cardiovascular Diseases: A Systematic Review. Angiology 2019, 70, 802–818. [Google Scholar] [CrossRef]
- Ganjali, S.; Gotto, A.M., Jr.; Ruscica, M.; Atkin, S.L.; Butler, A.E.; Banach, M.; Sahebkar, A. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J. Cell. Physiol. 2018, 233, 9237–9246. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ. Arrhythm. Electrophysiol. 2008, 1, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Voskoboinik, A.; Gerche, A.; Marwick, T.H.; McMullen, J.R. Prevention of Pathological Atrial Remodeling and Atrial Fibrillation: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 2846–2864. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, X.; Yuan, M.; Tian, C.; Li, H.; Yang, X.; Li, X.; Li, Y.; Yang, Y.; Liu, N.; et al. Mechanisms and Treatments of Oxidative Stress in Atrial Fibrillation. Curr. Pharm. Des. 2018, 24, 3062–3071. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Yan, B.P.; Chan, Y.W.; Tian, X.Y.; Huang, Y. Reactive Oxygen Species, Endoplasmic Reticulum Stress and Mitochondrial Dysfunction: The Link with Cardiac Arrhythmogenesis. Front. Physiol. 2016, 7, 313. [Google Scholar] [CrossRef]
- Korantzopoulos, P.; Letsas, K.; Fragakis, N.; Tse, G.; Liu, T. Oxidative stress and atrial fibrillation: An update. Free Radic. Res. 2018, 52, 1199–1209. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Korantzopoulos, P.; Kolettis, T.; Siogas, K.; Goudevenos, J. Atrial fibrillation and electrical remodeling: The potential role of inflammation and oxidative stress. Med. Sci. Monit. 2003, 9, RA225–RA229. [Google Scholar]
- Van Wagoner, D.R. Molecular basis of atrial fibrillation: A dream or a reality? J. Cardiovasc. Electrophysiol. 2003, 14, 667–669. [Google Scholar] [CrossRef]
- Van Wagoner, D.R. Electrophysiological remodeling in human atrial fibrillation. Pacing Clin. Electrophysiol. 2003, 26, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- St. Clair, D.K.; Oberley, T.D.; Muse, K.E.; Clair, W.H.S. Expression of manganese superoxide dismutase promotes cellular differentiation. Free Radic. Biol. Med. 1994, 16, 275–282. [Google Scholar] [CrossRef]
- Wispé, J.R.; Warner, B.B.; Clark, J.C.; Dey, C.R.; Neuman, J.; Glasser, S.W.; Crapo, J.D.; Chang, L.Y.; Whitsett, J.A. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J. Biol. Chem. 1992, 267, 23937–23941. [Google Scholar] [CrossRef]
- Beznă, M.C.; Cârstea, D.; Beznă, M.; Pisoschi, C.; Istrătoaie, O.; Alexandru, D.O.; Efrem, C.; Melinte, R.P. Estimation of Oxidative Stress Involvement by Superoxide Dismutase Variation in Cardiac Arrhythmias. Curr. Health Sci. J. 2017, 43, 119–126. [Google Scholar]
- Liu, T.; Shao, Q.; Korantzopoulos, P.; Liu, E.; Xu, G.; Li, G. Serum levels of nicotinamide-adenine dinucleotide phosphate oxidase 4 are associated with non-valvular atrial fibrillation. Biomed. Rep. 2015, 3, 864–868. [Google Scholar] [CrossRef][Green Version]
| Paroxysmal AF (n = 87) | Persistent AF (n = 43) | Controls (n = 58) | p Value | |
|---|---|---|---|---|
| Basic clinical features | ||||
| Female, n (%) | 47 (54.0%) | 14 (32.6%) | 37 (63.8%) | 0.007 *** |
| Age, years | 65.8 ± 9.7 | 63.0 ± 8.9 | 62.1 ± 11.0 | 0.069 |
| BMI, kg/m2 | 25.9 ± 2.9 | 26.7 ± 3.5 | 25.6 ± 3.8 | 0.284 |
| CHA2DS2-VASc score | 2.3 ± 1.5 | 2.5 ± 1.6 | — | 0.158 |
| HAS-BLED score | 1.5 ± 0.9 | 1.3 ± 0.9 | — | 0.271 |
| Drinking, n (%) | 20 (23%) | 16 (37.2%) | 5 (8.6%) | 0.003 ** |
| Smoking, n (%) | 11 (12.6%) | 8 (18.6%) | 8 (13.8%) | 0.653 |
| Arterial hypertension, n (%) | 59 (67.8%) | 23 (53.5%) | 42 (72.4%) | 0.123 |
| Diabetes mellitus, n (%) | 21 (24.1%) | 8 (18.6%) | 13 (22.4%) | 0.776 |
| Coronary heart disease, n (%) | 24 (27.6%) | 14 (32.6%) | 31 (53.4%) | 0.005 ** |
| Stroke, n (%) | 16 (18.4%) | 6 (14%) | 4 (6.9%) | 0.145 |
| Hyperlipidemia, n (%) | 6 (6.9%) | 2 (4.7%) | 6 (10.3%) | 0.589 |
| Neoplasm, n (%) | 6 (6.9%) | 5 (11.6%) | 0 (0.0%) | 0.504 |
| Preoperative AF medication | ||||
| Rivaroxaban, n (%) | 69 (79.3%) | 39 (90.7%) | — | 0.103 |
| β-blockers, n (%) | 31 (35.6%) | 18 (41.9%) | — | 0.491 |
| Amiodarone, n (%) | 38 (43.7%) | 20 (46.5%) | — | 0.760 |
| Propafenone, n (%) | 11 (12.6%) | 1 (2.3%) | — | 0.103 |
| Sotalol, n (%) | 19 (21.8%) | 8 (18.6%) | — | 0.669 |
| Paroxysmal AF (n = 87) | Persistent AF (n = 43) | Controls (n = 58) | p Value | |
|---|---|---|---|---|
| Echocardiogram parameters | ||||
| IVST, mm | 9.2 ± 1.6 | 9.3 ± 1.4 | 9.1 ± 1.2 | 0.827 |
| LAD, mm | 40.5 ± 4.3 | 45.4 ± 4.5 | 37.2 ± 5.1 | <0.001 */**/*** |
| LVEDD, mm | 47.6 ± 3.8 | 48.5 ± 4.2 | 46.5 ± 4.2 | 0.048 *** |
| LVESD, mm | 26.7 ± 5.3 | 29.7 ± 5.7 | 25.7 ± 3.7 | <00.001 */*** |
| RVEDD, mm | 20.7 ± 2.2 | 21.6 ± 2.6 | 20.2 ± 2.1 | 0.010 */*** |
| PAD, mm | 22.5 ± 3.1 | 22.4 ± 2.9 | 21.4 ± 2.7 | 0.063 |
| LVEF, % | 62.4 ± 4.5 | 59.1 ± 5.0 | 63.5 ± 3.3 | <00.001 */*** |
| LAA-MFV, cm/s | 56.3 ± 17.4 | 45.0 ± 16.2 | — | 0.001 * |
| LAA-MEV, cm/s | 62.2 ± 22.0 | 40.1 ± 14.0 | — | <00.001 * |
| Laboratory examinations | ||||
| GLO, g/L | 27.9 ± 4.9 | 25.8 ± 4.9 | 26.8 ± 4.0 | 0.044 * |
| ALT, U/L | 19.4 (13.9, 28.9) | 19.4 (14.9, 32.0) | 19.3 (14.5, 24.8) | 0.672 |
| AST, U/L | 16.9 (13.2, 22.3) | 18.0 (13.6, 22.2) | 17.9 (15.0, 21.1) | 0.505 |
| DBIL, μmol/L | 4.1 (2.8, 5.6) | 4.3 (3.1, 5.9) | 3.7 (2.7, 4.3) | 0.029 *** |
| TC, mmol/L | 4.9 ± 1.0 | 4.8 ± 0.9 | 4.9 ± 0.7 | 0.697 |
| LDL-c, mmol/L | 2.84 ± 0.86 | 2.94 ± 0.82 | 3.07 ± 0.61 | 0.228 |
| BUN, mmol/L | 5.9 ± 1.5 | 6.7 ± 1.6 | 5.7 ± 1.2 | 0.005 */*** |
| Scr, μmol/L | 72.8 ± 17.9 | 82.3 ± 20.0 | 62.6 ± 14.1 | <00.001 */**/*** |
| Ccr, mL/min | 87.80 ± 25.09 | 89.43 ± 26.98 | 97.93 ± 32.95 | 0.096 |
| eGFR, mL/min/1.73 m2 | 84.24 ± 15.57 | 81.07 ± 17.07 | 94.14 ± 12.54 | <00.001 **/*** |
| WBC, ×109/L | 6.32 ± 1.44 | 6.66 ± 1.60 | 6.52 ± 1.64 | 0.460 |
| RBC, ×1012/L | 4.52 ± 0.50 | 4.83 ± 0.47 | 4.55 ± 0.43 | 0.002 */*** |
| HCT, % | 41.5 ± 4.7 | 44.0 ± 4.7 | 41.3 ± 3.7 | 0.005 */*** |
| RDW-CV, % | 13.1 ± 1.0 | 13.1 ± 0.9 | 12.7 ± 0.5 | 0.007 **/*** |
| PLT, ×109/L | 221 ± 56 | 226 ± 51 | 234 ± 60 | 0.393 |
| PDW, % | 15.9 (12.2, 16.3) | 16.0 (13.7, 16.3) | 16.0 (15.8, 16.3) | 0.192 |
| PLR | 135.30 ± 50.52 | 129.82 ± 48.41 | 142.06 ± 51.90 | 0.475 |
| NLR | 2.44 ± 1.05 | 2.62 ± 1.07 | 2.49 ± 0.85 | 0.636 |
| MHR | 0.34 ± 0.13 | 0.38 ± 0.12 | 0.34 ± 0.15 | 0.336 |
| Oxidative stress biomarkers | ||||
| NOX4, ng/mL | 8.51 ± 1.59 | 8.17 ± 1.17 | 8.12 ± 1.54 | 0.246 |
| MnSOD, ug/mL | 322.84 (165.46, 547.61) | 234.55 (149.21, 427.09) | 201.83 (129.53, 301.93) | 0.002 ** |
| β | SE | Wald | p Value | OR | 95% CI | |
|---|---|---|---|---|---|---|
| LAD | 0.142 | 0.045 | 10.062 | 0.002 * | 1.153 | 1.056–1.259 |
| Scr | 0.035 | 0.013 | 7.047 | 0.008 * | 1.036 | 1.009–1.063 |
| RDW-CV | 0.709 | 0.318 | 4.988 | 0.026 * | 2.033 | 1.091–3.788 |
| MnSOD | 0.003 | 0.001 | 9.762 | 0.002 * | 1.003 | 1.001–1.005 |
| β | SE | Wald | p Value | OR | 95% CI | |
|---|---|---|---|---|---|---|
| LAD | 0.981 | 0.439 | 4.981 | 0.026 * | 2.667 | 1.127–6.310 |
| LVESD | −0.920 | 0.500 | 3.382 | 0.066 | 0.399 | 0.150–1.062 |
| BUN | 0.718 | 0.601 | 1.426 | 0.232 | 2.050 | 0.631–6.659 |
| Scr | 0.060 | 0.132 | 0.209 | 0.648 | 1.062 | 0.820–1.376 |
| RDW-CV | 0.080 | 0.084 | 0.905 | 0.341 | 1.083 | 0.919–1.277 |
| MnSOD | ||
|---|---|---|
| r | p Value | |
| Age | 0.043 | 0.626 |
| LAD | −0.232 | 0.008 * |
| LAA-MFV | −0.013 | 0.882 |
| LAA-MEV | −0.077 | 0.385 |
| RDW-CV | 0.214 | 0.014 * |
| PLR | 0.054 | 0.544 |
| NLR | 0.104 | 0.240 |
| MHR | 0.066 | 0.365 |
| NOX4 | −0.045 | 0.609 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, Q.; Liu, D.; Li, Z.; Fu, Y.; Tse, G.; Li, G.; Liu, T.; Xu, G. Manganese Superoxide Dismutase as a Novel Oxidative Stress Biomarker for Predicting Paroxysmal Atrial Fibrillation. J. Clin. Med. 2022, 11, 5131. https://doi.org/10.3390/jcm11175131
Liu H, Wang Q, Liu D, Li Z, Fu Y, Tse G, Li G, Liu T, Xu G. Manganese Superoxide Dismutase as a Novel Oxidative Stress Biomarker for Predicting Paroxysmal Atrial Fibrillation. Journal of Clinical Medicine. 2022; 11(17):5131. https://doi.org/10.3390/jcm11175131
Chicago/Turabian StyleLiu, Hao, Qiao Wang, Daiqi Liu, Ziqi Li, Yulin Fu, Gary Tse, Guangping Li, Tong Liu, and Gang Xu. 2022. "Manganese Superoxide Dismutase as a Novel Oxidative Stress Biomarker for Predicting Paroxysmal Atrial Fibrillation" Journal of Clinical Medicine 11, no. 17: 5131. https://doi.org/10.3390/jcm11175131
APA StyleLiu, H., Wang, Q., Liu, D., Li, Z., Fu, Y., Tse, G., Li, G., Liu, T., & Xu, G. (2022). Manganese Superoxide Dismutase as a Novel Oxidative Stress Biomarker for Predicting Paroxysmal Atrial Fibrillation. Journal of Clinical Medicine, 11(17), 5131. https://doi.org/10.3390/jcm11175131








