Hand Motor Fatigability Induced by a Simple Isometric Task in Spinal Cord Injury
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Demographic and Clinical Data
2.3. Motor Performance: Hand Function and Motor Fatigability
2.4. Statistics
3. Results
3.1. Demographic and Clinical Variables
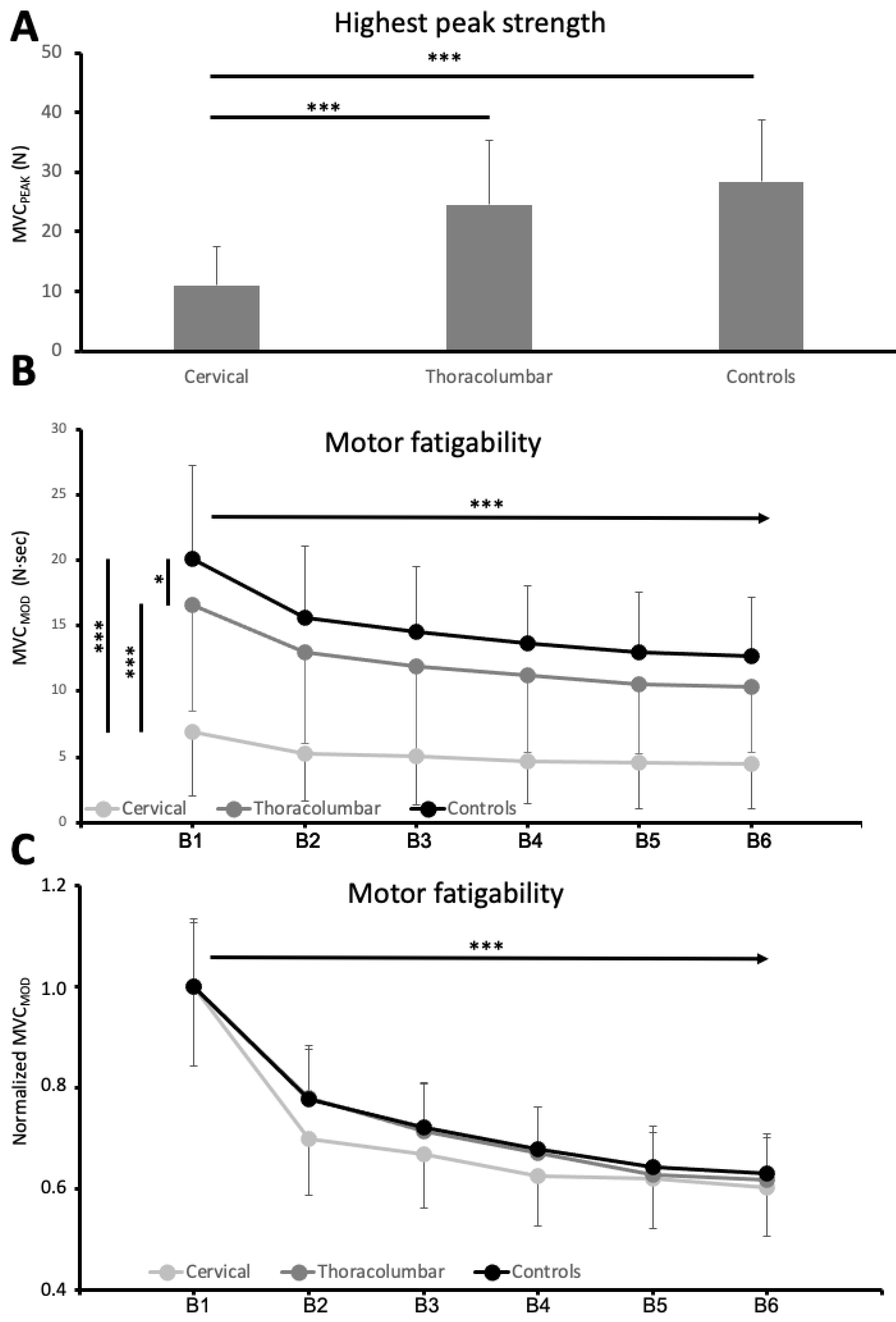
3.2. Motor Performance: Hand Function and Motor Fatigability
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wyndaele, M.; Wyndaele, J.J. Incidence, prevalence and epidemiology of spinal cord injury: What learns a worldwide literature survey? Spinal Cord 2006, 44, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Molton, I.R.; Groah, S.L.; Campbell, M.L.; Charlifue, S.; Chiodo, A.; Forchheimer, M.; Krause, J.S.; Tate, D. Secondary health conditions in individuals aging with SCI: Terminology, concepts and analytic approaches. Spinal Cord 2012, 50, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Tran, Y.; Wijesuriya, N.; Middleton, J. Fatigue and tiredness in people with spinal cord injury. J. Psychosom. Res. 2012, 73, 205–210. [Google Scholar] [CrossRef] [PubMed]
- McKinley, W.O.; Jackson, A.B.; Cardenas, D.D.; De Vivo, M.J. Long-term medical complications after traumatic spinal cord injury: A regional model systems analysis. Arch. Phys. Med. Rehabil. 1999, 80, 1402–1410. [Google Scholar] [CrossRef]
- Anton, H.A.; Miller, W.C.; Townson, A.F. Measuring fatigue in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 2008, 89, 538–542. [Google Scholar] [CrossRef]
- Fawkes-Kirby, T.M.; Wheeler, M.A.; Anton, H.A.; Miller, W.C.; Townson, A.F.; Weeks, C.A. Clinical correlates of fatigue in spinal cord injury. Spinal Cord 2008, 46, 21–25. [Google Scholar] [CrossRef]
- Lee, A.K.; Miller, W.C.; Townson, A.F.; Anton, H.A.; the F2N2 Research Group. Medication use is associated with fatigue in a sample of community-living individuals who have a spinal cord injury: A chart review. Spinal Cord 2010, 48, 429–433. [Google Scholar] [CrossRef]
- Anton, H.A.; Miller, W.C.; Townson, A.F.; Imam, B.; Silverberg, N.; Forwell, S. The course of fatigue after acute spinal cord injury. Spinal Cord 2016, 55, 94–97. [Google Scholar] [CrossRef]
- Figuérez, A.O.; Coy, J.A.; Fernández-Canosa, S.; León, V.S.; Molina, M.I.L.; Oliviero, A. Factors associated with fatigue in people with spinal cord injury: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-Based Management Strategies for Fatigue in Multiple Sclerosis; Paralyzed Veterans of America: Washington, DC, USA, 1998. [Google Scholar]
- Enoka, R.M.; Duchateau, J. Translating Fatigue to Human Performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef]
- Enoka, R.M.; Almuklass, A.M.; Alenazy, M.; Alvarez, E.; Duchateau, J. Distinguishing between Fatigue and Fatigability in Multiple Sclerosis. Neurorehabilit. Neural Repair 2021, 35, 960–973. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Tran, Y.; Wijesuriya, N.; Boord, P. A controlled investigation into the psychological determinants of fatigue. Biol. Psychol. 2006, 72, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Mahjoub, S.Z. Fatigue in healthy and diseased individuals. Am. J. Hosp. Palliat. Med. 2014, 31, 562–575. [Google Scholar] [CrossRef]
- Bigland-Ritchie, B.; Woods, J.J. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 1984, 7, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.K.; Enoka, R.M. The neurobiology of muscle fatigue: 15 years later. Integr. Comp. Biol. 2007, 47, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Tonali, P.A.; Mazzone, P.; Insola, A.; Pilato, F.; Saturno, E.; Dileone, M.; Rothwell, J.C. Direct demonstration of reduction of the output of the human motor cortex induced by a fatiguing muscle contraction. Exp. Brain Res. 2003, 149, 535–538. [Google Scholar] [CrossRef]
- Arias, P.; Robles-García, V.; Corral-Bergantiños, Y.; Madrid, A.; Espinosa, N.; Valls-Solé, J.; Grieve, K.L.; Oliviero, A.; Cudeiro, J. Central fatigue induced by short-lasting finger tapping and isometric tasks: A study of silent periods evoked at spinal and supraspinal levels. Neuroscience 2015, 305, 316–327. [Google Scholar] [CrossRef]
- Barat, M.; Dehail, P.; de Seze, M. Fatigue after spinal cord injury. Ann. Readapt. Med. Phys. 2006, 49, 277–282. [Google Scholar] [CrossRef]
- Hammell, K.W.; Miller, W.C.; Forwell, S.J.; Forman, B.E.; Jacobsen, B.A. Fatigue and spinal cord injury: A qualitative analysis. Spinal Cord 2009, 47, 44–49. [Google Scholar] [CrossRef]
- Freixes, O.; Rivas, M.E.; Agrati, P.E.; Bochkezanian, V.; Waldman, S.V.; Olmos, L.E. Fatigue level in spinal cord injury AIS D community ambulatory subjects. Spinal Cord 2012, 50, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.L.; Krause, J.S. Behavioral factors related to fatigue among persons with spinal cord injury. Arch. Phys. Med. Rehabil. 2012, 93, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Tran, Y.; Siddall, P.; Wijesuriya, N.; Lovas, J.; Bartrop, R.; Middleton, J. Developing a model of associations between chronic pain, depressive mood, chronic fatigue, and self-efficacy in people with spinal cord injury. J. Pain 2013, 14, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Cudeiro-Blanco, J.; Onate-Figuérez, A.; Soto-León, V.; Avendaño-Coy, J.; Mordillo-Mateos, L.; Brocalero-Camacho, A.; Esclarin-Ruz, A.; Rotondi, M.; Aguilar, J.; Arias, P.; et al. Prevalence of Fatigue and Associated Factors in a Spinal Cord Injury Population: Data from an Internet-Based and Face-to-Face Surveys. J. Neurotrauma 2017, 34, 2335–2341. [Google Scholar] [CrossRef]
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef]
- Alschuler, K.N.; Jensen, M.P.; Sullivan-Singh, S.J.; Borson, S.; Smith, A.E.; Molton, I.R. The association of age, pain, and fatigue with physical functioning and depressive symptoms in persons with spinal cord injury. J. Spinal Cord Med. 2013, 36, 483–491. [Google Scholar] [CrossRef]
- Tawashy, A.E.; Eng, J.J.; Lin, K.H.; Tang, P.F.; Hung, C. Physical activity is related to lower levels of pain, fatigue and depression in individuals with spinal-cord injury: A correlational study. Spinal Cord 2009, 47, 301–306. [Google Scholar] [CrossRef]
- Jensen, M.P.; Kuehn, C.M.; Amtmann, D.; Cardenas, D.D. Symptom burden in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 2007, 88, 638–645. [Google Scholar] [CrossRef]
- Wijesuriya, N.; Tran, Y.; Middleton, J.; Craig, A. Impact of fatigue on the health-related quality of life in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 2012, 93, 319–324. [Google Scholar] [CrossRef]
- Michaelis, L. International inquiry on neurological terminology and prognosis in paraplegia and tetraplegia. Spinal Cord 1969, 7, 1–5. [Google Scholar] [CrossRef][Green Version]
- Maynard, F.M.; Bracken, M.B.; Creasey, G.; Ditunno, J.F.; Donovan, W.H.; Ducker, T.B.; Garber, S.L.; Marino, R.J.; Stover, S.L.; Tator, C.H.; et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord 1997, 35, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Kuppuswamy, A.; Rothwell, J.; Ward, N. A model of poststroke fatigue based on sensorimotor deficits. Curr. Opin. Neurol. 2015, 28, 582–586. [Google Scholar] [CrossRef]
- Mordillo-Mateos, L.; Soto-Leon, V.; Torres-Pareja, M.; Peinado-Palomino, D.; Mendoza-Laiz, N.; Alonso-Bonilla, C.; Dileone, M.; Rotondi, M.; Aguilar, J.; Oliviero, A. Fatigue in Multiple Sclerosis: General and Perceived Fatigue Does Not Depend on Corticospinal Tract Dysfunction. Front. Neurol. 2019, 10, 339. [Google Scholar] [CrossRef]
- Siciliano, M.; Trojano, L.; Santangelo, G.; De Micco, R.; Tedeschi, G.; Tessitore, A. Fatigue in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2018, 33, 1712–1723. [Google Scholar] [CrossRef]
- Soto-Leon, V.; Alonso-Bonilla, C.; Peinado-Palomino, D.; Torres-Pareja, M.; Mendoza-Laiz, N.; Mordillo-Mateos, L.; Onate-Figuerez, A.; Arias, P.; Aguilar, J.; Oliviero, A. Effects of fatigue induced by repetitive movements and isometric tasks on reaction time. Hum. Mov. Sci. 2020, 73, 102679. [Google Scholar] [CrossRef]
- Andraszewicz, S.; Scheibehenne, B.; Rieskamp, J.; Grasman, R.; Verhagen, J.; Wagenmakers, E.-J. An Introduction to Bayesian Hypothesis Testing for Management Research. J. Manag. 2015, 41, 521–543. [Google Scholar] [CrossRef]
- Westfall, P. A Bayesian perspective on the Bonferroni adjustment. Biometrika 1997, 84, 419–427. [Google Scholar] [CrossRef]
- Lin, K.H.; Chen, Y.C.; Luh, J.J.; Wang, C.H.; Chang, Y.J. H-reflex, muscle voluntary activation level, and fatigue index of flexor carpi radialis in individuals with incomplete cervical cord injury. Neurorehabilit. Neural Repair 2012, 26, 68–75. [Google Scholar] [CrossRef]
- Prak, R.F.; Doestzada, M.; Thomas, C.K.; Tepper, M.; Zijdewind, I. Reduced voluntary drive during sustained but not during brief maximal voluntary contractions in the first dorsal interosseous weakened by spinal cord injury. J. Appl. Physiol. 2015, 119, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M. Mechanisms of muscle fatigue: Central factors and task dependency. J. Electromyogr. Kinesiol. 1995, 5, 141–149. [Google Scholar] [CrossRef]
- Enoka, R.M.; Stuart, D.G. Neurobiology of muscle fatigue. J. Appl. Physiol. 1992, 72, 1631–1648. [Google Scholar] [CrossRef] [PubMed]
- Madrid, A.; Valls-Solé, J.; Oliviero, A.; Cudeiro, J.; Arias, P. Differential responses of spinal motoneurons to fatigue induced by short-lasting repetitive and isometric tasks. Neuroscience 2016, 339, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Madrid, A.; Madinabeitia-Mancebo, E.; Cudeiro, J.; Arias, P. Effects of a Finger Tapping Fatiguing Task on M1-Intracortical Inhibition and Central Drive to the Muscle. Sci. Rep. 2018, 8, 9326. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Thomas, C.K. Effects of sustained stimulation on the excitability of motoneurons innervating paralyzed and control muscles. J. Appl. Physiol. 2003, 94, 567–575. [Google Scholar] [CrossRef]
- Krieger, S.R.; Pierotti, D.J.; Coast, J.R. Spinal cord injury and contractile properties of the human tibialis anterior. J. Sports Sci. Med. 2005, 4, 124–133. [Google Scholar]
- Celichowski, J.; Mrówczyński, W.; Krutki, P.; Górska, T.; Majczyński, H.; Sławińska, U. Changes in contractile properties of motor units of the rat medial gastrocnemius muscle after spinal cord transection. Exp. Physiol. 2006, 91, 887–895. [Google Scholar] [CrossRef]
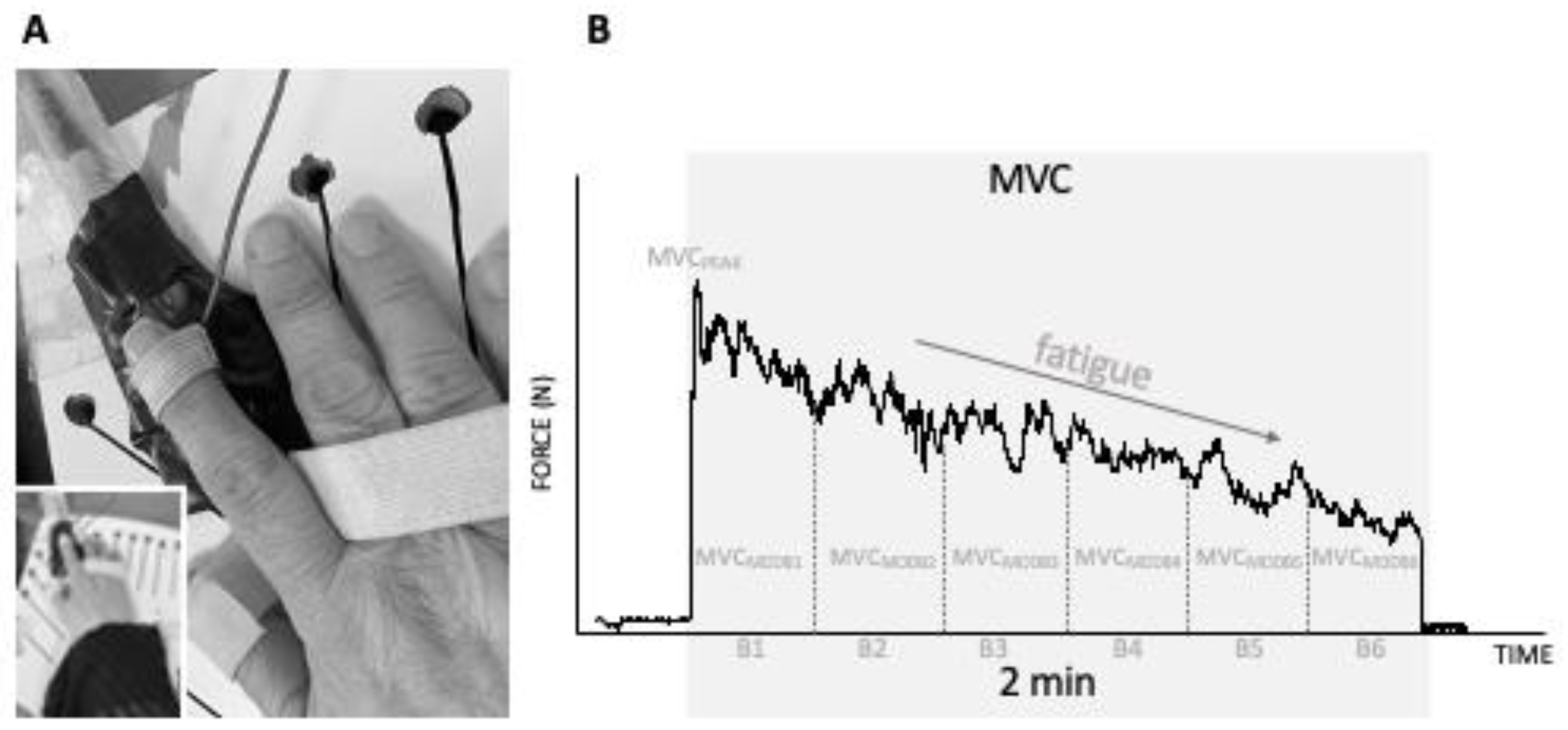
| Variable | Thoracolumbar | Cervical | p Value |
|---|---|---|---|
| N | 56 | 40 | - |
| Demographic | |||
| AGE (mean ± SD years) | 47.43 ± 17.56 | 47.18 ± 17.89 | 0.945 ** |
| SEX (Male/Female) | 33/23 | 26/14 | 0.547 * |
| Clinical Data | |||
| SCI etiology (Traumatic/non-Traumatic) | 34/22 | 30/10 | 0.143 * |
| TIME SINCE INJURY (mean ± SD months) | 27.37 ± 82.07 | 18.30 ± 39.45 | 0.519 ** |
| AIS (A/B/C/D) | 30/2/15/9 | 3/5/17/15 | <0.001 *** |
| UEMS (median, 95% CI) | 50.00 | 39.50 (36.15–41.54) | |
| LEMS (median, 95% CI) | 6.00 (10.00–18.57) | 31.50 (20.99–31.91) | 0.001 *** |
| UEMS_preferred_hand (median, 95% CI) | 25.00 | 22.00 (20.72–22.67) | |
| MAS (median, 95% CI) | 1.00 (0.60–1.13) | 1.25 (0.94–1.53) | 0.038 *** |
| FSS (mean ± SD) | 2.92 ± 1.54 | 3.34 ± 1.40 | 0.117 *** |
| CSF | 14 (25.00%) | 12 (30.00%) | 0.587 * |
| PAIN-NRS (median, 95% CI) | 3.00 (2.29–4.03) | 2.50 (2.31–4.64) | 0.741 *** |
| DEPRESSIVE MOOD | 11 (19.64%) | 4 (10.00%) | 0.200 * |
| Controls | Thoracolumbar | Cervical | p value | |
|---|---|---|---|---|
| MVCPEAK | 28.44 ± 10.28 N | 24.56 ± 10.70 N | 11.04 ± 6.58 N | <0.001 |
| MVCMOD | 20.11 ± 7.13 N·s | 16.53 ± 8.07 N·s | 6.90 ± 4.91 N·s | <0.001 |
| MVCMODF | 65.97 ± 19.40% | 64.67 ± 20.71% | 63.71 ± 23.00% | 0.862 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onate-Figuérez, A.; Soto-León, V.; Avendaño-Coy, J.; Mordillo-Mateos, L.; Pérez-Borrego, Y.A.; Redondo-Galán, C.; Arias, P.; Oliviero, A. Hand Motor Fatigability Induced by a Simple Isometric Task in Spinal Cord Injury. J. Clin. Med. 2022, 11, 5108. https://doi.org/10.3390/jcm11175108
Onate-Figuérez A, Soto-León V, Avendaño-Coy J, Mordillo-Mateos L, Pérez-Borrego YA, Redondo-Galán C, Arias P, Oliviero A. Hand Motor Fatigability Induced by a Simple Isometric Task in Spinal Cord Injury. Journal of Clinical Medicine. 2022; 11(17):5108. https://doi.org/10.3390/jcm11175108
Chicago/Turabian StyleOnate-Figuérez, Ana, Vanesa Soto-León, Juan Avendaño-Coy, Laura Mordillo-Mateos, Yolanda A. Pérez-Borrego, Carolina Redondo-Galán, Pablo Arias, and Antonio Oliviero. 2022. "Hand Motor Fatigability Induced by a Simple Isometric Task in Spinal Cord Injury" Journal of Clinical Medicine 11, no. 17: 5108. https://doi.org/10.3390/jcm11175108
APA StyleOnate-Figuérez, A., Soto-León, V., Avendaño-Coy, J., Mordillo-Mateos, L., Pérez-Borrego, Y. A., Redondo-Galán, C., Arias, P., & Oliviero, A. (2022). Hand Motor Fatigability Induced by a Simple Isometric Task in Spinal Cord Injury. Journal of Clinical Medicine, 11(17), 5108. https://doi.org/10.3390/jcm11175108






