Treatment Effect and Safety of Nanoliposomal Irinotecan with Fluorouracil and Folinic Acid after Gemcitabine-Based Therapy in Patients with Advanced Pancreatic Cancer: A Multicenter, Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and the Study Design
2.2. Treatment
2.3. Evaluation of Efficacy and Toxicity
2.4. Ethical Standards
2.5. Statistics
3. Results
3.1. Patient Characteristics
3.2. Treatment Delivery and Dose Reduction or Discontinuation
3.3. Treatment Response
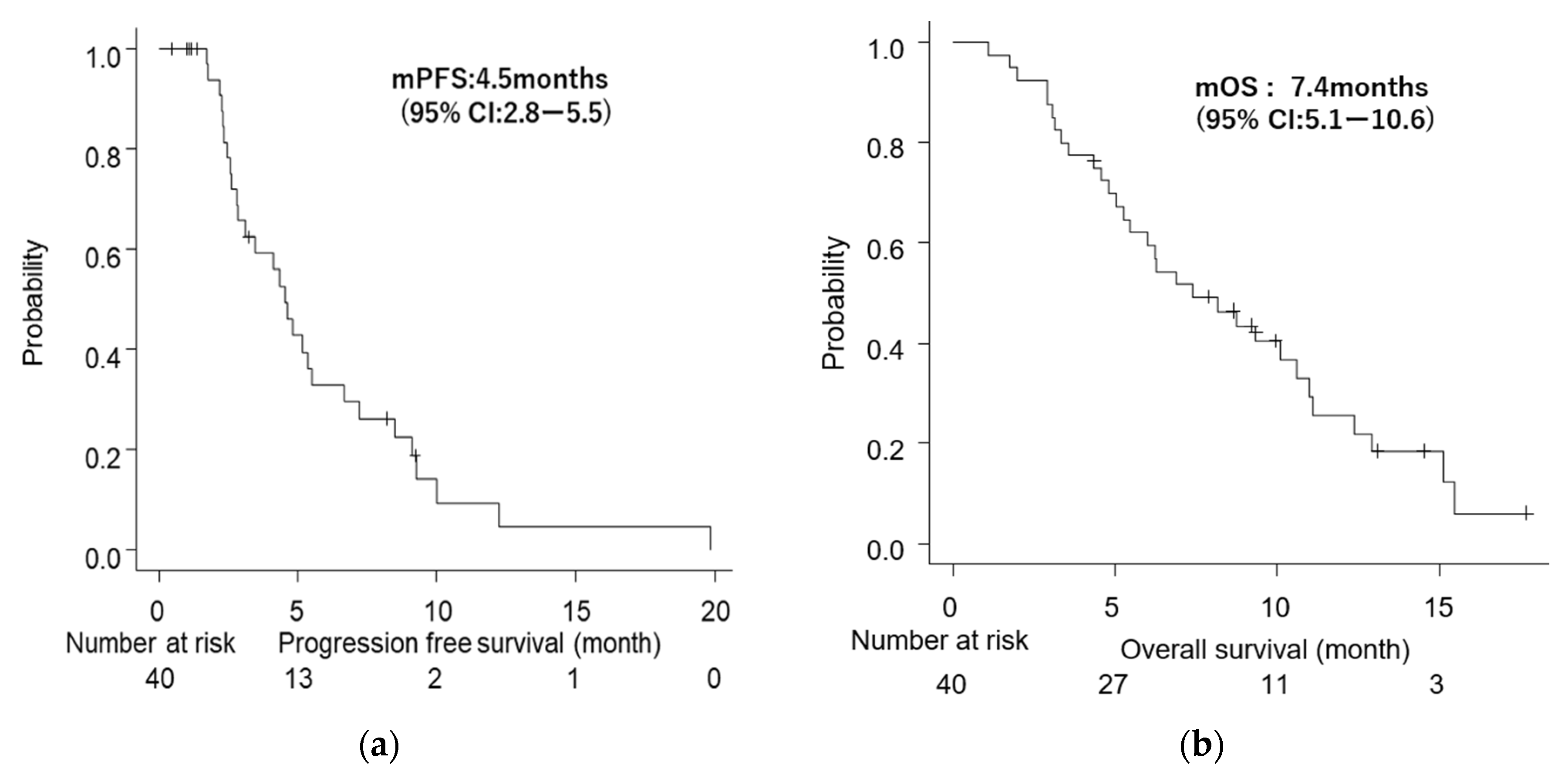
3.4. Survival
3.5. Safety
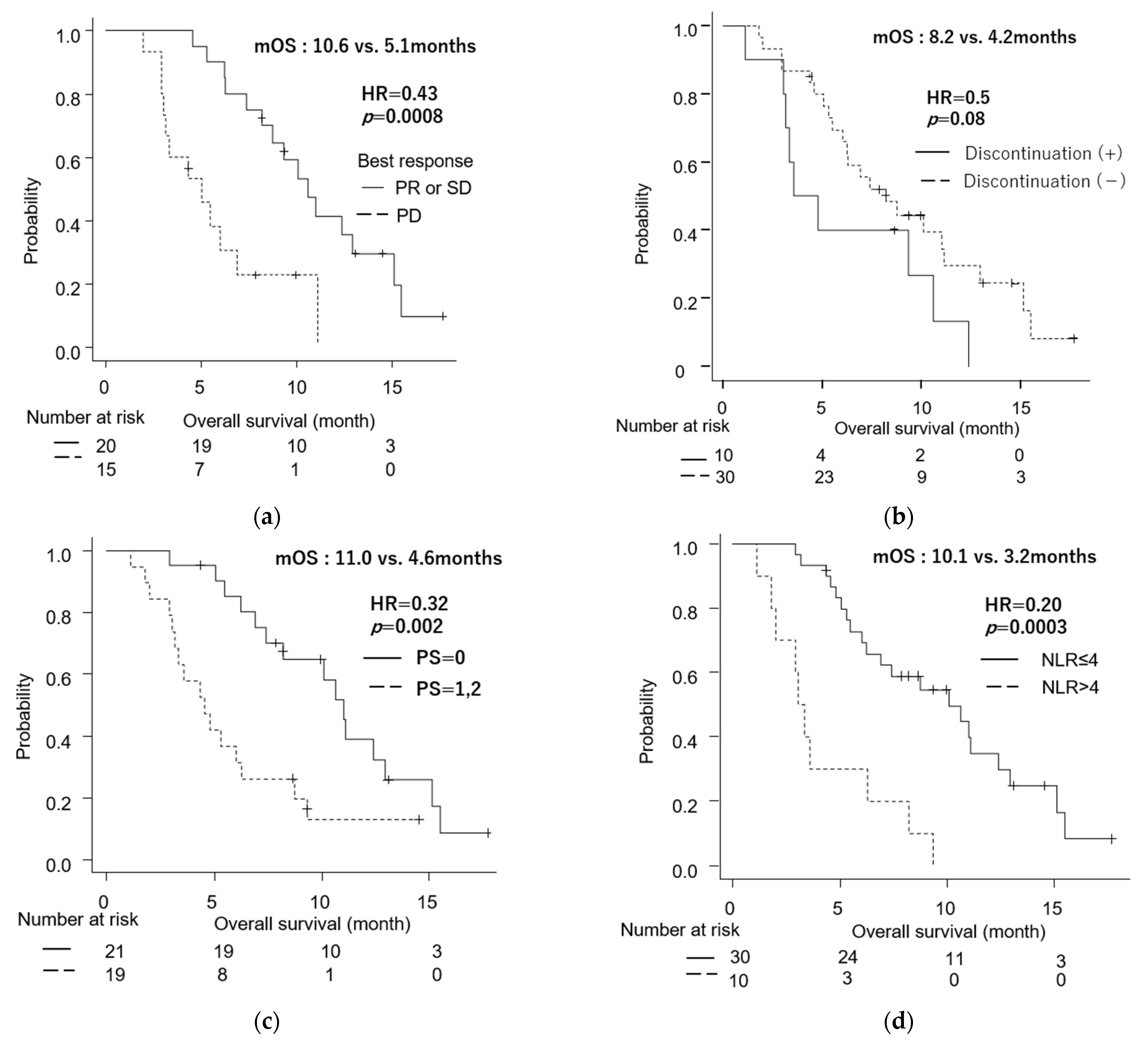
3.6. Comparison between Patients Who Continued and Discontinued the Treatment Regimen
3.7. Univariate and Multivariate Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic Cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX Versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2015, 36918, 1691–1703. [Google Scholar]
- Drummond, D.C.; Noble, C.O.; Guo, Z.; Hong, K.; Park, J.W.; Kirpotin, D.B. Development of a Highly Active Nanoliposomal Irinotecan Using a Novel Intraliposomal Stabilization Strategy. Cancer Res. 2006, 66, 3271–3277. Available online: http://www.aacrjournals.org (accessed on 10 August 2022). [CrossRef]
- Kalra, A.V.; Kim, J.; Klinz, S.G.; Paz, N.; Cain, J.; Drummond, D.C.; Nielsen, U.B.; Fitzgerald, J.B. Preclinical Activity of Nanoliposomal Irinotecan Is Governed by Tumor Deposition and Intratumor Prodrug Conversion. Available online: http://cancerres.aacrjournals.org/ (accessed on 10 August 2022).
- Chiang, N.J.; Chao, T.Y.; Hsieh, R.K.; Wang, C.H.; Wang, Y.W.; Yeh, C.G.; Chen, L.T. A Phase I Dose-Escalation Study of PEP02 (Irinotecan Liposome Injection) in Combination with 5-Fluorouracil and Leucovorin in Advanced Solid Tumors. BMC Cancer 2016, 16, 907. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Li, C.P.; Bodoky, G.; Dean, A.; Shan, Y.S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Schwartsmann, G.; et al. Nanoliposomal Irinotecan with Fluorouracil and Folinic Acid in Metastatic Pancreatic Cancer After Previous Gemcitabine-Based Therapy (NAPOLI-1): A Global, Randomised, Open-Label, Phase 3 Trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Ueno, M.; Nakamori, S.; Sugimori, K.; Kazuya, K.M.; Kawabe, K.; Furuse, J.; Komatsu, Y.; Ishii, H.; Sato, S.; Shimizu, S.; et al. Nal-IRI+5-FU/LV Versus 5-FU/LV in Post-Gemcitabine Metastatic Pancreatic Cancer: Randomized Phase 2 Trial in Japanese Patients. Cancer Med. 2020, 924, 9396–9408. [Google Scholar]
- Lee, J.C.; Kim, J.W.; Ahn, S.; Kim, H.W.; Lee, J.; Kim, Y.H.; Paik, K.H.; Kim, J.; Hwang, J.H. Optimal Dose Reduction of FOLFIRINOX for Preserving Tumour Response in Advanced Pancreatic Cancer: Using Cumulative Relative Dose Intensity. Eur. J. Cancer 2017, 76, 125–133. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Bang, Y.J.; Li, C.P.; Lee, K.H.; Chiu, C.F.; Park, J.O.; Shan, Y.S.; Kim, J.S.; Chen, J.S.; Shim, H.J.; Rau, K.M.; et al. Liposomal Irinotecan in Metastatic Pancreatic Adenocarcinoma in Asian Patients: Subgroup Analysis of the NAPOLI-1 Study. Cancer Sci. 2020, 111, 513–527. [Google Scholar] [CrossRef]
- Smith, C.J.; Bekaii-Saab, T.S.; Cook, K.D.; Eiring, R.A.; Halfdanarson, T.R.; Hanna, M.; Jin, Z.; Jochum, J.A.; Ma, W.W.; Mitchell, J.L.; et al. Nanoliposomal Irinotecan (Nal-IRI)-Based Chemotherapy After Irinotecan -Based Chemotherapy in Patients with Pancreas Cancer. Pancreatology 2021, 21, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Lee, C.Y.; Lin, L.G.; Chao, Y.; Li, C.P. Nanoliposomal irinotecan with 5-fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy: A real-world experience. J. Chin. Med. Assoc. 2022, 85, 42–50. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, H.; Shin, K.; Hong, T.H.; Suh, J.H.; Lee, M.A. Nanoliposomal Irinotecan plus Fluorouracil and Folinic Acid as a Second-Line Treatment Option in Patients with Metastatic Pancreatic Ductal Adenocarcinoma: A Retrospective Cohort Study. BMC Cancer 2021, 21, 211. [Google Scholar] [CrossRef]
- Su, Y.Y.; Chiang, N.J.; Tsai, H.J.; Yen, C.J.; Shan, Y.S.; Chen, L.T. The Impact of Liposomal Irinotecan on the Treatment of Advanced Pancreatic Adenocarcinoma: Real-World Experience in a Taiwanese Cohort. Sci. Rep. 2020, 10, 101. [Google Scholar] [CrossRef]
- Kasi, A.; McGinnis, T.; Naik, G.; Handa, S.; Williams, G.; Paluri, R. Efficacy and Tolerability of the Combination of Nano-liposomal Irinotecan and 5-Fluorouracil/Leucovorin in Advanced Pancreatic Adenocarcinoma: Post-approval Clinic Experience. J. Gastrointest. Oncol. 2021, 12, 464–473. [Google Scholar] [CrossRef]
- Glassman, D.C.; Palmaira, R.L.; Covington, C.M.; Desai, A.M.; Ku, G.Y.; Li, J.; Harding, J.J.; Varghese, A.M.; O’Reilly, E.M.; Yu, K.H. Nanoliposomal Irinotecan with Fluorouracil for the Treatment of Advanced Pancreatic Cancer, a Single Institution Experience. BMC Cancer 2018, 18, 693. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Feeney, K.; Lee, M.A.; Muñoz, A.; Gracián, A.C.; Lonardi, S.; Ryoo, B.Y.; Hung, A.; Lin, Y.; Bendell, J.; et al. Meta-analysis Examining Overall Survival in Patients with Pancreatic Cancer Treated with Second-Line 5-Fluorouracil and Oxaliplatin-Based Therapy After Failing First-Line Gemcitabine-Containing Therapy: Effect of Performance Status and Comparison with Other Regimens. BMC Cancer 2020, 20, 633. [Google Scholar] [CrossRef]
- Oh, D.; Pyo, J.S.; Son, B.K. Prognostic Roles of Inflammatory Markers in Pancreatic Cancer: Comparison Between the Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio. Gastroenterol. Res. Pract. 2018, 2018, 9745601. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.J.; Ma, J.Y.; Hu, G. Lymphocyte-to-Monocyte Ratio in Pancreatic Cancer: Prognostic Significance and Meta-analysis. Clin. Chim. Acta 2018, 481, 142–146. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Hubner, R.A.; Siveke, J.T.; Von Hoff, D.D.; Belanger, B.; de Jong, F.A.; Mirakhur, B.; Chen, L.-T. NAPOLI-1 Phase 3 Study of Liposomal Irinotecan in Metastatic Pancreatic Cancer: Final Overall Survival Analysis and Characteristics of Long-Term Survivors. Eur. J. Cancer 2019, 108, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| n/Median | (%)/[IQR] | |
|---|---|---|
| Age | 70.5 | [62.5–72.0] |
| Sex-Male | 19 | (47.5) |
| Body Mass Index | 20.9 | [14.3–26.5] |
| Primary site | ||
| Head/Body/Tail | 17/12/11 (43/30/27) | |
| Diameter of the primary tumor (mm) | 32 | [18–75] |
| Presence of history of biliary drainage | 9 | (23) |
| History of surgical resection | 9 | (23) |
| Distant metastases | 31 | (78) |
| Metastatic site | ||
| Liver | 16 | (40) |
| Peritoneum | 13 | (3) |
| Lung | 6 | (15) |
| Number of metastatic sites | ||
| 1 | 12 | (30) |
| 2 | 14 | (35) |
| ≥3 | 5 | (13) |
| ECOG PS | ||
| 0/1/2 | 21/18/1 (52/45/3) | |
| Presence of UGT1A1 polymorphism | 2 | (5) |
| Reason for the discontinuation of the previous therapy | ||
| Progressive disease | 36 | (90) |
| Toxicity | 4 | (10) |
| Line of therapy where nal-IRI/FF was administered | ||
| Second-line | 36 | (90) |
| Third-line | 4 | (10) |
| First-line regimen | ||
| Gemcitabine + nab-paclitaxel | 39 | (98) |
| Gemcitabine + S-1 | 1 | (3) |
| Second-line regimen * | ||
| S-1 | 3 | (8) |
| Gemcitabine + nab-paclitaxel | 1 | (3) |
| The duration between the diagnosis and initiation of nal-IRI/FF (months) | ||
| 6.9 | [5.0–10.7] | |
| Post-study anticancer therapy | ||
| modified FOLFIRINOX | 7 | (18) |
| Gemcitabine + nab-paclitaxel | 3 | (8) |
| Oxaliplatin + fluorouracil/folinic acid | 7 | (18) |
| S-1 | 1 | (3) |
| Observation period (months) | 7.3 | [4.4–10.6] |
| Median | (%)/[IQR] | |
|---|---|---|
| The initial dose of nal-IRI | ||
| 100% | 28 | (70) |
| 90% | 0 | (0) |
| 80% | 9 | (21.5) |
| 70% | 3 | (7.5) |
| Duration of treatment (months) | 3.2 | [1.7–6.2] |
| Cycles of treatment (n) | 6 | [3–10] |
| Relative dose intensity (%) | 69.0 | [54.5–86.5] |
| Dose reduction during all cycles | 20 | (50) |
| Dose reduction due to AE in the first 4 cycles | 14 | (35) |
| Discontinuation due to AE | 10 | (40) |
| Tumor Response § | n | (%) |
|---|---|---|
| PR | 3 | (9) |
| SD | 17 | (49) |
| PD | 15 | (43) |
| DCR | 20 | (57) |
| CA19-9 response | n | (%) |
| responder | 8 | (25) |
| non-responder | 24 | (75) |
| All Grade | Grade 3/4 | Required Dose Reduction | Required Discontinuation | |||||
|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | |||||
| Hematologic | ||||||||
| Neutropenia | 25 | (63) | 11 | (28) | 9 | (23) | ||
| Leukocytopenia | 24 | (60) | 5 | (13) | ||||
| Thrombocytopenia | 11 | (28) | 0 | (0) | ||||
| Anemia | 14 | (35) | 2 | (5) | ||||
| Non-hematologic | ||||||||
| General fatigue | 25 | (63) | 9 | (23) | 9 | (23) | 6 | (15) |
| Anorexia | 16 | (40) | 2 | (5) | ||||
| Diarrhea | 11 | (28) | 1 | (3) | 2 | (5) | 1 | (3) |
| Hypokalemia | 2 | (5) | 0 | (0) | ||||
| Dysgeusia | 2 | (5) | 0 | (0) | ||||
| Hemobilia | 1 | (3) | 1 | (3) | ||||
| Septic shock | 1 | (3) | 1 | (3) | ||||
| Ileus | 1 | (3) | 1 | (3) | ||||
| Infusion reaction | 1 | (3) | 0 | (0) | ||||
| Variables at the Start of nal-IRI/FF Treatment | n | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | ||
| Age | |||||||
| ≥70 y.o. | 20 | 1.17 | (0.57–2.38) | 0.67 | |||
| <70 y.o. | 20 | ||||||
| Sex | |||||||
| male | 19 | 1.06 | (0.52–2.17) | 0.86 | |||
| female | 21 | ||||||
| ECOG PS | |||||||
| =1 or 2 | 19 | 3.08 | (1.43–6.63) | 0.004 | 2.24 | 0.86–5.73 | 0.09 |
| =0 | 21 | ||||||
| Stage | |||||||
| metastatic | 31 | 1.40 | (0.57–3.46) | 0.51 | |||
| locally advanced | 9 | ||||||
| Liver metastasis | |||||||
| present | 19 | 1.12 | (0.54–2.32) | 0.67 | |||
| absent | 21 | ||||||
| Peritoneal metastasis | |||||||
| present | 14 | 1.12 | (0.54–2.33) | 0.76 | |||
| absent | 26 | ||||||
| Carcinomatosis * | |||||||
| present | 19 | 1.44 | (0.71–2.93) | 0.31 | |||
| absent | 21 | ||||||
| NLR | |||||||
| >4 | 10 | 4.88 | (2.10–11.3) | 0.0002 | 3.08 | 1.21–7.85 | 0.02 |
| ≤4 | 30 | ||||||
| CA19-9 | |||||||
| >1000 U/dL | 20 | 1.38 | (0.67–2.80) | 0.37 | |||
| ≤1000 U/dL | 16 | ||||||
| GPS | |||||||
| =2 | 11 | 2.08 | (0.94–4.59) | 0.07 | |||
| =0.1 | 29 | ||||||
| LMR | |||||||
| <3 | 27 | 1.18 | (0.55–2.52) | 0.67 | |||
| ≥3 | 13 | ||||||
| PLR | |||||||
| >150 | 32 | 0.80 | (0.35–1.83) | 0.60 | |||
| ≤150 | 8 | ||||||
| PNI | |||||||
| <45 | 30 | 0.78 | (0.35–1.71) | 0.53 | |||
| ≥45 | 10 | ||||||
| Bodyweight decrease from the diagnosis as an unresectable disease | |||||||
| >5% | 14 | 2.29 | (1.09–4.85) | 0.03 | 1.46 | 0.64–3.32 | 0.37 |
| ≤5% | 23 | ||||||
| The interval from the diagnosis to the administration of nal-IRI/FF | |||||||
| >6.6months | 20 | 1.04 | 0.51–2.14 | 0.95 | |||
| ≤6.6months | 20 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miki, M.; Fujimori, N.; Ueda, K.; Lee, L.; Murakami, M.; Takamatsu, Y.; Shimokawa, Y.; Niina, Y.; Oono, T.; Hisano, T.; et al. Treatment Effect and Safety of Nanoliposomal Irinotecan with Fluorouracil and Folinic Acid after Gemcitabine-Based Therapy in Patients with Advanced Pancreatic Cancer: A Multicenter, Prospective Observational Study. J. Clin. Med. 2022, 11, 5084. https://doi.org/10.3390/jcm11175084
Miki M, Fujimori N, Ueda K, Lee L, Murakami M, Takamatsu Y, Shimokawa Y, Niina Y, Oono T, Hisano T, et al. Treatment Effect and Safety of Nanoliposomal Irinotecan with Fluorouracil and Folinic Acid after Gemcitabine-Based Therapy in Patients with Advanced Pancreatic Cancer: A Multicenter, Prospective Observational Study. Journal of Clinical Medicine. 2022; 11(17):5084. https://doi.org/10.3390/jcm11175084
Chicago/Turabian StyleMiki, Masami, Nao Fujimori, Keijiro Ueda, Lingaku Lee, Masatoshi Murakami, Yu Takamatsu, Yuzo Shimokawa, Yusuke Niina, Takamasa Oono, Terumasa Hisano, and et al. 2022. "Treatment Effect and Safety of Nanoliposomal Irinotecan with Fluorouracil and Folinic Acid after Gemcitabine-Based Therapy in Patients with Advanced Pancreatic Cancer: A Multicenter, Prospective Observational Study" Journal of Clinical Medicine 11, no. 17: 5084. https://doi.org/10.3390/jcm11175084
APA StyleMiki, M., Fujimori, N., Ueda, K., Lee, L., Murakami, M., Takamatsu, Y., Shimokawa, Y., Niina, Y., Oono, T., Hisano, T., Furukawa, M., & Ogawa, Y. (2022). Treatment Effect and Safety of Nanoliposomal Irinotecan with Fluorouracil and Folinic Acid after Gemcitabine-Based Therapy in Patients with Advanced Pancreatic Cancer: A Multicenter, Prospective Observational Study. Journal of Clinical Medicine, 11(17), 5084. https://doi.org/10.3390/jcm11175084







