The Mortality Risk and Pulmonary Fibrosis Investigated by Time-Resolved Fluorescence Spectroscopy from Plasma in COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Characteristics of the Study Patients
2.2. Sample Preparation
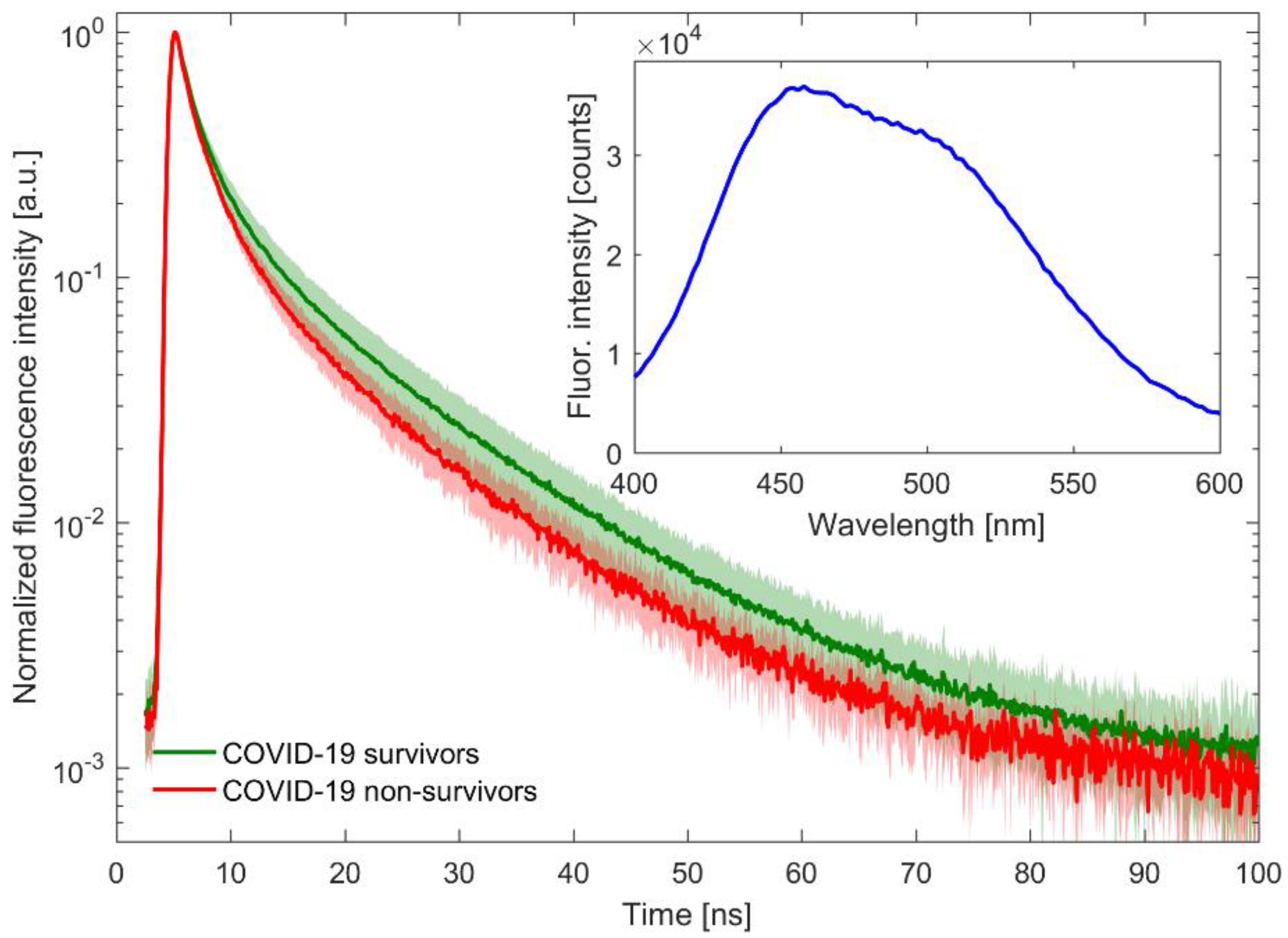
2.3. Time-Resolved Fluorescence Spectroscopy Measurements
2.4. Statistical Analysis
3. Results
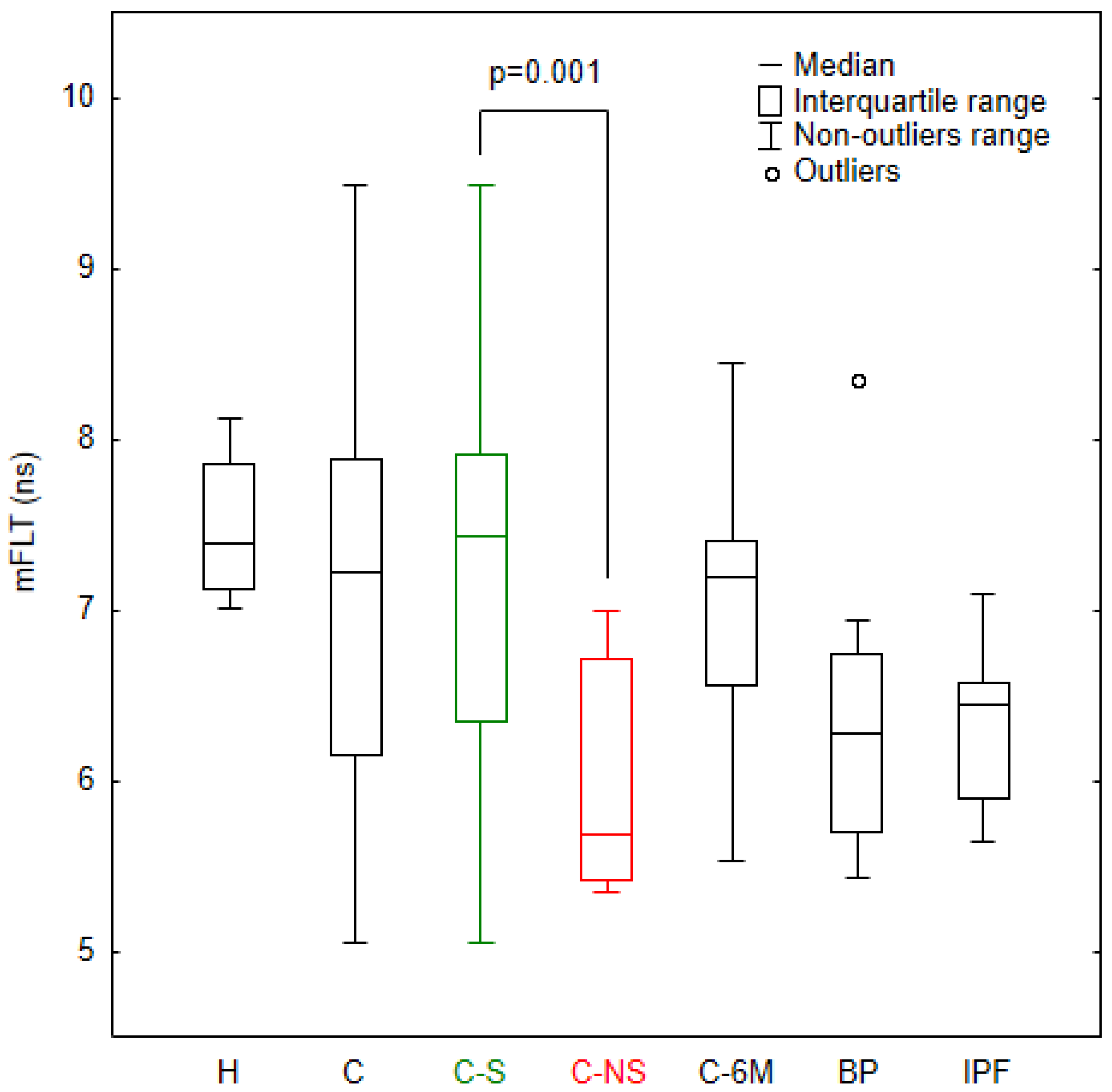
3.1. Analysis of mFLT in the Study Groups
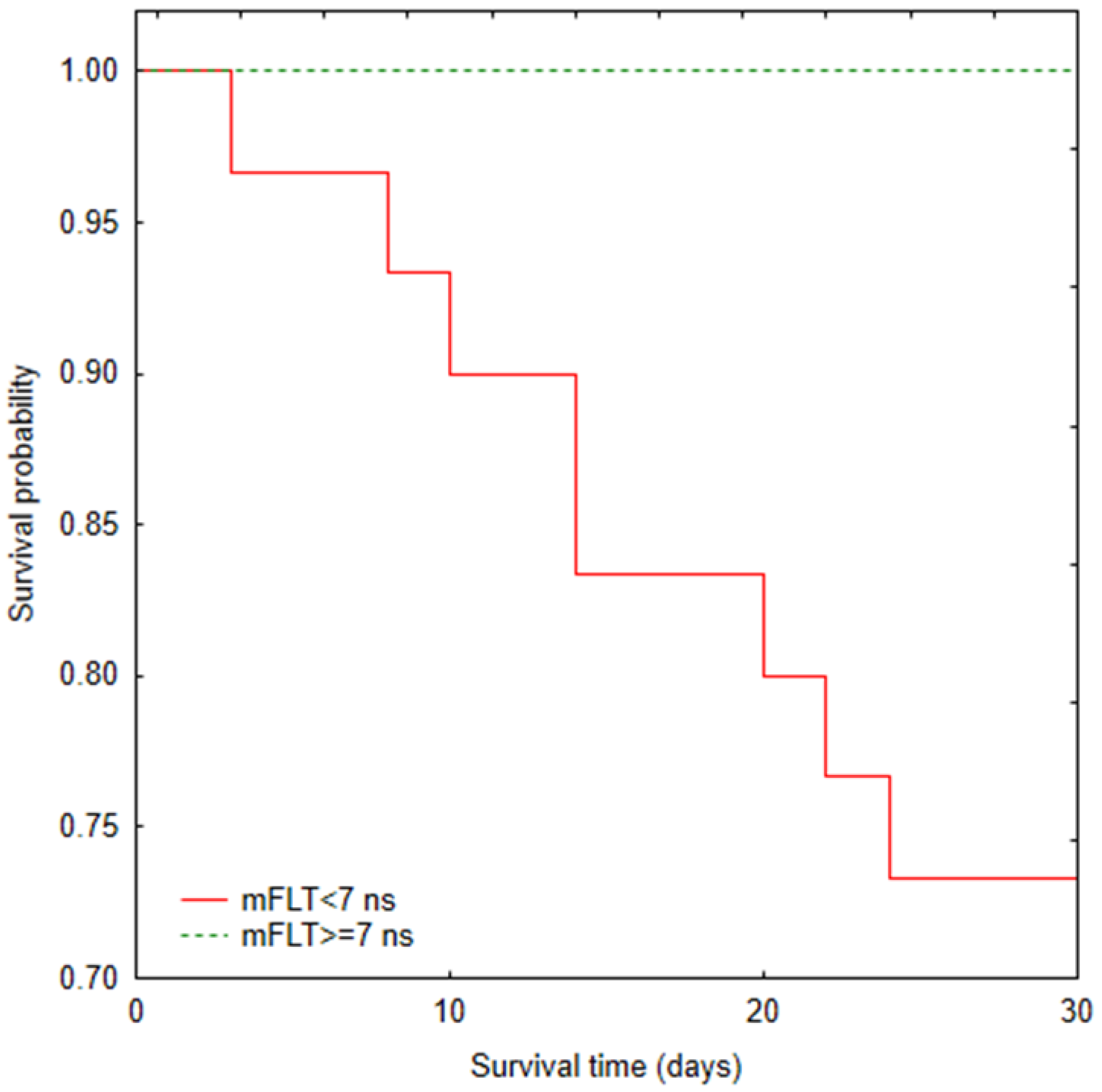
3.2. Mortality Analysis
3.3. A Correlation Study in COVID-19 Patients
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Coronavirus Disease 2019 (COVID-19) Situation Report-98; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200427-sitrep-98-covid-19.pdf?sfvrsn=90323472_4 (accessed on 27 April 2020).
- Zumla, A.; Hui, D.S.; Azhar, E.I.; Memish, Z.A.; Maeurer, M. Reducing mortality from 2019-nCoV: Host-directed therapies should be an option. Lancet 2020, 395, e35–e36. [Google Scholar] [CrossRef]
- Wilson, M.S.; Wynn, T.A. Pulmonary fibrosis: Pathogenesis, etiology and regulation. Mucosal Immunol. 2009, 2, 103–121. [Google Scholar] [PubMed]
- Strieter, R.M.; Mehrad, B. New Mechanisms of Pulmonary Fibrosis. Chest 2009, 136, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Taskar, V.; Coultas, D. Exposures and Idiopathic Lung Disease. Semin. Respir. Crit. Care Med. 2008, 29, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, X.; Tong, Q.; Li, W.; Wang, B.; Sutter, K.; Trilling, M.; Lu, M.; Dittmer, U.; Yang, D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020, 92, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients after Acute COVID-19. JAMA 2020, 324, 603. [Google Scholar] [CrossRef]
- George, P.M.; Patterson, C.M.; Reed, A.K.; Thillai, M. Lung transplantation for idiopathic pulmonary fibrosis. Lancet Respir. Med. 2019, 7, 271–282. [Google Scholar]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- McDonald, L.T. Healing after COVID-19: Are survivors at risk for pulmonary fibrosis? Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L257–L265. [Google Scholar] [CrossRef]
- Borczuk, A.C.; Salvatore, S.P.; Seshan, S.V.; Patel, S.S.; Bussel, J.B.; Mostyka, M.; Elsoukkary, S.; He, B.; del Vecchio, C.; Fortarezza, F.; et al. COVID-19 pulmonary pathology: A multi-institutional autopsy cohort from Italy and New York City. Mod. Pathol. 2020, 33, 2156–2168. [Google Scholar]
- Baratella, E.; Bussani, R.; Zanconati, F.; Marrocchio, C.; Fabiola, G.; Braga, L.; Maiocchi, S.; Berlot, G.; Volpe, M.C.; Moro, E.; et al. Radiological–pathological signatures of patients with COVID-19-related pneumomediastinum: Is there a role for the Sonic hedgehog and Wnt5a pathways? ERJ Open Res. 2021, 7, 00346–02021. [Google Scholar] [CrossRef] [PubMed]
- Simioli, F.; Annunziata, A.; Polistina, G.E.; Coppola, A.; di Spirito, V.; Fiorentino, G. The Role of High Flow Nasal Cannula in COVID-19 Associated Pneumomediastinum and Pneumothorax. Healthcare 2021, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P.; Amrane, S.; Million, M.; Cortaredona, S.; Parola, P.; Lagier, J.-C.; Raoult, D. Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int. J. Infect. Dis. 2021, 102, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, S.; Amati, F.; Pappalettera, M.; di Pasquale, M.; D’Adda, A.; Mantero, M.; Gramegna, A.; Simonetta, E.; Oneta, A.M.; Privitera, E.; et al. COVID-19 multidisciplinary high dependency unit: The Milan model. Respir. Res. 2020, 21, 260. [Google Scholar]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar]
- Jenkins, R.G.; Simpson, J.K.; Saini, G.; Bentley, J.H.; Russell, A.-M.; Braybrooke, R.; Molyneaux, P.L.; McKeever, T.M.; Wells, A.U.; Flynn, A.; et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: An analysis from the prospective, multicentre PROFILE study. Lancet Respir. Med. 2015, 3, 462–472. [Google Scholar] [CrossRef]
- Dancer, R.C.A.; Wood, A.M.; Thickett, D.R. Metalloproteinases in idiopathic pulmonary fibrosis. Eur. Respir. J. 2011, 38, 1461–1467. [Google Scholar] [CrossRef]
- Organ, L.A.; Duggan, A.-M.R.; Oballa, E.; Taggart, S.C.; Simpson, J.K.; Kang’ombe, A.R.; Braybrooke, R.; Molyneaux, P.L.; North, B.; Karkera, Y.; et al. Biomarkers of collagen synthesis predict progression in the PROFILE idiopathic pulmonary fibrosis cohort. Respir. Res. 2019, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Shrirao, A.B.; Schloss, R.S.; Fritz, Z.; Shrirao, M.V.; Rosen, R.; Yarmush, M.L. Autofluorescence of blood and its application in biomedical and clinical research. Biotechnol. Bioeng. 2021, 118, 4550–4576. [Google Scholar] [CrossRef] [PubMed]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [PubMed]
- O’Connor, D.V.; Phillips, D. Time-Correlated Single Photon Counting; Academic Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Millar, D.P. Time-resolved fluorescence spectroscopy. Curr. Opin. Struct. Biol. 1996, 6, 637–642. [Google Scholar] [CrossRef]
- Sikora, J.; Cyrankiewicz, M.; Wybranowski, T.; Ziomkowska, B.; Osmialowski, B.; Obonska, E.; Augustynska, B.; Kruszewski, S.; Kubica, J. Use of time-resolved fluorescence spectroscopy to evaluate diagnostic value of collagen degradation products. J. Biomed. Opt. 2015, 20, 051039. [Google Scholar] [CrossRef]
- Sikora, J.; Cyrankiewicz, M.; Wybranowski, T.; Ziomkowska, B.; Kasprzak, M.; Krintus, M.; Odrowąż-Sypniewska, G.; Augustyńska, B.; Kruszewski, S.; Kubica, J. Fluorescence lifetime of collagen degradation products in plasma of patients with left ventricular remodeling. Med. Res. J. 2014, 3, 20–25. [Google Scholar]
- WHO. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected: Interim Guidance; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf (accessed on 13 March 2020).
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Delpino, M.V.; Quarleri, J. SARS-CoV-2 Pathogenesis: Imbalance in the Renin-Angiotensin System Favors Lung Fibrosis. Front. Cell. Infect. Microbiol. 2020, 10, 340. [Google Scholar]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; Veen, W.; Brüggen, M.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef]
- Sivashanmugam, K.; Kandasamy, M.; Subbiah, R.; Ravikumar, V. Repurposing of histone deacetylase inhibitors: A promising strategy to combat pulmonary fibrosis promoted by TGF-β signalling in COVID-19 survivors. Life Sci. 2021, 266, 118883. [Google Scholar]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Bai, L.; Li, A.; Gong, C.; Ning, X.; Wang, Z. Protective effect of rutin against bleomycin induced lung fibrosis: Involvement of TGF-β1/α-SMA//Col I and III pathway. BioFactors 2020, 46, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Snijder, J.; Peraza, J.; Padilla, M.; Capaccione, K.; Salvatore, M.M. Pulmonary fibrosis: A disease of alveolar collapse and collagen deposition. Expert Rev. Respir. Med. 2019, 13, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Cao, R.; Ma, J.; Mou, D.; Zhu, Y.; Li, W.; Lv, L.; Gao, D.; Zhang, S.; Gong, F.; et al. Pathological features of COVID-19-associated lung injury: A preliminary proteomics report based on clinical samples. Signal Transduct. Target. Ther. 2020, 5, 240. [Google Scholar] [PubMed]
- Aschner, Y.; Zemans, R.L.; Yamashita, C.M.; Downey, G.P. Matrix Metalloproteinases and Protein Tyrosine Kinases: Potential novel targets in acute lung injury and ARDS. Chest 2014, 146, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Lanchou, J.; Corbel, M.; Tanguy, M.; Germain, N.; Boichot, E.; Theret, N.; Clement, B.; Lagente, V.; Malledant, Y. Imbalance between matrix metalloproteinases (MMP-9 and MMP-2) and tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in acute respiratory distress syndrome patients. Crit. Care Med. 2003, 31, 536–542. [Google Scholar] [CrossRef]
- Gelzo, M.; Cacciapuoti, S.; Pinchera, B.; de Rosa, A.; Cernera, G.; Scialò, F.; Comegna, M.; Mormile, M.; Fabbrocini, G.; Parrella, R.; et al. Matrix metalloproteinases (MMP) 3 and 9 as biomarkers of severity in COVID-19 patients. Sci. Rep. 2022, 12, 1212. [Google Scholar] [CrossRef]
- D’Avila-Mesquita, C.; Couto, A.E.S.; Campos, L.C.B.; Vasconcelos, T.F.; Michelon-Barbosa, J.; Corsi, C.A.C.; Mestriner, F.; Petroski-Moraes, B.C.; Garbellini-Diab, M.J.; Couto, D.M.S.; et al. MMP-2 and MMP-9 levels in plasma are altered and associated with mortality in COVID-19 patients. Biomed. Pharmacother. 2021, 142, 112067. [Google Scholar] [CrossRef]
- Lerum, T.V.; Maltzahn, N.N.; Aukrust, P.; Trøseid, M.; Henriksen, K.N.; Kåsine, T.; Dyrhol-Riise, A.-M.; Stiksrud, B.; Haugli, M.; Blomberg, B.; et al. Persistent pulmonary pathology after COVID-19 is associated with high viral load, weak antibody response, and high levels of matrix metalloproteinase-9. Sci. Rep. 2021, 11, 23205. [Google Scholar]
- Kadry, R.; Newsome, A.S.; Somanath, P.R. Pharmacological Inhibition of MMP3 as a Potential Therapeutic Option for COVID-19 Associated Acute Respiratory Distress Syndrome. Infect. Disord. Drug Targets 2021, 21, e170721187996. [Google Scholar] [CrossRef]
- Shi, S.; Su, M.; Shen, G.; Hu, Y.; Yi, F.; Zeng, Z.; Zhu, P.; Yang, G.; Zhou, H.; Li, Q.; et al. Matrix metalloproteinase 3 as a valuable marker for patients with COVID-19. J. Med. Virol. 2021, 93, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z.; Ai, T.; Wu, S.; Xia, L. Association of “initial CT” findings with mortality in older patients with coronavirus disease 2019 (COVID-19). Eur. Radiol. 2020, 30, 6186. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Barisione, E.; Mastracci, L.; Campora, M.; Costa, D.; Robba, C.; Battaglini, D.; Micali, M.; Costantino, F.; Cittadini, G.; et al. Extension of Collagen Deposition in COVID-19 Post Mortem Lung Samples and Computed Tomography Analysis Findings. Int. J. Mol. Sci. 2021, 22, 7498. [Google Scholar] [CrossRef]
- Sand, J.M.B.; Tanino, Y.; Karsdal, M.A.; Nikaido, T.; Misa, K.; Sato, Y.; Togawa, R.; Wang, X.; Leeming, D.J.; Munakata, M. A Serological Biomarker of Versican Degradation is Associated with Mortality Following Acute Exacerbations of Idiopathic Interstitial Pneumonia. Respir. Res. 2018, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Eastin, C.; Eastin, T. Clinical Characteristics of Coronavirus Disease 2019 in China. J. Emerg. Med. 2020, 58, 711–712. [Google Scholar] [CrossRef]
- Reiner, R.C.; Barber, R.M.; Collins, J.K.; Zheng, P.; Adolph, C.; Albright, J.; Antony, C.M.; Aravkin, A.Y.; Bachmeier, S.D.; Bang-Jensen, B.; et al. Modeling COVID-19 scenarios for the United States. Nat. Med. 2021, 27, 94–105. [Google Scholar]
- Guler, S.A.; Ebner, L.; Aubry-Beigelman, C.; Bridevaux, P.-O.; Brutsche, M.; Clarenbach, C.; Garzoni, C.; Geiser, T.K.; Lenoir, A.; Mancinetti, M.; et al. Pulmonary function and radiological features 4 months after COVID-19: First results from the national prospective observational Swiss COVID-19 lung study. Eur. Respir. J. 2021, 57, 2003690. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, L.; Moss, S.; Khan, F.; Chi, W.; Xia, J.; Robinson, K.; Smyth, A.; Jenkins, G.; Stewart, I. Post-viral parenchymal lung disease of COVID-19 and viral pneumonitis: A systematic review and meta-analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- John, A.E.; Joseph, C.; Jenkins, G.; Tatler, A.L. COVID-19 and pulmonary fibrosis: A potential role for lung epithelial cells and fibroblasts. Immunol. Rev. 2021, 302, 228–240. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study Group—COVID-19 (C) | Study Group—COVID-19 after 6 Months (C-6M) | Study Group [Bacterial Pneumonia; Non-COVID-19] (BP) | Study Group [Idiopathic Pulmonary Fibrosis] (IPF) | Control Group (H) | |
|---|---|---|---|---|---|
| Number | 66 | 30 | 10 | 9 | 15 |
| Mean age (range) [years] | 62.3 (25–98) | 60.6 (30–87) | 62.4 (41–90) | 68.3 (62–87) | 40.8 (25–61) |
| Gender | |||||
| Women | 13 (20%) | 5 (17%) | 1 (10%) | 3 (33%) | 12 (80%) |
| Men | 53 (80%) | 25 (83%) | 9 (90%) | 6 (67%) | 3 (20%) |
| Smoking | |||||
| Yes | 16 (24%) | 5 (17%) | 7 (70%) | 4 (44%) | 3 (20%) |
| No | 50 (76%) | 25 (83%) | 3 (30%) | 5 (56%) | 12 (80%) |
| Common symptoms: | |||||
| Dyspnoea | 53 (80%) | 6 (60%) | |||
| Cough | 51 (77%) | 3 (30%) | |||
| Fever | 60 (91%) | 1 (10%) | |||
| Myalgia | 21 (32%) | 1 (10%) | |||
| Changes in the sense of smell and/or taste | 11 (16%) | 0 | |||
| Common comorbidities: | |||||
| Cardiovascular diseases | 34 (51%) | 4 (13%) | 7 (70%) | ||
| Type 2 diabetes | 14 (21%) | 3 (10%) | 3 (30%) | ||
| Previous lung diseases | 6 (9%) | 2 (7%) | 4 (40%) | ||
| Cancer | 4 (6%) | 2 (7%) | 3 (30%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wybranowski, T.; Pyskir, J.; Bosek, M.; Napiórkowska, M.; Cyrankiewicz, M.; Ziomkowska, B.; Pilaczyńska-Cemel, M.; Pyskir, M.; Rogańska, M.; Kruszewski, S.; et al. The Mortality Risk and Pulmonary Fibrosis Investigated by Time-Resolved Fluorescence Spectroscopy from Plasma in COVID-19 Patients. J. Clin. Med. 2022, 11, 5081. https://doi.org/10.3390/jcm11175081
Wybranowski T, Pyskir J, Bosek M, Napiórkowska M, Cyrankiewicz M, Ziomkowska B, Pilaczyńska-Cemel M, Pyskir M, Rogańska M, Kruszewski S, et al. The Mortality Risk and Pulmonary Fibrosis Investigated by Time-Resolved Fluorescence Spectroscopy from Plasma in COVID-19 Patients. Journal of Clinical Medicine. 2022; 11(17):5081. https://doi.org/10.3390/jcm11175081
Chicago/Turabian StyleWybranowski, Tomasz, Jerzy Pyskir, Maciej Bosek, Marta Napiórkowska, Michał Cyrankiewicz, Blanka Ziomkowska, Marta Pilaczyńska-Cemel, Małgorzata Pyskir, Milena Rogańska, Stefan Kruszewski, and et al. 2022. "The Mortality Risk and Pulmonary Fibrosis Investigated by Time-Resolved Fluorescence Spectroscopy from Plasma in COVID-19 Patients" Journal of Clinical Medicine 11, no. 17: 5081. https://doi.org/10.3390/jcm11175081
APA StyleWybranowski, T., Pyskir, J., Bosek, M., Napiórkowska, M., Cyrankiewicz, M., Ziomkowska, B., Pilaczyńska-Cemel, M., Pyskir, M., Rogańska, M., Kruszewski, S., & Przybylski, G. (2022). The Mortality Risk and Pulmonary Fibrosis Investigated by Time-Resolved Fluorescence Spectroscopy from Plasma in COVID-19 Patients. Journal of Clinical Medicine, 11(17), 5081. https://doi.org/10.3390/jcm11175081





