Clinical Factors Contributing to Cognitive Function in the Acute Stage after Treatment of Intracranial Aneurysms: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Standard Protocol Approvals, Registrations, and Patient Consent
2.3. Distribution of Intracranial Aneurysms
2.4. Procedures
2.5. Neuropsychological Tests
2.6. Statistical Analyses
3. Results
3.1. Neuropsychiatric Features after Treatment of Intracranial Aneurysms
3.2. Factors Contributing to Cognitive Impairment after Treatment of Intracranial Aneurysms
3.3. Mediation of Clinical Factors on Cognitive Function (Figure 1)
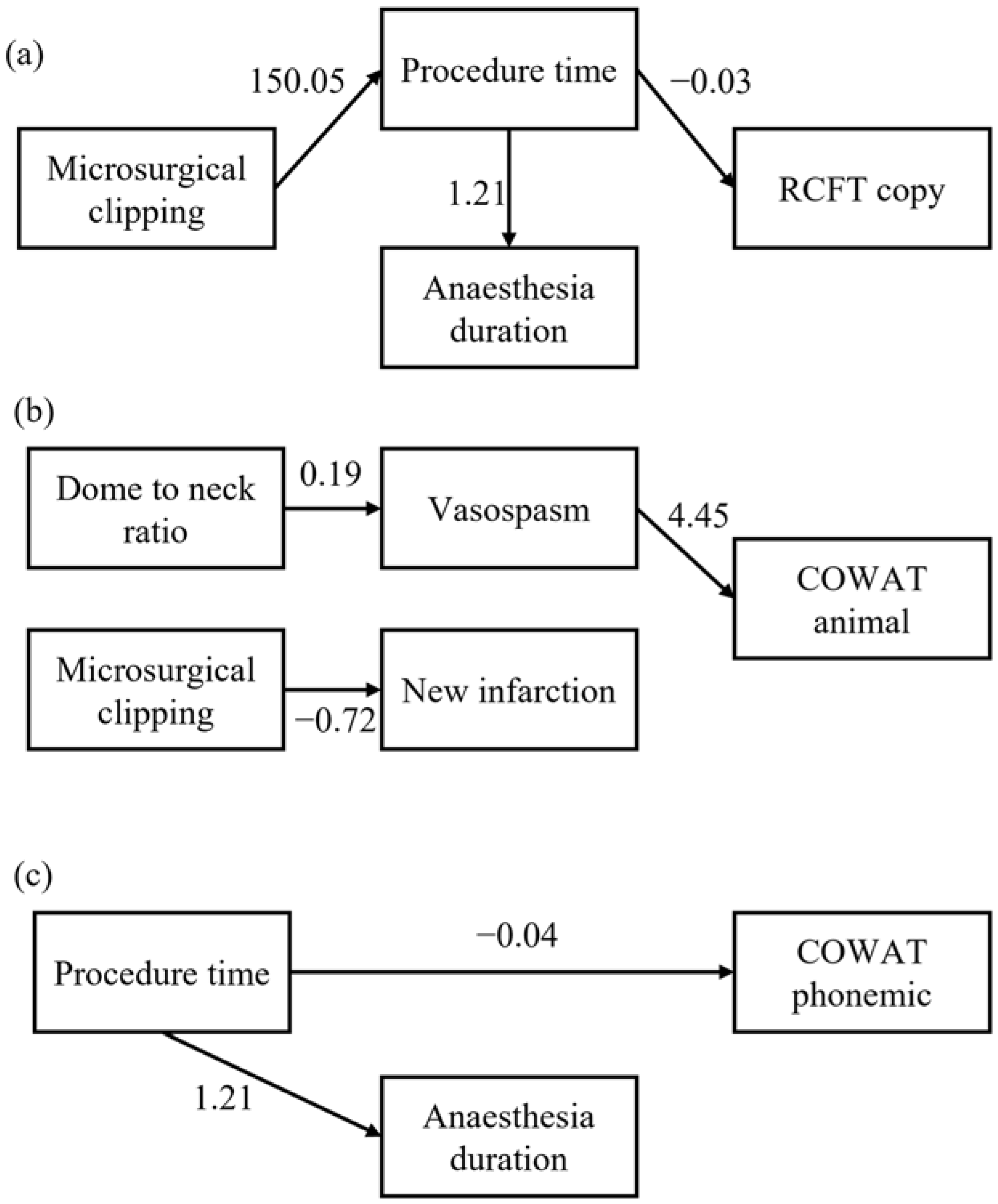
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hutter, B.O.; Kreitschmann-Andermahr, I.; Gilsbach, J.M. Cognitive deficits in the acute stage after subarachnoid hemorrhage. Neurosurgery 1998, 43, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.I.; Tarr, R.W.; Selman, W.R. Aneurysmal subarachnoid hemorrhage. N. Engl. J. Med. 2006, 354, 387–396. [Google Scholar] [CrossRef]
- Towgood, K.; Ogden, J.A.; Mee, E. Neurological, neuropsychological, and psychosocial outcome following treatment of unruptured intracranial aneurysms: A review and commentary. J. Int. Neuropsychol. Soc. 2004, 10, 114–134. [Google Scholar] [CrossRef] [PubMed]
- The International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—Risk of rupture and risks of surgical intervention. N. Engl. J. Med. 1998, 339, 1725–1733. [Google Scholar] [CrossRef]
- Alexander, M.P.; Freedman, M. Amnesia after anterior communicating artery aneurysm rupture. Neurology 1984, 34, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Stenhouse, L.M.; Knight, R.G.; Longmore, B.E.; Bishara, S.N. Long-term cognitive deficits in patients after surgery on aneurysms of the anterior communicating artery. J. Neurol. Neurosurg. Psychiatry 1991, 54, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.S.; Cipolotti, L.; Yousry, T.; Shallice, T. Qualitatively different memory impairments across frontal lobe subgroups. Neuropsychologia 2007, 45, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Sonesson, B.; Ljunggren, B.; Saveland, H.; Brandt, L. Cognition and adjustment after late and early operation for ruptured aneurysm. Neurosurgery 1987, 21, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Haug, T.; Sorteberg, A.; Sorteberg, W.; Lindegaard, K.F.; Lundar, T.; Finset, A. Cognitive functioning and health related quality of life after rupture of an aneurysm on the anterior communicating artery versus middle cerebral artery. Br. J. Neurosurg. 2009, 23, 507–515. [Google Scholar] [CrossRef]
- Tekin, S.; Cummings, J.L. Frontal–subcortical neuronal circuits and clinical neuropsychiatry. J. Psychosom. Res. 2002, 53, 647–654. [Google Scholar] [CrossRef]
- Barbarotto, R.; De Santis, A.; Laiacona, M.; Basso, A.; Spagnoli, D.; Capitani, E. Neuropsychological Follow-up of Patients Operated for Aneurysms of the Middle Cerebral Artery and Posterior Communicating Artery. Cortex 1989, 25, 275–288. [Google Scholar] [CrossRef]
- Frazer, D.; Ahuja, A.; Watkins, L.; Cipolotti, L. Coiling versus clipping for the treatment of aneurysmal subarachnoid hemorrhage: A longitudinal investigation into cognitive outcome. Neurosurgery 2007, 60, 434–441. [Google Scholar] [CrossRef]
- Zaki Ghali, M.G.; Srinivasan, V.M.; Wagner, K.; Rao, C.; Chen, S.R.; Johnson, J.N.; Kan, P. Cognitive Sequelae of Unruptured and Ruptured Intracranial Aneurysms and their Treatment: Modalities for Neuropsychological Assessment. World Neurosurg. 2018, 120, 537–549. [Google Scholar] [CrossRef]
- Murphy, T.H.; Corbett, D. Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef]
- Koivisto, T.; Vanninen, R.; Hurskainen, H.; Saari, T.; Hernesniemi, J.; Vapalahti, M. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke 2000, 31, 2369–2377. [Google Scholar] [CrossRef]
- Bonares, M.J.; Egeto, P.; de Oliveira Manoel, A.L.; Vesely, K.A.; Macdonald, R.L.; Schweizer, T.A. Unruptured intracranial aneurysm treatment effects on cognitive function: A meta-analysis. J. Neurosurg. 2016, 124, 784–790. [Google Scholar] [CrossRef]
- Hillis, A.E.; Anderson, N.; Sampath, P.; Rigamonti, D. Cognitive impairments after surgical repair of ruptured and unruptured aneurysms. J. Neurol. Neurosurg. Psychiatry 2000, 69, 608–615. [Google Scholar] [CrossRef]
- Egeto, P.; Loch Macdonald, R.; Ornstein, T.J.; Schweizer, T.A. Neuropsychological function after endovascular and neurosurgical treatment of subarachnoid hemorrhage: A systematic review and meta-analysis. J. Neurosurg. 2017, 128, 768–776. [Google Scholar] [CrossRef]
- Hunt, W.E.; Hess, R.M. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J. Neurosurg. 1968, 28, 14–20. [Google Scholar] [CrossRef]
- Alfonso, M.; Aftab, S.; Hamadneh, T.; Sherali, N.; Tsouklidis, N. Understanding Cognitive Deficit After Subarachnoid Hemorrhage: A Memory Focused Approach. Cureus 2020, 12, e11513. [Google Scholar] [CrossRef]
- Stephens, S.; Kenny, R.A.; Rowan, E.; Allan, L.; Kalaria, R.N.; Bradbury, M.; Ballard, C.G. Neuropsychological characteristics of mild vascular cognitive impairment and dementia after stroke. Int. J. Geriatr. Psychiatry 2004, 19, 1053–1057. [Google Scholar] [CrossRef]
- Rasquin, S.M.; Verhey, F.R.; Lousberg, R.; Winkens, I.; Lodder, J. Vascular cognitive disorders: Memory, mental speed and cognitive flexibility after stroke. J. Neurol. Sci. 2002, 203–204, 115–119. [Google Scholar] [CrossRef]
- Prins, N.D.; van Dijk, E.J.; den Heijer, T.; Vermeer, S.E.; Jolles, J.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005, 128, 2034–2041. [Google Scholar] [CrossRef]
- Tuffiash, E.; Tamargo, R.J.; Hillis, A.E. Craniotomy for treatment of unruptured aneurysms is not associated with long-term cognitive dysfunction. Stroke 2003, 34, 2195–2199. [Google Scholar] [CrossRef]
- Pereira-Filho, A.A.; Pereira, A.G.; Pereira-Filho, N.A.; Lima, L.C.; Costa, J.C.D.; Kraemer, J.L.; Portuguez, M.W. Long-term behavioral and cognitive outcomes following clipping for incidental unruptured intracranial aneurysms. Neuropsychology 2014, 28, 75–83. [Google Scholar] [CrossRef]
- Otawara, Y.; Ogasawara, K.; Ogawa, A.; Yamadate, K. Cognitive function before and after surgery in patients with unruptured intracranial aneurysm. Stroke 2005, 36, 142–143. [Google Scholar] [CrossRef]
- Lim, C.; Alexander, M.P. Stroke and episodic memory disorders. Neuropsychologia 2009, 47, 3045–3058. [Google Scholar] [CrossRef]
- Richardson, J.T. Cognitive performance following rupture and repair of intracranial aneurysm. Acta Neurol. Scand. 1991, 83, 110–122. [Google Scholar] [CrossRef]
- Su, J.; Tongzhou, E.; Guo, Q.; Lei, Y.; Gu, Y. Memory Deficits After Aneurysmal Subarachnoid Hemorrhage: A Functional Magnetic Resonance Imaging Study. World Neurosurg. 2018, 111, e500–e506. [Google Scholar] [CrossRef]
- Maher, M.; Churchill, N.W.; de Oliveira Manoel, A.L.; Graham, S.J.; Macdonald, R.L.; Schweizer, T.A. Altered Resting-State Connectivity within Executive Networks after Aneurysmal Subarachnoid Hemorrhage. PLoS ONE 2015, 10, e0130483. [Google Scholar] [CrossRef]
- Da Costa, L.; Shah-Basak, P.P.; Dunkley, B.T.; Robertson, A.D.; Pang, E.W. Visual Working Memory Encoding and Recognition in Good Outcome Aneurysmal Subarachnoid Patients. Front. Neurol. 2018, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, A.J.; Kerr, R.S.C.; Yu, L.-M.; Clarke, M.; Sneade, M.; Yarnold, J.A.; Sandercock, P. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005, 366, 809–817. [Google Scholar] [CrossRef]
- Burns, J.D.; Brown, R.D., Jr. Treatment of unruptured intracranial aneurysms: Surgery, coiling, or nothing? Curr. Neurol. Neurosci. Rep. 2009, 9, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, A.; Uchida, K.; Hashimoto, J.; Kawase, T. Neuropsychological evaluation and cerebral blood flow study of 30 patients with unruptured cerebral aneurysms before and after surgery. Surg. Neurol. 1999, 51, 132–139. [Google Scholar] [CrossRef]
- Lee, S.H.; Jang, M.U.; Kang, J.; Kim, Y.J.; Kim, C.; Sohn, J.H.; Yang, J.; Jeon, J.P.; Cho, Y.; Choi, H.J. Impact of Reducing the Procedure Time on Thromboembolism After Coil Embolization of Cerebral Aneurysms. Front. Neurol. 2018, 9, 1125. [Google Scholar] [CrossRef] [PubMed]
- Ravi, B.; Pincus, D.; Choi, S.; Jenkinson, R.; Wasserstein, D.N.; Redelmeier, D.A. Association of Duration of Surgery With Postoperative Delirium Among Patients Receiving Hip Fracture Repair. JAMA Netw. Open 2019, 2, e190111. [Google Scholar] [CrossRef] [PubMed]
- Kotekar, N.; Shenkar, A.; Nagaraj, R. Postoperative cognitive dysfunction—Current preventive strategies. Clin. Interv. Aging 2018, 13, 2267–2273. [Google Scholar] [CrossRef]
- Hindman, B.J. Emboli, inflammation, and CNS impairment: An overview. Heart Surg. Forum 2002, 5, 249–253. [Google Scholar]
- Goettel, N.; Burkhart, C.S.; Rossi, A.; Cabella, B.C.; Berres, M.; Monsch, A.U.; Czosnyka, M.; Steiner, L.A. Associations Between Impaired Cerebral Blood Flow Autoregulation, Cerebral Oxygenation, and Biomarkers of Brain Injury and Postoperative Cognitive Dysfunction in Elderly Patients After Major Noncardiac Surgery. Anesth. Analg. 2017, 124, 934–942. [Google Scholar] [CrossRef]
- Kim, J.; Shim, J.K.; Song, J.W.; Kim, E.K.; Kwak, Y.L. Postoperative Cognitive Dysfunction and the Change of Regional Cerebral Oxygen Saturation in Elderly Patients Undergoing Spinal Surgery. Anesth. Analg. 2016, 123, 436–444. [Google Scholar] [CrossRef]
- Ziolkowski, N.; Rogers, A.D.; Xiong, W.; Hong, B.; Patel, S.; Trull, B.; Jeschke, M.G. The impact of operative time and hypothermia in acute burn surgery. Burns 2017, 43, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, T.J.; Turner, M.J. The effects of hypocapnia and the cerebral autoregulatory response on cerebrovascular resistance and apparent zero flow pressure during isoflurane anesthesia. Anesth. Analg. 2009, 108, 1284–1290. [Google Scholar] [CrossRef]
- Martin, J.F.; Melo, R.O.; Sousa, L.P. Postoperative cognitive dysfunction after cardiac surgery. Rev. Bras. Cir. Cardiovasc. 2008, 23, 245–255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ille, R.; Lahousen, T.; Schweiger, S.; Hofmann, P.; Kapfhammer, H.P. Influence of patient-related and surgery-related risk factors on cognitive performance, emotional state, and convalescence after cardiac surgery. Cardiovasc. Revasc. Med. 2007, 8, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Yaffe, K.; Biller, J.; Bratzke, L.C.; Faraci, F.M.; Gorelick, P.B.; Gulati, M.; Kamel, H.; Knopman, D.S.; Launer, L.J.; et al. Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension 2016, 68, e67–e94. [Google Scholar] [CrossRef] [PubMed]
- Sharp, S.I.; Aarsland, D.; Day, S.; Sonnesyn, H.; Alzheimer’s Society Vascular Dementia Systematic Review Group; Ballard, C. Hypertension is a potential risk factor for vascular dementia: Systematic review. Int. J. Geriatr. Psychiatry 2011, 26, 661–669. [Google Scholar] [CrossRef]
- Zaal, I.J.; Devlin, J.W.; Peelen, L.M.; Slooter, A.J. A systematic review of risk factors for delirium in the ICU. Crit. Care Med. 2015, 43, 40–47. [Google Scholar] [CrossRef]
- Feinkohl, I.; Winterer, G.; Pischon, T. Hypertension and Risk of Post-Operative Cognitive Dysfunction (POCD): A Systematic Review and Meta-Analysis. Clin. Pract. Epidemiol. Ment. Health 2017, 13, 27–42. [Google Scholar] [CrossRef]
- Wong, G.K.; Lam, S.W.; Wong, A.; Ngai, K.; Mok, V.; Poon, W.S. Early Cognitive Domain Deficits in Patients with Aneurysmal Subarachnoid Hemorrhage Correlate with Functional Status. Acta Neurochir. Suppl. 2016, 122, 129–132. [Google Scholar] [CrossRef]
| Characteristics | UIA (n = 14) | SAH (n = 34) |
|---|---|---|
| Age (years) a | 65.5 ± 8.79 | 56.6 ± 7.74 |
| Gender (% b, female) | 11 (78.6) | 25 (73.5) |
| Years of education (years) a | 7.00 ± 4.038 | 9.22 ± 5.119 |
| PHASES score a | 7.2 ± 1.81 | 8.4 ± 1.91 |
| WFNS grade (%) | ||
| 1 | 14 (41.2) | |
| 2 | 14 (41.2) | |
| 3 | 5 (14.7) | |
| 4 | 1 (2.9) | |
| Vascular risk factor b | ||
| Hypertension (%) | 10 (71.4) | 11 (32.4) |
| Diabetes mellitus (%) | 2 (14.3) | 0 (0.0) |
| Dyslipidemia (%) | 3 (21.4) | 2 (5.9) |
| Procedural time (min) a | 120.4 ± 54.30 | 122.7 ± 101.31 |
| Anaesthetic duration (min) a | 173.2 ± 61.04 | 166.9 ± 125.51 |
| Interval between disease onset and NP test (days) a | 21.7 ± 13.08 | 23.3 ± 13.76 |
| Microsurgical clipping (%) | 11 (78.6) | 14 (41.2) |
| Complications b | ||
| Hydrocephalus | 0 (0.0) | 5 (14.7) |
| Vasospasm | 0 (0.0) | 4 (11.8) |
| New infarction | 3 (21.4) | 19 (55.9) |
| Delayed cerebral ischemia | 0 (0) | 0 (0) |
| UIA | SAH | |
|---|---|---|
| Single aneurysm | 12 (85.7%) | 30 (88.2%) |
| ICA | 2 | 2 |
| AcomA | 2 | 12 |
| ACA | 0 | 1 |
| MCA | 5 | 6 |
| PCA | 0 | 0 |
| PcomA | 3 | 7 |
| PICA | 0 | 1 |
| VA | 0 | 1 |
| Multiple aneurysms | 2 (14.3%) | 4 (11.8%) |
| MCA + Acom | MCA + PcomA | |
| ICA + MCA | AcomA + ICA | |
| MCA + MCA | ||
| PCA + ICA |
| UIA (n = 14) | SAH (n = 34) | |||
|---|---|---|---|---|
| Mean Raw Score (SD) | Mean z Score (SD) | Mean Raw Score (SD) | Mean z Score (SD) | |
| Digit span forward | 4.9 ± 1.77 | −0.44 ± 1.189 | 5.6 ± 1.65 | −0.41 ± 1.020 |
| Digit span backward | 3.1 ± 1.70 | −0.46 ± 1.505 | 3.9 ± 1.59 | −0.50 ± 1.651 |
| K-BNT | 41.6 ± 11.77 | −0.42 ± 1.252 | 46.5 ± 9.55 | −0.62 ± 1.472 |
| RCFT copy | 27.07 ± 7.516 | −0.58 ± 0.929 | 29.38 ± 6.700 | −1.10 ± 1.922 |
| SVLT immediate recall | 15.8 ± 5.96 | −0.75 ± 1.278 | 17.0 ± 5.52 | −1.12 ± 1.142 |
| SVLT delayed recall | 3.7 ± 2.87 | −0.99 ± 1.147 | 4.0 ± 3.10 | −1.45 ± 1.227 |
| SVLT recognition | 19.9 ± 3.08 | −0.28 ± 1.593 | 19.7 ± 2.66 | −0.99 ± 1.322 |
| RCFT immediate recall | 6.71 ± 6.275 | −1.02 ± 1.025 | 13.58 ± 7.633 | −0.56 ± 1.044 |
| RCFT delayed recall | 7.21 ± 6.284 | −0.97 ± 1.057 | 12.61 ± 7.369 | −0.73 ± 1.033 |
| RCFT recognition | 18.4 ± 2.31 | −0.51 ± 1.057 | 19.0 ± 2.50 | −0.64 ± 1.321 |
| COWAT animal | 9.6 ± 4.96 | −1.29 ± 1.176 | 11.7 ± 5.31 | −1.18 ± 1.098 |
| COWAT supermarket | 12.9 ± 6.74 | −0.58 ± 1.277 | 14.5 ± 6.48 | −0.81 ± 0.960 |
| COWAT phonemic | 14.7 ± 10.17 | −0.91 ± 1.196 | 16.8 ± 10.05 | −1.23 ± 0.875 |
| Stroop test colour reading correct | 51.7 ± 34.12 | −1.60 ± 1.833 | 65.0 ± 34.12 | −1.83 ± 2.050 |
| RCFT Immediate Recall | COWAT Animal | Stroop Test | ||||
|---|---|---|---|---|---|---|
| β (SE) | p Value | β (SE) | p Value | β (SE) | p Value | |
| Size of aneurysm | 0.565 (1.155) | 0.635 | 1.279 (0.776) | 0.130 | 7.997 (5.656) | 0.195 |
| Dome-to-neck ratio of aneurysm | 0.094 (0.330) | 0.788 | −0.004 (0.249) | 0.987 | 2.486 (1.591) | 0.162 |
| Located in the post. circulation | N/A | N/A | N/A | |||
| Interval between disease onset and NP test | −0.014 (0.146) | 0.926 | −0.001 (0.109) | 0.996 | −0.118 (0.923) | 0.901 |
| Microsurgical clipping | −0.969 (4.476) | 0.833 | 1.021 (3.344) | 0.766 | −15.171 (24.448) | 0.552 |
| Procedure time | −0.026 (0.033) | 0.453 | −0.017 (0.025) | 0.522 | −0.244 (0.164) | 0.175 |
| Anaesthetic duration | −0.011 (0.031) | 0.720 | −0.001 (0.023) | 0.957 | −0.148 (0.160) | 0.381 |
| Multiple procedure | −8.634 (6.383) | 0.206 | −0.491 (5.197) | 0.927 | −22.365 (36.062) | 0.552 |
| Hypertension | −8.235 (3.682) | 0.049 | −5.314 (2.930) | 0.100 | −45.909 (23.146) | 0.083 |
| Diabetes mellitus | 0.283 (5.408) | 0.959 | −1.384 (4.027) | 0.738 | −13.277 (33.967) | 0.706 |
| Dyslipidaemia | −0.724 (4.762) | 0.882 | 1.741 (3.528) | 0.632 | −10.077 (30.106) | 0.746 |
| Hydrocephalus | N/A | N/A | N/A | |||
| Vasospasm | N/A | N/A | N/A | |||
| New infarction | 1.832 (4.398) | 0.686 | −2.021 (3.260) | 0.549 | −18.186 (28.516) | 0.541 |
| RCFT Copy | SVLT Immediate Recall | SVLT Delayed Recall | COWAT Animal | COWAT Phonemic | Stroop Test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | p Value | β (SE) | p Value | β (SE) | p Value | β (SE) | p Value | β (SE) | p Value | β (SE) | p Value | |
| Size of aneurysm | 0.110 (0.431) | 0.800 | 0.174 (0.386) | 0.656 | −0.218 (0.221) | 0.331 | 0.326 (0.325) | 0.324 | −0.085 (0.688) | 0.902 | −1.375 (2.206) | 0.538 |
| Dome-to-neck ratio of aneurysm | 0.671 (0.987) | 0.502 | 0.852 (0.895) | 0.350 | 0.094 (0.518) | 0.857 | 2.109 (0.639) | 0.002 | 1.033 (1.510) | 0.501 | 3.697 (4.924) | 0.460 |
| Located in the post. circulation | −5.450 (3.391) | 0.119 | −3.458 (3.124) | 0.277 | −3.433 (1.737) | 0.057 | −2.753 (2.671) | 0.311 | −8.667 (5.055) | 0.099 | −39.191 (16.207) | 0.022 |
| Interval between disease onset and NP test | −0.051 (0.080) | 0.531 | 0.006 (0.071) | 0.928 | −0.053 (0.040) | 0.196 | −0.053 (0.060) | 0.385 | −0.071 (0.132) | 0.592 | −0.390 (0.389) | 0.325 |
| Microsurgical clipping | −4.192 (1.892) | 0.035 | −1.122 (1.781) | 0.533 | −0.517 (1.034) | 0.621 | −4.074 (1.335) | 0.005 | −5.619 (3.024) | 0.075 | −17.471 (9.784) | 0.085 |
| Procedure time | −0.030 (0.008) | 0.001 | −0.008 (0.009) | 0.363 | −0.006 (0.005) | 0.276 | −0.009 (0.007) | 0.226 | −0.035 (0.014) | 0.020 | −0.090 (0.049) | 0.075 |
| Anaesthetic duration | −0.024 (0.007) | 0.001 | −0.005 (0.007) | 0.521 | −0.004 (0.004) | 0.303 | −0.006 (0.006) | 0.306 | −0.026 (0.012) | 0.034 | −0.066 (0.040) | 0.108 |
| Multiple procedure | −4.132 (2.470) | 0.105 | 0.336 (2.328) | 0.886 | −0.333 (1.348) | 0.807 | 0.287 (1.985) | 0.886 | −4.583 (4.137) | 0.279 | −8.910 (14.046) | 0.531 |
| Hypertension | −1.466 (2.248) | 0.519 | −0.901 (1.997) | 0.655 | −1.334 (1.135) | 0.249 | 0.847 (1.702) | 0.623 | 0.985 (3.587) | 0.786 | −11.808 (11.180) | 0.300 |
| Diabetes mellitus | N/A | N/A | N/A | N/A | N/A | N/A | ||||||
| Dyslipidemia | 1.110 (4.246) | 0.796 | −1.422 (3.809) | 0.712 | −1.950 (2.184) | 0.379 | 0.897 (3.252) | 0.785 | −6.816 (6.221) | 0.284 | −24.677 (20.855) | 0.247 |
| Hydrocephalus | −0.315 (3.070) | 0.919 | −5.123 (2.389) | 0.040 | −3.237 (1.364) | 0.024 | -−3.112 (2.113) | 0.151 | −5.320 (5.092) | 0.306 | −25.910 (14.889) | 0.093 |
| Vasospasm | −4.304 (2.980) | 0.159 | 1.779 (2.730) | 0.520 | 0.971 (1.583) | 0.544 | 6.226 (2.051) | 0.005 | 1.559 (5.502) | 0.779 | 6.745 (17.617) | 0.705 |
| New infarction | 2.725 (1.919) | 0.166 | 2.168 (1.713) | 0.215 | 0.585 (1.013) | 0.568 | 3.746 (1.334) | 0.009 | 3.923 (3.040) | 0.209 | 14.334 (9.759) | 0.153 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Lee, S.-H.; Jeon, J.P.; Choi, H.-C.; Choi, H.J. Clinical Factors Contributing to Cognitive Function in the Acute Stage after Treatment of Intracranial Aneurysms: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 5053. https://doi.org/10.3390/jcm11175053
Kim YJ, Lee S-H, Jeon JP, Choi H-C, Choi HJ. Clinical Factors Contributing to Cognitive Function in the Acute Stage after Treatment of Intracranial Aneurysms: A Cross-Sectional Study. Journal of Clinical Medicine. 2022; 11(17):5053. https://doi.org/10.3390/jcm11175053
Chicago/Turabian StyleKim, Yeo Jin, Sang-Hwa Lee, Jin Pyeong Jeon, Hui-Chul Choi, and Hyuk Jai Choi. 2022. "Clinical Factors Contributing to Cognitive Function in the Acute Stage after Treatment of Intracranial Aneurysms: A Cross-Sectional Study" Journal of Clinical Medicine 11, no. 17: 5053. https://doi.org/10.3390/jcm11175053
APA StyleKim, Y. J., Lee, S.-H., Jeon, J. P., Choi, H.-C., & Choi, H. J. (2022). Clinical Factors Contributing to Cognitive Function in the Acute Stage after Treatment of Intracranial Aneurysms: A Cross-Sectional Study. Journal of Clinical Medicine, 11(17), 5053. https://doi.org/10.3390/jcm11175053






