Higher Thyroid fT3-to-fT4 Ratio Is Associated with Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes
Abstract
:1. Introduction
2. Materials and Methods
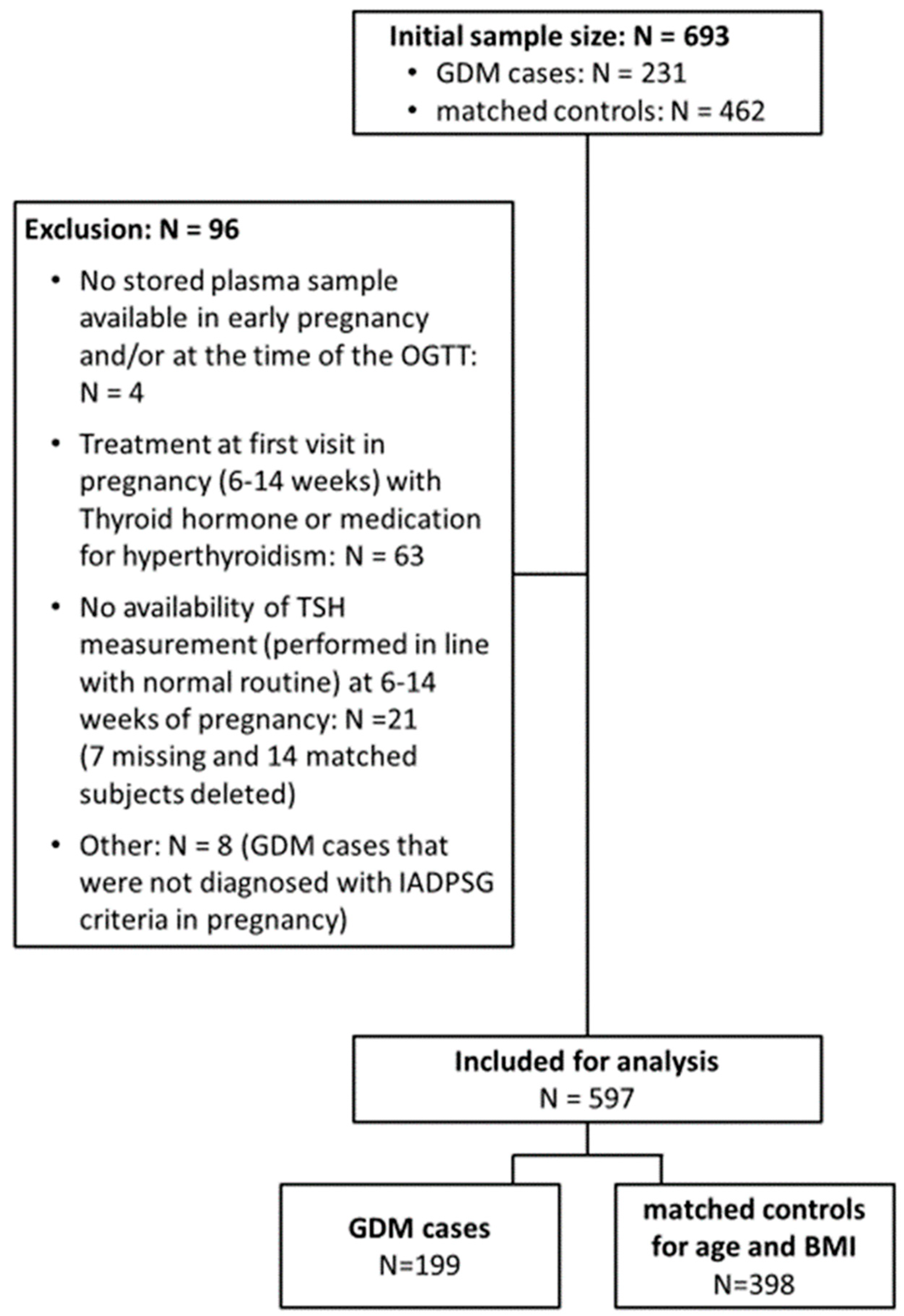
2.1. Study Design and Setting
2.2. Study Visits and Measurements
2.3. Pregnancy and Delivery Outcome Data
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| NGT N = 398 (66.7%) | GDM N = 199 (33.3%) | p-Value | |
|---|---|---|---|
| General | |||
| Age (years) | 31.3 ± 4.3 | 31.9 ± 4.7 | 0.174 |
| % Ethnic minorities | 0.432 | ||
| Asian | 3.8 (15) | 6.1 (12) | |
| Northern African | 6.0 (24) | 4.1 (8) | |
| Turkish | 1.5 (6) | 1.5 (3) | |
| Black African | 1.3 (5) | 2.0 (4) | |
| Middle East | 1.5 (6) | 0.5 (1) | |
| Latin American | 2.0 (8) | 0.5 (1) | |
| Other | 2.5 (10) | 4.1 (8) | |
| % Multiparity | 49.5 (194) | 52.8 (105) | 0.488 |
| % Higher degree diploma | 82.0 (300) | 76.0 (136) | 0.111 |
| % Highest education: | 0.220 | ||
| primary school | 0.8 (3) | 3.2 (6) | |
| till 15 years | 4.9 (19) | 4.7 (9) | |
| high school | 18.0 (70) | 20.5 (39) | |
| bachelor | 36.0 (140) | 36.3 (69) | |
| master | 40.4 (157) | 35.3 (67) | |
| % Paid job | 87.4 (346) | 91.3 (178) | 0.170 |
| % Smoking before pregnancy | 26.5 (105) | 35.5 (70) | 0.028 |
| % Smoking during pregnancy | 2.5 (10) | 5.6 (11) | 0.096 |
| % First-degree family history of diabetes | 11.4 (43) | 18.6 (34) | 0.026 |
| % First-degree family history of GDM | 4.4 (16) | 7.6 (14) | 0.163 |
| % History of GDM * | 5.1 (10) | 29.8 (31) | <0.001 |
| % History of impaired glucose intolerance | 2.0 (7) | 2.7 (5) | 0.551 |
| 6–14 weeks visit | |||
| Weeks of gestation first visit with FPG | 11.9 ± 1.6 | 11.9 ± 1.7 | 0.571 |
| BMI (kg/m2) | 25.7 ± 5.4 | 26.4 ± 5.3 | 0.057 |
| % Overweight | 45.5 (181) | 51.0 (101) | 0.223 |
| % Obesity | 19.6 (78) | 22.2 (44) | 0.453 |
| Weight gain (first visit till OGTT) (kg) | 6.8 ± 3.2 | 6.9 ± 3.7 | 0.642 |
| Delivery | |||
| Total weight gain (delivery—first visit) (kg) | 12.5 ± 5.2 | 8.7 ± 5.0 | <0.001 |
| % Excessive weight gain | 35.1 (124) | 15.8 (26) | <0.001 |
| fT3 Lower Tertile (3.0–4.1) 26–28 Weeks N = 53 (38.4%) | fT3 Upper Tertile (4.6–7.2) 26–28 Weeks N = 85 (61.6%) | p-Value | |
|---|---|---|---|
| Postpartum | |||
| Glucose intolerance at postpartum OGTT | 1.000 | ||
| % IFG | 4.2 (2) | 5.6 (4) | |
| % IGT | 12.5 (6) | 11.1 (8) | |
| % Both IFG and IGT | 0 (0) | 1.4 (1) | |
| BMI (kg/m2) * | 23.6 ± 4.0 | 28.8 ± 5.6 | <0.001 |
| % Overweight * | 27.1 (13) | 73.9 (51) | <0.001 |
| % Obesity * | 6.2 (3) | 34.8 (24) | <0.001 |
| Systolic blood pressure (mmHg) * | 112.3 ± 10.1 | 120.0 ± 12.4 | 0.002 |
| Diastolic blood pressure (mmHg) * | 70.5 ± 9.1 | 75.6 ± 8.9 | 0.006 |
| % Hypertension (≥140/90 mmHg) | 2.1 (1) | 8.7 (6) | 0.237 |
| Fasting glycaemia (mmol/L) * | 4.72 (4.47–4.91) | 4.94 (4.69–5.16) | 0.003 |
| 30 min glucose OGTT (mmol/L) * | 7.80 (6.94–8.74) | 7.66 (6.77–8.60) | 0.637 |
| 1 h glucose OGTT (mmol/L) * | 7.30 (5.99–8.63) | 7.08 (5.80–8.21) | 0.488 |
| 2 h glucose OGTT (mmol/L) * | 5.50 (4.94–6.66) | 6.10 (5.00–6.91) | 0.423 |
| Fasting insulin (pmol/L) * | 38.5 (23.6–58.8) | 54.3 (39.5–84.2) | 0.001 |
| 30 min insulin OGTT (pmol/L) * | 332.2 (228.0–418.0) | 388.2 (258.0–571.5) | 0.104 |
| 1 h insulin OGTT (pmol/L) * | 364.5 (273.3–501.4) | 451.7 (269.2–694.8) | 0.081 |
| 2 h insulin OGTT (pmol/L) * | 249.0 (164.4–424.7) | 338.4 (228.6–546.2) | 0.028 |
| HbA1c (%) * | 5.3 (5.1–5.5) | 5.2 (5.1–5.5) | 0.820 |
| Matsuda insulin sensitivity * | 0.9 (0.5–1.4) | 0.7 (0.4–0.9) | 0.006 |
| Stumvoll index * | −135.6 (−346.9–220.9) | 95.2 (−164.6–534.1) | 0.017 |
| Oral disposition index * | −90.0 (−338.5–159.0) | 69.0 (−136.3–212.3) | 0.020 |
| HOMA-IR * | 8.1 (4.6–12.6) | 11.2 (8.9–19.2) | <0.001 |
| HOMA-B * | 642.9 (447.7–826.5) | 784.0 (582.4–1210.8) | 0.033 |
| ISSI-2 * | 0.3 (0.2–0.7) | 0.2 (0.1–0.4) | 0.013 |
| Insulinogenic index/Homa-IR * | 0.3 (0.2–0.4) | 0.2 (0.2–0.3) | 0.043 |
| Fasting total cholesterol (mmol/L) * | 4.80 (4.13–5.21) | 4.60 (4.11–5.08) | 0.421 |
| Fasting HDL (mmol/L) * | 1.67 (1.44–1.95) | 4.41 (1.26–1.67) | 0.003 |
| Fasting LDL (mmol/L) * | 2.64 (2.16–3.13) | 2.67 (2.13–3.13) | 0.967 |
| Fasting TG (mmol/L) * | 0.75 (0.62–0.96) | 0.89 (0.69–1.25) | 0.008 |
| GDM and fT3-to-fT4 Ratio Lower Tertile (0.21 to 0.33) 26–28 Weeks N = 53 (40.1%) | GDM and fT3-to-fT4 Ratio Upper Tertile (0.38 to 0.66) 26–28 Weeks N = 79 (59.8%) | p-Value | |
|---|---|---|---|
| Postpartum | |||
| Glucose intolerance at postpartum OGTT | 17.8 (8) | 19.1 (13) | 0.721 |
| % IFG | 6.7 (3) | 2.9 (2) | |
| % IGT | 8.9 (4) | 13.2 (9) | |
| % Both IFG and IGT | 2.2 (1) | 2.9 (2) | |
| BMI (kg/m2) * | 23.1 ± 3.8 | 28.4 ± 5.6 | <0.001 |
| % Overweight * | 22.2 (10) | 68.7 (46) | <0.001 |
| % Obesity * | 6.7 (3) | 31.3 (21) | 0.002 |
| Systolic blood pressure (mmHg) * | 111.1 ± 14.0 | 120.6 ± 13.6 | <0.001 |
| Diastolic blood pressure (mmHg) * | 70.6 ± 9.0 | 75.5 ± 8.5 | 0.004 |
| % Hypertension (≥140/90 mmHg) | 6.7 (3) | 7.5 (5) | 1.000 |
| Fasting glycaemia (mmol/L) * | 4.72 (4.50–4.94) | 4.94 (4.74–5.19) | 0.011 |
| 30 min glucose OGTT (mmol/L) * | 7.66 (6.55–8.94) | 7.83 (6.83–8.60) | 0.887 |
| 1 h glucose OGTT (mmol/L) * | 6.94 (6.10–8.60) | 7.35 (5.72–8.38) | 0.808 |
| 2 h glucose OGTT (mmol/L) * | 5.55 (4.99–6.27) | 5.91 (4.99–7.13) | 0.480 |
| Fasting insulin (pmol/L) * | 38.8 (26.3–56.1) | 55.4 (38.1–101.0) | <0.001 |
| 30 min insulin OGTT (pmol/L) * | 332.2 (230.7–454.7) | 418.5 (289.3–565.5) | 0.055 |
| 1 h insulin OGTT (pmol/L) * | 364.6 (229.2–554.1) | 487.1 (324.5–790.0) | 0.036 |
| 2 h insulin OGTT (pmol/L) * | 280.4 (189.9–453.6) | 342.8 (233.9–653.5) | 0.034 |
| HbA1c (%) * | 5.2 (5.1–5.5) | 5.3 (5.0–5.5) | 0.590 |
| Matsuda insulin sensitivity * | 0.8 (0.5–1.3) | 0.6 (0.4–0.9) | 0.002 |
| Stumvoll index * | −55.1 (−328.2–311.1) | 185.8 (−30.8–660.7) | 0.016 |
| Oral disposition index * | −81.9 (−349.3–190.3) | 97.6 (−35.8–254.9) | 0.018 |
| HOMA-IR * | 8.3 (5.8–12.2) | 12.1 (8.4–22.6) | <0.001 |
| HOMA-B * | 675.0 (445.9–1074.5) | 787.7 (563.7–1287.3) | 0.050 |
| ISSI-2 * | 0.3 (0.2–0.7) | 0.2 (0.1–0.4) | 0.004 |
| Insulinogenic index/Homa-IR * | 0.3 (0.2–0.4) | 0.2 (0.1–0.3) | 0.047 |
| Fasting total cholesterol (mmol/L) * | 4.87 (4.13–5.2) | 4.74 (4.34–5.16) | 0.869 |
| Fasting HDL (mmol/L) * | 1.63 (1.30–1.95) | 1.39 (1.18–1.77) | 0.009 |
| Fasting LDL (mmol/L) * | 2.66 (2.12–3.20) | 2.81 (2.31–3.13) | 0.516 |
| Fasting TG (mmol/L) * | 0.80 (0.64–0.97) | 0.94 (0.64–1.43) | 0.062 |
| fT4 (pmol/L) | 16.2 (14.7–17.9) | 15.5 (14.1–16.6) | 0.040 |
| TSH (mIU/L) | 1.5 (0.9–1.9) | 1.5 (1.1–2.1) | 0.733 |
| Lifestyle score: physical activity * | 1.0 (0–2.0) | 1.0 (0–2.0) | 0.879 |
| Lifestyle score: diet * | 3.0 (0–6.0) | 2.0 (0–4.0) | 0.293 |
| % breastfeeding * | 81.4 (35) | 80.6 (54) | 1.000 |
| METs category at time of the OGTT * | 0.161 | ||
| % Low | 19.5 (8) | 12.3 (8) | |
| % Moderate | 53.6 (22) | 43.1 (28) | |
| % High | 26.8 (11) | 44.6 (29) | |
| % MET category low * | 19.5 (8) | 12.3 (8) | 0.405 |
References
- Thorpe-Beeston, J.G.; Nicolaides, K.H.; Felton, C.V.; Butler, J.; McGregor, A.M. Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. N. Engl. J. Med. 1991, 324, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Visser, W.E.; Peeters, R.P. Interpretation of thyroid function tests during pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101431. [Google Scholar] [CrossRef] [PubMed]
- Krassas, G.; Poppe, K.; Glinoer, D. Thyroid function and human reproductive health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef]
- Toulis, K.A.; Stagnaro-Green, A.; Negro, R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: A meta-analysis. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2014, 20, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Tsai, M.Y.; Hinkle, S.N.; Zhu, Y.; Bao, W.; Lin, Y.; Panuganti, P.; Albert, P.S.; Ma, R.C.W.; Zhang, C. A Longitudinal Study of Thyroid Markers Across Pregnancy and the Risk of Gestational Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 2447–2456. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S165–S172. [Google Scholar] [CrossRef] [PubMed]
- Tudela, C.M.; Casey, B.M.; McIntire, D.D.; Cunningham, F.G. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obs. Gynecol. 2012, 119, 983–988. [Google Scholar] [CrossRef]
- Sahu, M.T.; Das, V.; Mittal, S.; Agarwal, A.; Sahu, M. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch. Gynecol. Obstet. 2010, 281, 215–220. [Google Scholar] [CrossRef]
- Ying, H.; Tang, Y.P.; Bao, Y.R.; Su, X.J.; Cai, X.; Li, Y.H.; Wang, D.F. Maternal TSH level and TPOAb status in early pregnancy and their relationship to the risk of gestational diabetes mellitus. Endocrine 2016, 54, 742–750. [Google Scholar] [CrossRef]
- Leng, J.; Li, W.; Wang, L.; Zhang, S.; Liu, H.; Li, W.; Wang, S.; Shao, P.; Pan, L.; Wang, S.; et al. Higher thyroid-stimulating hormone levels in the first trimester are associated with gestational diabetes in a Chinese population. Diabet. Med. A J. Br. Diabet. Assoc. 2019, 36, 1679–1685. [Google Scholar] [CrossRef]
- Li, G.; Wei, T.; Ni, W.; Zhang, A.; Zhang, J.; Xing, Y.; Xing, Q. Incidence and Risk Factors of Gestational Diabetes Mellitus: A Prospective Cohort Study in Qingdao, China. Front. Endocrinol. 2020, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, P.; Zhou, H.; Li, L. A longitudinal study of thyroid markers during pregnancy and the risk of gestational diabetes mellitus and post-partum glucose metabolism. Diabetes/Metab. Res. Rev. 2021, 37, e3441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-M.; Li, G.; Wu, Y.; Zhang, D.; Zhang, S.; Hao, Y.-T.; Chen, W.; Huang, Q.; Li, S.; Xie, Y.; et al. Increased Central and Peripheral Thyroid Resistance Indices During the First Half of Gestation Were Associated With Lowered Risk of Gestational Diabetes—Analyses Based on Huizhou Birth Cohort in South China. Front. Endocrinol. 2022, 13, 806256. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, K.; Van Crombrugge, P.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Dufraimont, E.; De Block, C.; Jacquemyn, Y.; Mekahli, F.; et al. The Belgian Diabetes in Pregnancy Study (BEDIP-N), a multi-centric prospective cohort study on screening for diabetes in pregnancy and gestational diabetes: Methodology and design. BMC Pregnancy Childbirth 2014, 14, 226. [Google Scholar] [CrossRef]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. The sensitivity and specificity of the glucose challenge test in a universal two-step screening strategy for gestational diabetes mellitus using the 2013 world health organization criteria. Diabetes Care 2018, 41, e111–e112. [Google Scholar] [CrossRef]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. A Modified Two-Step Screening Strategy for Gestational Diabetes Mellitus Based on the 2013 WHO Criteria by Combining the Glucose Challenge Test and Clinical Risk Factors. J. Clin. Med. 2018, 7, 351. [Google Scholar] [CrossRef]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia 2019, 62, 2118–2128. [Google Scholar] [CrossRef]
- Benhalima, K.; Van Crombrugge, P.; Moysonl, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammens, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. Estimating the risk of gestational diabetes mellitus based on the 2013 WHO criteria: A prediction model based on clinical and biochemical variables in early pregnancy. Acta Diabetol. 2020, 57, 661–671. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S14. [Google Scholar] [CrossRef]
- World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Huston-Presley, L.; Kalhan, S.C.; Catalano, P.M. Clinically useful estimates of insulin sensitivity during pregnancy: Validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care 2001, 24, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Qi, Y.; Goran, M.I.; Hamilton, J.K. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet. Med. A J. Br. Diabet. Assoc. 2009, 26, 1198–1203. [Google Scholar] [CrossRef]
- Bekaert, A.; Devlieger, H.; Eeckels, R.; Martens, G. Standaarden van geboortegewicht-voor-zwangerschapsduur voor de Vlaamse boreling. Tijdschr. Voor Geneeskd. 2000, 56, 1–4. [Google Scholar]
- Institute of Medicine (US); National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. The National Academies Collection: Reports funded by National Institutes of Health. In Weight Gain during Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academy of Sciences: Washington, DC, USA, 2009. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, F.; Wu, P.; Huang, Y.; Ye, Y.; Yang, X.; Yuan, J.; Liu, Y.; Zeng, H.; Wen, Y.; et al. A prospective study of early-pregnancy thyroid markers, lipid species, and risk of gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2021, 107, e804–e814. [Google Scholar] [CrossRef]
- Yang, S.; Shi, F.T.; Leung, P.C.; Huang, H.F.; Fan, J. Low Thyroid Hormone in Early Pregnancy Is Associated with an Increased Risk of Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2016, 101, 4237–4243. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, X.; Yang, S.; Zhang, C.; Han, M.; Huang, H.F.; Fan, J. Maternal low thyroxin levels are associated with adverse pregnancy outcomes in a Chinese population. PLoS ONE 2017, 12, e0178100. [Google Scholar] [CrossRef]
- Eerdekens, A.; Verhaeghe, J.; Darras, V.; Naulaers, G.; Van den Berghe, G.; Langouche, L.; Vanhole, C. The placenta in fetal thyroid hormone delivery: From normal physiology to adaptive mechanisms in complicated pregnancies. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obs. 2020, 33, 3857–3866. [Google Scholar] [CrossRef]
- Bassols, J.; Prats-Puig, A.; Soriano-Rodríguez, P.; García-González, M.M.; Reid, J.; Martínez-Pascual, M.; Mateos-Comerón, F.; de Zegher, F.; Ibáñez, L.; López-Bermejo, A. Lower Free Thyroxin Associates with a Less Favorable Metabolic Phenotype in Healthy Pregnant Women. J. Clin. Endocrinol. Metab. 2011, 96, 3717–3723. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Ding, X.; Xu, Y.; Liu, R.; Gu, M.; Chen, Y.; Yin, Y.; Wang, Y.; Sun, H.; Peng, Y. Different levels of thyroid hormones between impaired fasting glucose and impaired glucose tolerance: Free T3 affects the prevalence of impaired fasting glucose and impaired glucose tolerance in opposite ways. Clin. Endocrinol. 2014, 80, 890–898. [Google Scholar] [CrossRef]
- Roef, G.L.; Rietzschel, E.R.; Van Daele, C.M.; Taes, Y.E.; De Buyzere, M.L.; Gillebert, T.C.; Kaufman, J.-M. Triiodothyronine and Free Thyroxine Levels are Differentially Associated with Metabolic Profile and Adiposity-Related Cardiovascular Risk Markers in Euthyroid Middle-Aged Subjects. Thyroid Off. J. Am. Thyroid Assoc. 2014, 24, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Mentuccia, D.; Proietti-Pannunzi, L.; Tanner, K.; Bacci, V.; Pollin, T.I.; Poehlman, E.T.; Shuldiner, A.R.; Celi, F.S. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: Evidence of interaction with the Trp64Arg variant of the beta-3-adrenergic receptor. Diabetes 2002, 51, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Kaartokallio, T.; Cervera, A.; Kyllönen, A.; Laivuori, K.; Kere, J.; Laivuori, H.; Laivuori, H.; Heinonen, S.; Kajantie, E.; Kere, J.; et al. Gene expression profiling of pre-eclamptic placentae by RNA sequencing. Sci. Rep. 2015, 5, 14107. [Google Scholar] [CrossRef] [PubMed]
- Strączkowski, M.; Nikołajuk, A.; Stefanowicz, M.; Matulewicz, N.; Fernandez-Real, J.M.; Karczewska-Kupczewska, M. Adipose Tissue and Skeletal Muscle Expression of Genes Associated with Thyroid Hormone Action in Obesity and Insulin Resistance. Thyroid Off. J. Am. Thyroid Assoc. 2021, 32, 206–214. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Knight, B.A.; Shields, B.M.; Hattersley, A.T.; Vaidya, B. Maternal hypothyroxinaemia in pregnancy is associated with obesity and adverse maternal metabolic parameters. Eur. J. Endocrinol. 2016, 174, 51–57. [Google Scholar] [CrossRef]
- Wang, J.; Gong, X.H.; Peng, T.; Wu, J.N. Association of Thyroid Function During Pregnancy With the Risk of Pre-eclampsia and Gestational Diabetes Mellitus. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2021, 27, 819–825. [Google Scholar] [CrossRef]
- Lucaccioni, L.; Ficara, M.; Cenciarelli, V.; Berardi, A.; Predieri, B.; Iughetti, L. Long term outcomes of infants born by mothers with thyroid dysfunction during pregnancy. Acta Bio-Med. Atenei Parm. 2020, 92, e2021010. [Google Scholar] [CrossRef]
- Mahadik, K.; Choudhary, P.; Roy, P.K. Study of thyroid function in pregnancy, its feto-maternal outcome; a prospective observational study. BMC Pregnancy Childbirth 2020, 20, 769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, X.; Zhang, Y.; Guo, F.; Yang, S.; Peeters, R.P.; Korevaar, T.I.M.; Fan, J.; Huang, H.F. Association Between Maternal Thyroid Hormones and Birth Weight at Early and Late Pregnancy. J. Clin. Endocrinol. Metab. 2019, 104, 5853–5863. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.M.; Dashe, J.S.; Wells, C.E.; McIntire, D.D.; Byrd, W.; Leveno, K.J.; Cunningham, F.G. Subclinical Hypothyroidism and Pregnancy Outcomes. Obs. Gynecol. 2005, 105, 239–245. [Google Scholar] [CrossRef] [PubMed]
| Total Cohort N = 597 | GDM N = 199 (33.3%) | NGT N = 398 (66.7%) | p-Value | |
|---|---|---|---|---|
| 6–14 weeks | ||||
| Gestational age at blood collection (weeks) | 11.9 ± 1.6 | 12.0 ± 1.7 | 11.9 ± 1.6 | 0.571 |
| TSH (mIU/L) | 1.3 ± 0.9 | 1.3 ± 0.9 | 1.3 ± 0.9 | 0.416 |
| fT4 (pmol/L) | 14.9 ± 2.4 | 14.6 ± 2.1 | 15.0 ±2.6 | 0.039 |
| fT3 (pmol/L) | 5.0 ± 0.8 | 5.0 ± 0.6 | 5.0 ± 0.8 | 0.288 |
| Anti-TPO ≥ 34 IU/L (%) | 6.5 (39) | 6.6 (13) | 6.5 (26) | 1.000 |
| fT3/fT4 (upper vs. lower tertile) | 0.34 ± 0.06 | 0.35 ± 0.05 | 0.34 ± 0.06 | 0.020 |
| 24–28 weeks | ||||
| Gestational age at blood collection (weeks) | 26.9 ± 1.1 | 27.1 ± 1.2 | 26.9 ± 1.0 | 0.034 |
| TSH (mIU/L) | 1.6 ± 0.8 | 1.5 ± 0.8 | 1.6 ± 0.8 | 0.104 |
| fT4 (pmol/L) | 12.2 ± 1.6 | 12.1 ± 1.6 | 12.3 ± 1.6 | 0.146 |
| fT3 (pmol/L) | 4.4 ± 0.5 | 4.5 ± 0.5 | 4.4 ± 0.5 | 0.013 |
| fT3/fT4 (upper vs. lower tertile) | 0.36 ± 0.06 | 0.37 ± 0.07 | 0.36 ± 0.06 | 0.010 |
| Change from 6–14 weeks to 26–28 weeks | ||||
| TSH (mIU/L) | 0.3 ± 0.8 | 0.3 ± 0.8 | 0.3 ± 0.8 | 0.394 |
| fT4 (pmol/L) | −2.6 ± 1.9 | −2.5 ± 1.5 | −2.7 ± 2.0 | 0.396 |
| fT3 (pmol/L) | −0.7 ± 0.6 | −0.6 ± 0.5 | −0.7 ± 0.7 | 0.075 |
| fT3/fT4 (upper vs. lower tertile) | 0.02 ± 0.1 | 0.02 ± 0.0 | 0.02 ± 0.1 | 0.336 |
| GDM N = 199 (33.3%) | NGT N = 398 (66.7%) | Crude Model | Multivariable Model a | |
|---|---|---|---|---|
| 6–14 weeks | ||||
| fT3 (pmol/L) | ||||
| Tertile 1: 3.3–4.6 | 28.6 (57) | 29.1 (116) | 1 | 1 |
| Tertile 2:4.7–5.1 | 30.6 (61) | 32.7 (130) | 0.96 (0.61; 1.49) | 1.36 (0.73; 2.55) |
| Tertile 3: 5.2–13.8 | 40.7 (81) | 38.2 (152) | 1.09 (0.71; 1.69) | 0.75 (0.38; 1.46) |
| Upper decile: 5.8–13.8 | 28.7 (23) | 25.2 (39) | 0.76 (0.32; 1.78) | 0.99 (0.16; 6.28) |
| p for trend | 0.977 | 0.249 | ||
| Per unit increment | 1.00 (0.80; 1.26) | 0.77 (0.50; 1.18) | ||
| fT4 (pmol/L) | ||||
| Tertile 1: 9.9–13.7 | 35.2 (70) | 29.9 (119) | 1 | 1 |
| Tertile 2: 13.8–15.3 | 32.7 (65) | 32.7 (130) | 0.84 (0.56; 1.27) | 0.56 (0.31; 1.01) |
| Tertile 3: 15.4–47.1 | 32.2 (64) | 37.4 (149) | 0.72 (0.47; 1.10) | 0.57 (0.32; 1.02) |
| Upper decile: 17.2–47.1 | 21.3 (19) | 27.4 (45) | 0.72 (0.32; 1.61) | 0.54 (0.17; 1.77) |
| p for trend | 0.078 | 0.111 | ||
| Per unit increment | 0.93 (0.85; 1.01) | 0.91 (0.81; 1.02) | ||
| fT3/fT4 ratio | ||||
| Tertile 1: 0.20–0.31 | 30.1 (60) | 34.4 (137) | 1 | 1 |
| Tertile 2: 0.32–0.35 | 31.2 (62) | 34.2 (136) | 1.07 (0.70; 1.65) | 1.29 (0.68; 2.42) |
| Tertile 3: 0.36–1.01 | 38.7 (77) | 31.4 (125) | 1.50 (0.95; 2.36) | 1.31 (0.69; 2.48) |
| Upper decile: 0.41–1.01 | 29.4 (25) | 20.3 (35) | 1.26 (0.45; 3.52) | 0.74 (0.14; 3.80) |
| p for trend | 0.070 | 0.467 | ||
| Per unit increment | 20.03 (0.079; 510.43) | 5.00 (0.06; 383.24) | ||
| TSH (mIU/L) | ||||
| Tertile 1: 0.00–0.85 | 36.7 (73) | 31.6 (124) | 1 | 1 |
| Tertile 2: 0.86–1.59 | 33.2 (66) | 37.2 (146) | 0.79 (0.52; 1.20) | 1.07 (0.59; 1.98) |
| Tertile 3: 1.60–8.00 | 30.1 (60) | 31.1 (122) | 0.80 (0.52; 1.23) | 1.15 (0.63; 2.10) |
| Upper decile: 2.40–8.00 | 18.0 (16) | 17.3 (26) | 0.84 (0.34; 2.06) | 1.79 (0.37; 8.74) |
| p for trend | 0.576 | 0.651 | ||
| Per unit increment | 0.95 (0.78; 1.15) | 1.06 (0.81; 1.39) | ||
| 24–28 weeks | ||||
| fT3 (pmol/L) | ||||
| Tertile 1: 3.0–4.1 | 26.6 (53) | 34.9 (139) | 1 | 1 |
| Tertile 2: 4.2–4.5 | 30.6 (61) | 32.2 (128) | 1.29 (0.83; 2.01) | 1.63 (0.89; 2.98) |
| Tertile 3: 4.6–7.2 | 42.7 (85) | 32.9 (131) | 1.74 (1.13; 2.67) | 1.70 (0.91; 3.16) |
| Upper decile: 5.0–7.2 | 39.8 (35) | 22.3 (40) | 1.52 (0.71; 3.24) | 0.82 (0.17; 3.92) |
| p for trend | 0.014 | 0.174 | ||
| Per unit increment | 1.55 (1.09; 2.21) | 1.43 (0.85; 2.38) | ||
| fT4 (pmol/L) | ||||
| Tertile 1: 8.0–11.4 | 33.2 (66) | 30.9 (123) | 1 | 1 |
| Tertile 2: 11.5–12.7 | 36.7 (73) | 32.7 (130) | 1.06 (0.70; 1.61) | 0.84 (0.44; 1.57) |
| Tertile 3: 12.8–24.0 | 30.1 (60) | 36.4 (145) | 0.78 (0.51; 1.18) | 0.77 (0.43; 1.39) |
| Upper decile: 14.3–24.0 | 18.5 (15) | 26.8 (45) | 0.65 (0.24; 1.80) | 1.30 (0.28; 6.00) |
| p for trend | 0.157 | 0.467 | ||
| Per unit increment | 0.92 (0.83; 1.03) | 0.94 (0.80; 1.11) | ||
| fT3/fT4 ratio | ||||
| Tertile 1: 0.21–0.33 | 26.6 (53) | 36.2 (144) | 1 | 1 |
| Tertile 2: 0.34–0.37 | 33.7 (67) | 32.7 (130) | 1.45 (0.94; 2.24) | 1.82 (0.98; 3.40) |
| Tertile 3: 0.38–0.66 | 39.7 (79) | 31.2 (124) | 1.85 (1.18; 2.92) | 2.12 (1.07; 4.23) |
| Upper decile: 0.44–0.66 | 37.6 (32) | 16.8 (29) | 1.92 (0.67; 5.52) | 2.12 (0.34; 13.00) |
| p for trend | 0.001 | 0.042 | ||
| Per unit increment | 190.42 (8.09; 4483.4) | 134.84 (1.19; 15302) | ||
| TSH (mIU/L) | ||||
| Tertile 1: 0.04–1.20 | 38.2 (76) | 30.0 (119) | 1 | 1 |
| Tertile 2: 1.21–1.84 | 29.1 (58) | 34.5 (137) | 0.64 (0.41; 0.98) | 0.54 (0.29; 1.04) |
| Tertile 3: 1.85–5.95 | 32.7 (65) | 35.5 (141) | 0.69 (0.45; 1.06) | 0.89 (0.49; 1.63) |
| Upper decile: 2.60–5.95 | 20.0 (19) | 25.6 (41) | 0.76 (0.31; 1.87) | 1.56 (0.38; 6.45) |
| p for trend | 0.145 | 0.713 | ||
| Per unit increment | 0.84 (0.66; 1.06) | 1.06 (0.77; 1.47) | ||
| fT3 | fT3/fT4 Ratio | |||
|---|---|---|---|---|
| r | p | r | p | |
| General | ||||
| Age (years) | −0.076 | 0.063 | 0.021 | 0.610 |
| 6–14 weeks | ||||
| BMI (kg/m2) | 0.327 | <0.001 | 0.365 | <0.001 |
| Waist circumference (cm) | 0.269 | <0.001 | 0.320 | <0.001 |
| Systolic blood pressure (mmHg) | 0.278 | <0.001 | 0.230 | <0.001 |
| Diastolic blood pressure (mmHg) | 0.225 | <0.001 | 0.195 | <0.001 |
| Fasting glycaemia (mmol/L) | 0.131 | 0.001 | 0.162 | <0.001 |
| Fasting insulin (pmol/L) | 0.249 | <0.001 | 0.294 | <0.001 |
| HOMA-IR | 0.257 | <0.001 | 0.305 | <0.001 |
| HOMA-B | 0.071 | 0.085 | 0.143 | <0.001 |
| HbA1c (mmol/mol and %) | 0.022 | 0.597 | 0.078 | 0.057 |
| Fasting Total cholesterol (mmol/L) | −0.045 | 0.276 | 0.112 | 0.006 |
| Fasting HDL (mmol/L) | −0.196 | <0.001 | −0.156 | <0.001 |
| Fasting LDL (mmol/L) | 0.011 | 0.793 | 0.135 | 0.001 |
| Fasting TG (mmol/L) | 0.126 | 0.002 | 0.252 | <0.001 |
| 26–28 weeks | ||||
| BMI (kg/m2) | 0.345 | <0.001 | 0.400 | <0.001 |
| Systolic blood pressure (mmHg) | 0.300 | <0.001 | 0.318 | <0.001 |
| Diastolic blood pressure (mmHg) | 0.273 | <0.001 | 0.265 | <0.001 |
| Glucose non-fasting 0 min on GCT (mmol/L) | 0.069 | 0.095 | 0.122 | 0.003 |
| Glucose 60 min on GCT (mmol/L) | 0.070 | 0.091 | 0.092 | 0.025 |
| Fasting glycaemia OGTT(mmol/L) | 0.173 | <0.001 | 0.242 | <0.001 |
| 30 min glucose OGTT (mmol/L) | 0.136 | 0.001 | 0.105 | 0.010 |
| 1 h glucose OGTT (mmol/L) | 0.120 | 0.003 | 0.171 | <0.001 |
| 2 h glucose OGTT (mmol/L) | 0.093 | 0.023 | 0.134 | 0.001 |
| Fasting insulin OGTT (pmol/L) | 0.285 | <0.001 | 0.379 | <0.001 |
| 30 min insulin OGTT (pmol/L) | 0.176 | <0.001 | 0.200 | <0.001 |
| 1 h insulin OGTT (pmol/L) | 0.207 | <0.001 | 0.319 | <0.001 |
| 2 h insulin OGTT (pmol/L) | 0.205 | <0.001 | 0.297 | <0.001 |
| HbA1c (mmol/mol and %) | 0.142 | <0.001 | 0.260 | <0.001 |
| Matsuda insulin sensitivity | −0.269 | <0.001 | −0.349 | <0.001 |
| HOMA-IR | 0.290 | <0.001 | 0.395 | <0.001 |
| HOMA-B | 0.106 | 0.009 | 0.124 | 0.002 |
| ISSI-2 | −0.194 | <0.001 | −0.259 | <0.001 |
| Insulinogenic index/HOMA-IR | −0.179 | <0.001 | −0.226 | <0.001 |
| Fasting total cholesterol (mmol/L) | −0.190 | <0.001 | −0.038 | 0.356 |
| Fasting HDL (mmol/L) | −0.213 | <0.001 | −0.180 | <0.001 |
| Fasting LDL (mmol/L) | −0.136 | 0.001 | −0.037 | 0.369 |
| Fasting TG (mmol/L) | 0.074 | 0.071 | 0.206 | <0.001 |
| Delivery | ||||
| Total weight gain (delivery—first visit) (kg) | −0.021 | 0.629 | 0.021 | 0.622 |
| Weight baby (g) | 0.014 | 0.734 | 0.008 | 0.838 |
| fT3 Lower Tertile (3.0–4.1) 26–28 Weeks N = 192 (47.1%) | fT3 Upper Tertile (4.6–7.2) 26–28 Weeks N = 216 (52.9%) | p-Value | |
|---|---|---|---|
| General | |||
| Age (years) | 32.1 ± 4.0 | 31.2 ± 4.7 | 0.047 |
| 6–14 weeks visit | |||
| BMI (kg/m2) | 23.9 ± 4.4 | 27.7 ± 6.0 | <0.001 |
| % Overweight | 30.2 (58) | 61.6 (133) | <0.001 |
| % Obesity | 8.3 (16) | 32.9 (71) | <0.001 |
| Waist circumference (cm) | 84.1 ± 11.0 | 91.9 ± 13.6 | <0.001 |
| % Waist 80–88 cm | 44.9 (84) | 26.5 (56) | <0.001 |
| % Waist ≥ 88 cm | 22.5 (42) | 53.5 (113) | |
| Weight gain (first visit till OGTT) (kg) | 6.7 ± 3.3 | 6.7 ± 3.4 | 0.525 |
| Systolic blood pressure (mmHg) | 112.3 ± 8.7 | 119.4 ± 12.1 | <0.001 |
| Diastolic blood pressure (mmHg) | 69.2 ± 7.1 | 73.5 ± 8.9 | <0.001 |
| % Hypertension (≥140/90 mmHg) | 0.5 (1) | 6.9 (15) | <0.001 |
| Fasting glycaemia (mmol/L) | 2.15 (2.02–2.22) | 2.17 (2.07–2.27) | 0.004 |
| Fasting insulin (pmol/L) | 41.1 (31.0–53.8) | 60.1 (43.0–86.8) | <0.001 |
| HOMA-IR | 8.2 (6.3–11.3) | 12.2 (8.6–18.5) | <0.001 |
| HOMA-B | 760.7 (582.1–1052.9) | 1015.4 (735.8–1519.6) | <0.001 |
| HbA1c (mmol/mol and %) | 31.1 (30.1–33.3) 5.0 (4.9–5.2) | 31.1 (30.1–33.3) 5.0 (4.9–5.2) | 0.779 |
| Fasting total cholesterol (mmol/L) | 2.2 (2.0–2.4) | 2.1 (1.9–2.4) | 0.400 |
| Fasting HDL (mmol/L) | 0.85 (0.75–0.96) | 0.77 (0.68–0.88) | <0.001 |
| Fasting LDL (mmol/L) | 1.15 (0.91–1.32) | 1.38 (0.94–1.33) | 0.655 |
| Fasting TG (mmol/L) | 0.96 (0.79–1.20) | 1.04 (0.86–1.45) | 0.002 |
| fT4 (pmol/L) | 14.7 (13.4–16.1) | 14.8 (13.5–16.0) | 0.477 |
| TSH (mIU/L) | 1.2 (0.8–1.8) | 1.1 (0.6–1.8) | 0.136 |
| % Anti-TPO-positive (≥34 IU/L) | 6.8 (13) | 6.0 (13) | 0.840 |
| Total score lifestyle | |||
| Physical activity | 1.0 (0–2.0) | 1.0 (0–2.0) | 0.073 |
| Diet | 3.0 (0–5.0) | 2.0 (0–4.0) | 0.009 |
| 24–28 weeks visit | |||
| BMI (kg/m2) | 26.3 ± 4.4 | 30.2 ± 5.7 | <0.001 |
| % Overweight | 51.6 (95) | 78.4 (167) | <0.001 |
| % Obesity | 16.3 (30) | 47.9 (102) | <0.001 |
| Systolic blood pressure (mmHg) | 109.5 ± 8.7 | 116.0 ± 11.4 | <0.001 |
| Diastolic blood pressure (mmHg) | 64.9 ± 6.5 | 69.6 ± 8.4 | <0.001 |
| % Hypertension (≥140/90 mmHg) | 0.5 (1) | 4.6 (10) | 0.012 |
| Weeks of gestation at OGTT | 26.9 ± 1.1 | 27.0 ± 1.1 | 0.685 |
| Fasting glycaemia OGTT (mmol/L) | 4.38 (4.16–4.66) | 4.50 (4.30–4.82) | <0.001 |
| 30 min glucose OGTT (mmol/L) | 7.21 (6.49–8.10) | 7.55 (6.72–8.49) | 0.003 |
| 1 h glucose OGTT (mmol/L) | 7.52 (6.19–9.02) | 8.27 (6.94–9.43) | 0.007 |
| 2 h glucose OGTT (mmol/L) | 6.60 (5.44–7.85) | 7.16 (5.88–8.27) | 0.008 |
| % GDM diagnosis | 27.6 (53) | 39.3 (85) | 0.016 |
| Fasting insulin OGTT (pmol/L) | 56.4 (41.2–78.2) | 79.9 (61.3–113.7) | <0.001 |
| 30 min insulin OGTT (pmol/L) | 442.4 (336.4–677.50) | 583.1 (407.7–872.1) | <0.001 |
| 1 h insulin OGTT (pmol/L) | 542.8 (382.3–828.8) | 693.7 (478.8–1014.0) | <0.001 |
| 2 h insulin OGTT (pmol/L) | 513.9 (344.3–786.0) | 692.0 (452.8–1005.0) | <0.001 |
| HbA1c (mmol/mol and %) | 30.1 (29.0–32.2) 4.9 (4.8–5.1) | 31.1 (30.1–33.3) 5.0 (4.9–5.2) | 0.002 |
| Matsuda insulin sensitivity | 0.6 (0.4–0.8) | 0.4 (0.3–0.5) | <0.001 |
| HOMA-IR | 11.0 (7.9–15.9) | 16.1 (11.7–23.5) | <0.001 |
| HOMA-B | 1281.4 (913.4–1759.6) | 1635.1 (1147.3–2292.2) | <0.001 |
| ISSI-2 | 0.1 (0.1–0.3) | 0.1 (0–0.2) | <0.001 |
| Insulinogenic index/HOMA-IR | 0.3 (0.2–0.5) | 0.3 (0.2–0.3) | <0.001 |
| Fasting total cholesterol (mmol/L) | 6.47 (5.74–7.15) | 5.97 (5.46–6.70) | <0.001 |
| Fasting HDL (mmol/L) | 2.03 (1.75–2.31) | 1.82 (1.59–2.08) | <0.001 |
| Fasting LDL (mmol/L) | 3.59 (2.93–4.29) | 3.21 (2.75–3.90) | 0.004 |
| Fasting TG (mmol/L) | 1.84 (1.46–2.25) | 1.95 (1.63–2.48) | 0.015 |
| GDM treatment * | |||
| GDM | 27.6 (53) | 39.3 (85) | 0.016 |
| % Need for treatment with insulin | 3.7 (7) | 4.6 (10) | 1.000 |
| Gestational age at start insulin | 30.7 ± 3.0 | 29.6 ± 2.0 | 0.551 |
| Delivery | |||
| % Excessive weight gain | 21.3 (38) | 36.2 (64) | 0.002 |
| Gestational age (weeks) | 39.3 ± 1.6 | 38.9 ± 1.9 | 0.036 |
| % Preeclampsia | 1.0 (2) | 4.6 (10) | 0.040 |
| % Gestational hypertension | 3.1 (6) | 8.3 (18) | 0.034 |
| % Cesarean sections (total) | 16.1 (31) | 29.4 (63) | 0.002 |
| % Planned CS | 4.7 (9) | 16.8 (36) | <0.001 |
| % Emergency CS (during labor) | 11.5 (2) | 12.6 (27) | 0.762 |
| % Macrosomia (>4 kg) | 6.2 (12) | 8.4 (18) | 0.451 |
| % LGA | 8.8 (17) | 13.9 (30) | 0.122 |
| % SGA | 5.7 (11) | 4.6 (10) | 0.659 |
| % Neonatal hypoglycaemia <2.22 mmol/L | 5.8 (10) | 9.8 (18) | 0.172 |
| % NICU admission | 6.8 (13) | 14.9 (32) | 0.011 |
| fT3-to-fT4 Ratio Lower Tertile (0.21 to 0.33) 26–28 Weeks N = 197 (49.2%) | fT3-to-fT4 Ratio Upper Tertile (0.38 to 0.66) 26–28 Weeks N = 203 (50.7%) | p-Value | |
|---|---|---|---|
| General | |||
| Age (years) | 31.6 ± 4.1 | 31.8 ± 4.6 | 0.752 |
| % History of impaired glucose intolerance | 0.6 (1) | 4.9 (9) | 0.020 |
| 6–14 weeks visit | |||
| Weeks of gestation first visit with FPG | 12.0 ± 1.6 | 11.9 ± 1.7 | 0.669 |
| BMI (kg/m2) | 23.3 ± 3.9 | 28.0 ± 5.6 | <0.001 |
| % Overweight | 27.4 (54) | 66.0 (134) | <0.001 |
| % Obesity | 6.1 (12) | 32.5 (66) | |
| Waist circumference (cm) | 83.4 ± 10.0 | 92.6 ± 12.5 | <0.001 |
| % Waist 80–88 cm | 42.0 (81) | 30.6 (61) | <0.001 |
| % Waist ≥ 88 cm | 22.3 (43) | 55.8 (111) | |
| Weight gain (first visit till OGTT) (kg) | 6.4 ± 3.3 | 7.2 ± 3.0 | 0.007 |
| Systolic blood pressure (mmHg) | 112.4 ± 9.0 | 118.2 ± 11.5 | <0.001 |
| Diastolic blood pressure (mmHg) | 69.3 ± 7.3 | 72.7 ± 9.0 | <0.001 |
| % Hypertension (≥140/90 mmHg) | 0.5 (1) | 6.9 (14) | <0.001 |
| Fasting glycaemia (mmol/L) | 4.61 (4.33–4.77) | 4.66 (4.50–4.88) | 0.002 |
| Fasting insulin (pmol/L) | 39.4 (30.6–53.6) | 62.5 (43.9–89.1) | <0.001 |
| HOMA-IR | 8.0 (6.1–11.2) | 12.9 (9.0–18.7) | <0.001 |
| HOMA-B | 742.9 (582.6–1052.5) | 1048.9 (774.0–1470.9) | <0.001 |
| HbA1c (mmol/mol and %) | 31.1 (30.1–33.3) 5.0 (4.9–5.2) | 31.1 (30.1–33.3) 5.0 (4.9–5.2) | 0.053 |
| Fasting total cholesterol (mmol/L) | 4.54 (3.98–5.11) | 4.75 (4.31–5.26) | 0.006 |
| Fasting HDL (mmol/L) | 1.82 (1.57–2.03) | 1.67 (1.44–1.90) | <0.001 |
| Fasting LDL (mmol/L) | 2.31 (1.85–2.70) | 2.49 (2.11–2.93) | <0.001 |
| Fasting TG (mmol/L) | 0.91 (0.77–1.13) | 1.10 (0.86–1.57) | <0.001 |
| fT4 (pmol/L) | 15.7 (14.4–16.9) | 13.8 (12.8–14.9) | <0.001 |
| TSH (mIU/L) | 1.1 (0.60; 1.75) | 1.2 (0.8–1.9) | 0.039 |
| % Anti-TPO-positive (≥34 IU/L) | 6.6 (13) | 7.9 (16) | 0.702 |
| 24–28 weeks visit | |||
| BMI (kg/m2) | 25.6 ± 3.9 | 30.7 ± 5.3 | <0.001 |
| % Overweight | 44.8 (7) | 86.4 (171) | <0.001 |
| % Obesity | 12.9 (25) | 50.0 (99) | <0.001 |
| Systolic blood pressure (mmHg) | 109.7 ± 9.0 | 116.6 ± 11.2 | <0.001 |
| Diastolic blood pressure (mmHg) | 65.2 ± 6.9 | 69.7 ± 8.2 | <0.001 |
| % Hypertension (≥140/90 mmHg) | 0 (0) | 4.5 (9) | 0.004 |
| Fasting glycaemia OGTT(mmol/L) | 4.38 (4.16–4.61) | 4.55 (4.33–4.88) | <0.001 |
| 30 min glucose OGTT (mmol/L) | 7.21 (6.44–7.99) | 7.46 (6.83–8.38) | 0.006 |
| 1 h glucose OGTT (mmol/L) | 7.49 (6.05–8.82) | 8.32 (6.99–9.49) | <0.001 |
| 2 h glucose OGTT (mmol/L) | 6.60 (5.38–7.88) | 7.16 (5.94–8.27) | 0.004 |
| Fasting insulin OGTT (pmol/L) | 53.7 (39.8–76.7) | 84.8 (64.3–124.9) | <0.001 |
| 30 min insulin OGTT (pmol/L) | 466.9 (325.5–661.2) | 591.9 (418.9–867.9) | <0.001 |
| 1 h insulin OGTT (pmol/L) | 518.9 (357.2–741.0) | 752.4 (563.2–1038.0) | <0.001 |
| 2 h insulin OGTT (pmol/L) | 497.8 (334.7–737.6) | 757.0 (499.2–1046.5) | <0.001 |
| % GDM diagnosis | 26.9 (53) | 38.9 (79) | 0.011 |
| HbA1c (mmol/mol and %) | 30.1 (29.0–32.2) 4.9 (4.8–5.1) | 31.1 (30.1–33.3) 5.0 (4.9–5.2) | <0.001 |
| Matsuda insulin sensitivity | 0.6 (0.4–0.8) | 0.4 (0.3–0.5) | <0.001 |
| HOMA-IR | 10.7 (7.7–15.4) | 17.5 (12.5–25.8) | <0.001 |
| HOMA-B | 1254.5 (889.6–1699.7) | 1668.0 (1146.0–2311.7) | <0.001 |
| ISSI-2 | 0.2 (0.1–0.3) | 0.1 (0.0–0.1) | <0.001 |
| Insulinogenic index/HOMA-IR | 0.3 (0.2–0.5) | 0.2 (0.2–0.3) | <0.001 |
| Fasting total cholesterol (mmol/L) | 6.09 (5.60–6.60) | 6.14 (5.62–6.91) | 0.937 |
| Fasting HDL (mmol/L) | 1.98 (1.72–2.29) | 1.77 (1.51–2.11) | <0.001 |
| Fasting LDL (mmol/L) | 3.36 (2.80–3.93) | 3.31 (2.80–3.98) | 0.961 |
| Fasting TG (mmol/L) | 1.73 (1.39–2.16) | 2.04 (1.69–2.56) | <0.001 |
| Increase (difference) in TG between the first and second visit (mmol/L) | 0.76 (0.50–1.08) | 0.87 (0.59–1.24) | 0.033 |
| fT4 (pmol/L) | 13.3 (12.5–14.3) | 11.1 (10.4–11.8) | <0.001 |
| TSH (mIU/L) | 1.4 (1.0–2.0) | 1.7 (1.1–2.1) | 0.005 |
| Total score lifestyle: | |||
| Physical activity | 1.0 (0–2.0) | 1.0 (0–2.0) | 0.216 |
| Diet | 3.0 (1.0–5.0) | 2.0 (0–4.0) | 0.004 |
| GDM treatment * | |||
| GDM | 26.9 (53) | 38.9 (79) | 0.011 |
| % Need for treatment with insulin | 3.0 (6) | 5.9 (12) | 0.540 |
| Gestational age at start insulin | 29.8 ± 1.2 | 30.2 ± 1.6 | 0.666 |
| Delivery | |||
| Total weight gain (delivery—first visit) (kg) | 10.7 ± 4.9 | 11.6 ± 5.7 | 0.038 |
| % Excessive weight gain | 15.3 (27) | 39.5 (70) | <0.001 |
| Gestational age (weeks) | 39.3 ± 1.5 | 39.1 ± 1.8 | 0.094 |
| % Preeclampsia | 0.5 (1) | 4.4 (9) | 0.020 |
| % Gestational hypertension | 2.0 (4) | 8.9 (18) | 0.003 |
| % Preterm delivery | 6.1 (12) | 7.4 (15) | 0.692 |
| % Induction labor | 27.9 (55) | 37.1 (75) | 0.055 |
| % Cesarean sections (total) | 12.7 (25) | 32.2 (65) | <0.001 |
| % Planned CS | 6.1 (12) | 17.3 (35) | <0.001 |
| % Emergency CS (during labor) | 6.6 (13) | 14.8 (30) | 0.009 |
| % Macrosomia (>4 kg) | 6.1 (12) | 9.9 (20) | 0.197 |
| % LGA | 8.1 (16) | 17.8 (36) | 0.005 |
| % SGA | 4.1 (8) | 3.0 (6) | 0.597 |
| % Neonatal hypoglycaemia <2.22 mmol/L | 5.6 (10) | 8.7 (15) | 0.304 |
| % NICU admission | 6.1 (12) | 16.8 (34) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raets, L.; Minschart, C.; Van den Bruel, A.; Van den Bogaert, E.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; et al. Higher Thyroid fT3-to-fT4 Ratio Is Associated with Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes. J. Clin. Med. 2022, 11, 5016. https://doi.org/10.3390/jcm11175016
Raets L, Minschart C, Van den Bruel A, Van den Bogaert E, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, Vercammen C, et al. Higher Thyroid fT3-to-fT4 Ratio Is Associated with Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes. Journal of Clinical Medicine. 2022; 11(17):5016. https://doi.org/10.3390/jcm11175016
Chicago/Turabian StyleRaets, Lore, Caro Minschart, Annick Van den Bruel, Emmelien Van den Bogaert, Paul Van Crombrugge, Carolien Moyson, Johan Verhaeghe, Sofie Vandeginste, Hilde Verlaenen, Chris Vercammen, and et al. 2022. "Higher Thyroid fT3-to-fT4 Ratio Is Associated with Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes" Journal of Clinical Medicine 11, no. 17: 5016. https://doi.org/10.3390/jcm11175016
APA StyleRaets, L., Minschart, C., Van den Bruel, A., Van den Bogaert, E., Van Crombrugge, P., Moyson, C., Verhaeghe, J., Vandeginste, S., Verlaenen, H., Vercammen, C., Maes, T., Dufraimont, E., Roggen, N., De Block, C., Jacquemyn, Y., Mekahli, F., De Clippel, K., Loccufier, A., Laenen, A., ... Benhalima, K. (2022). Higher Thyroid fT3-to-fT4 Ratio Is Associated with Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes. Journal of Clinical Medicine, 11(17), 5016. https://doi.org/10.3390/jcm11175016









