Analysis of Mortality in Unvaccinated Patients with COVID-19 and Cardiovascular Risk
Abstract
:1. Introduction
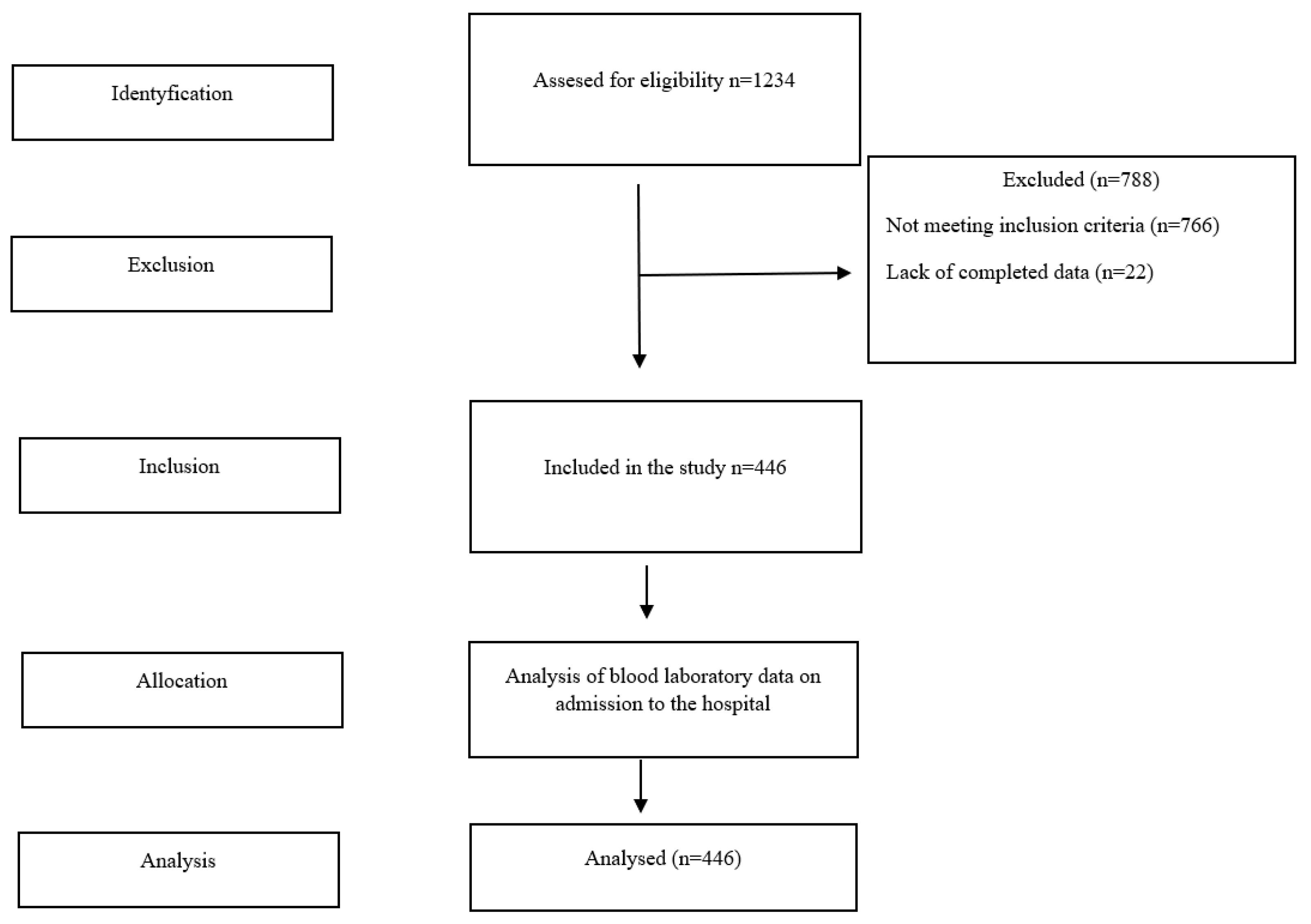
2. Materials and Methods
3. Results
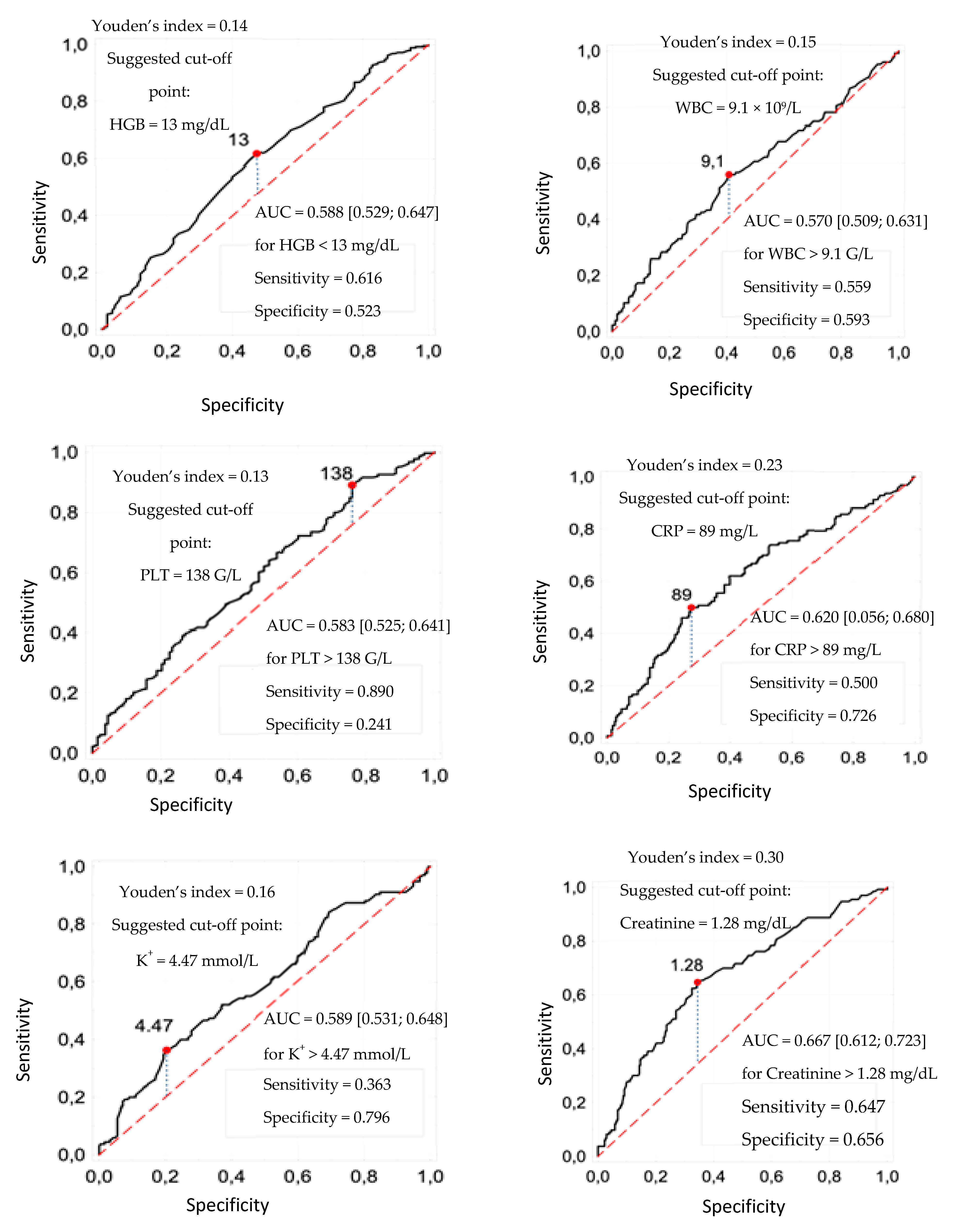
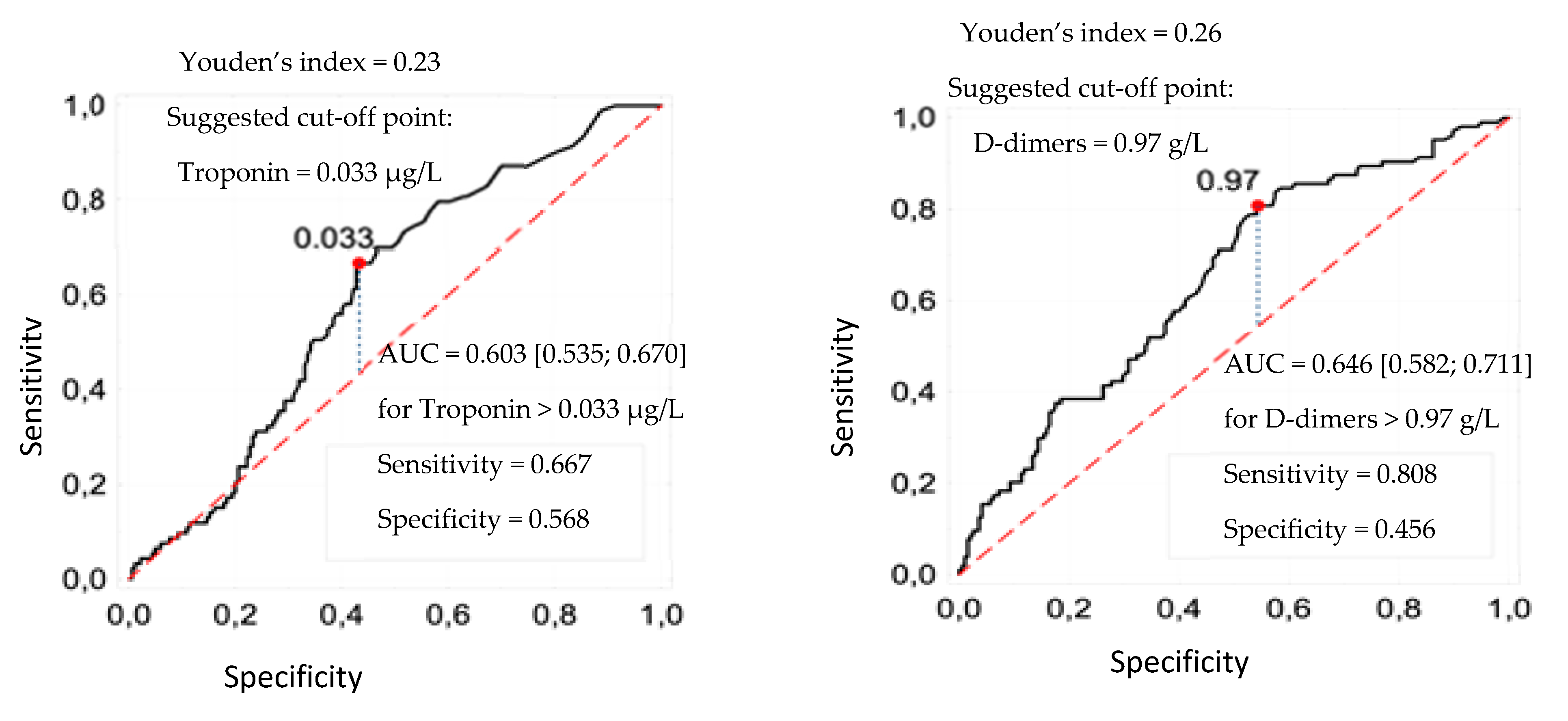
3.1. Cut-Off Points of Laboratory Parameters
3.2. Predictors of Death
3.3. Logistics Model Verification
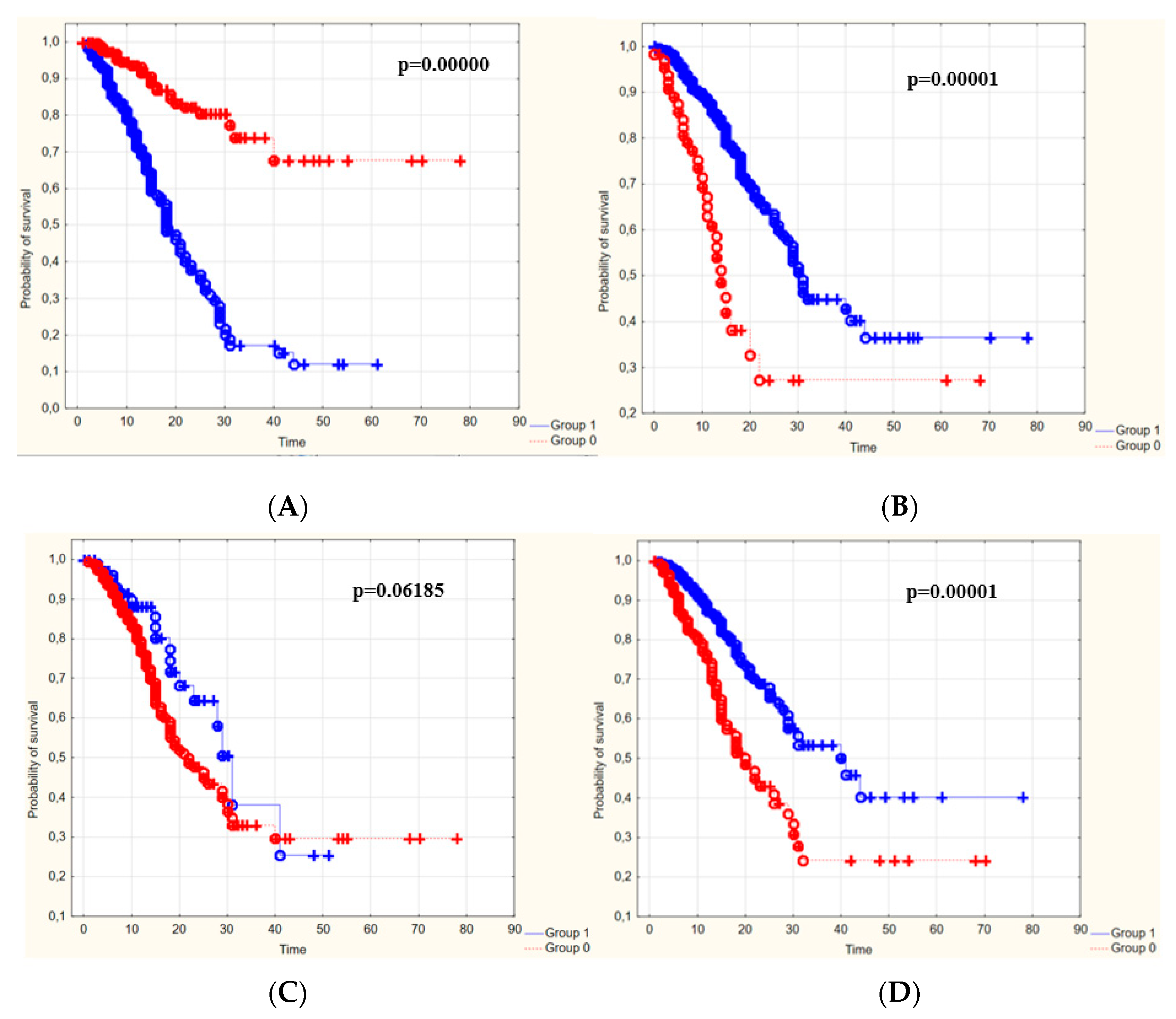
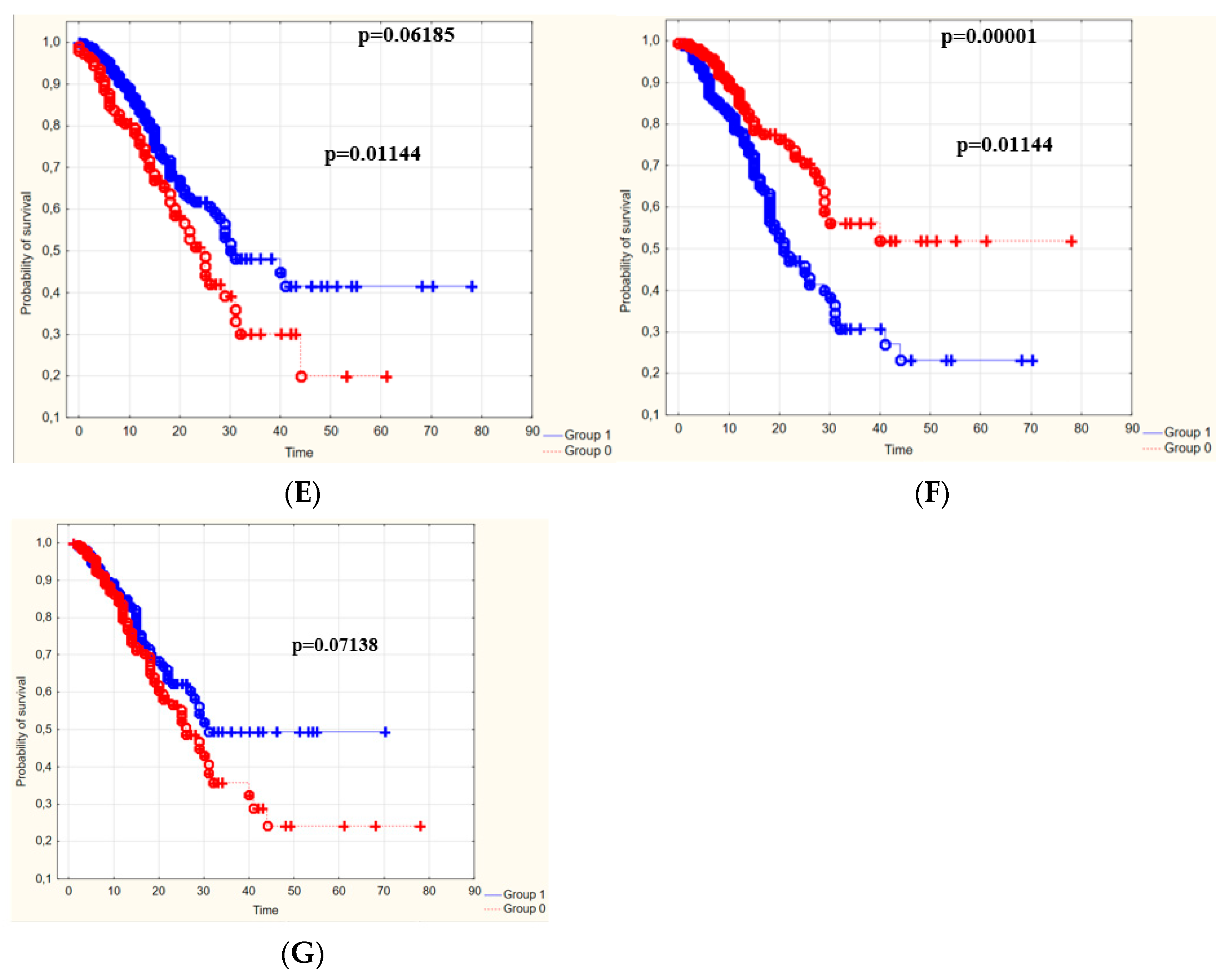
3.4. The Survival Rate of Patients Depending on the Duration of Hospitalization
4. Discussion
5. Conclusions
6. Practical Implications
7. Study Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.worldometers.info/coronavirus/ (accessed on 7 December 2021).
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059, Erratum in JAMA 2020, 323, 2098. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Zuliani, G.; Rigatelli, A.; Mazza, A.; Roncon, L. Arterial hypertension and risk of death in patients with COVID-19 infection: Systematic review and meta-analysis. J. Infect. 2020, 81, e84–e86. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Jayakumar, R.B.; Al-Jarallah, M.; Dashti, R.; Kobalava, Z.D.; Brady, P.A. COVID-19 and the heart. E-J.-Cardiol.-Pract. 2021, 21. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-21/covid-19-and-the-heart (accessed on 5 May 2022).
- Chang, W.T.; Toh, H.S.; Liao, C.T.; Yu, W.L. Cardiac Involvement of COVID-19: A Comprehensive Review. Am. J. Med. Sci. 2021, 361, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Combes, A.; Aissaoui, N. Cardiac injury in COVID-19. Intensive Care Med. 2022, 48, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Jonigk, D.; Markl, B.; Helms, J. COVID-19: What the clinician should know about post-mortem findings. Intensive Care Med. 2021, 47, 86–89. [Google Scholar] [CrossRef]
- Cheng, M.P.; Cau, A.; Lee, T.C.; Brodie, D.; Slutsky, A.; Marshall, J.; Murthy, S.; Lee, T.; Singer, J.; Demir, K.K.; et al. Angiotensin Receptor Blocker Coronavirus Study CI. Acute Cardiac Injury in Coronavirus Disease 2019 and Other Viral Infections-A Systematic Review and Meta-Analysis. Crit. Care Med. 2021, 49, 1558–1566. [Google Scholar] [CrossRef]
- Kiss, S.; Gede, N.; Hegyi, P.; Németh, D.; Földi, M.; Dembrovszky, F.; Nagy, B.; Juhász, M.F.; Ocskay, K.; Zádori, N.; et al. Early changes in laboratory parameters are predictors of mortality and ICU admission in patients with COVID-19: A systematic review and meta-analysis. Med. Microbiol. Immunol. 2021, 210, 33–47. [Google Scholar] [CrossRef]
- Yang, L.; Jin, J.; Luo, W.; Gan, Y.; Chen, B.; Li, W. Risk factors for predicting mortality of COVID-19 patients: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0243124. [Google Scholar] [CrossRef]
- de Almeida-Pititto, B.; Dualib, P.M.; Zajdenverg, L.; Rodrigues Dantas, J.; Dias de Souza, F.; Rodacki, M.; Casaccia Bertoluci, M. on behalf of Brazilian Diabetes Society Study Group (SBD). Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: A meta-analysis. Diabetol. Metab. Syndr. 2020, 12, 75. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Cenko, E.; Badimon, L.; Bugiardini, R.; Claeys, M.J.; De Luca, G.; de Wit, C.; Derumeaux, G.; Dorobantu, M.; Duncker, D.J.; Eringa, E.C.; et al. Cardiovascular disease and COVID-19: A consensus paper from the ESC Working Group on Coronary Pathophysiology & Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA). Cardiovasc. Res. 2021, 117, 2705–2729. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.M.; Zhu, H.Q.; Xie, Y.N.; Zou, Z.S.; Zuo, H.M.; Rao, Y.W.; Liu, X.Y.; Zhong, B.; Chen, X. Temporal changes in laboratory markers of survivors and non-survivors of adult inpatients with COVID-19. BMC Infect. Dis. 2020, 20, 952. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, W.; Guo, Y.; Chen, L.; Zhang, L.; Zhao, S.; Long, D.; Yu, L. Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets 2020, 31, 490–496. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Q.; Wang, Y.; Wu, Y.; Xu, J.; Yu, Y.; Shang, Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1469–1472. [Google Scholar] [CrossRef]
- Liao, D.; Zhou, F.; Luo, L.; Xu, M.; Wang, H.; Xia, J.; Gao, Y.; Cai, L.; Wang, Z.; Yin, P.; et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study. Lancet Haematol. 2020, 7, e671–e678. [Google Scholar] [CrossRef]
- Zhang, J.J.; Cao, Y.Y.; Tan, G.; Dong, X.; Wang, B.C.; Lin, J.; Yan, Y.Q.; Liu, G.H.; Akdis, M.; Akdis, C.A.; et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy 2021, 76, 533–550. [Google Scholar] [CrossRef]
- Aksel, G.; İslam, M.M.; Algın, A.; Eroğlu, S.E.; Yaşar, G.B.; Ademoğlu, E.; Dölek, Ü.C. Early predictors of mortality for moderate to severely ill patients with COVID-19. Am. J. Emerg. Med. 2021, 45, 290–296. [Google Scholar] [CrossRef]
- Huang, I.; Pranata, R.; Lim, M.A.; Oehadian, A.; Alisjahbana, B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: A meta-analysis. Ther. Adv. Respir. Dis. 2020, 14, 1753466620937175. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Peiró, Ó.M.; Carrasquer, A.; Sánchez-Gimenez, R.; Lal-Trehan, N.; del-Moral-Ronda, V.; Bonet, G.; Fort-Gallifa, I.; Picó-Plana, E.; Bastón-Paz, N.; Gutiérrez, C.; et al. Biomarkers and short-term prognosis in COVID-19. Biomarkers 2021, 26, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Castro-Castro, M.J.; García-Tejada, L.; Arbiol-Roca, A.; Sánchez-Navarro, L.; Rapún-Mas, L.; Cachon-Suárez, I.; Álvarez-Álvarez, M.; Dot-Bach, D.; Güell-Miró, R.; Cortés-Bosch de Bassea, A.; et al. Dynamic profiles and predictive values of some biochemical and haematological quantities in COVID-19 inpatients. Biochem. Med. 2022, 32, 74–84. [Google Scholar]
- Tahtasakal, C.A.; Oncul, A.; Sevgi, D.Y.; Celik, E.; Ocal, M.; Turkkan, H.M.; Bayraktar, B.; Oba, S.; Dokmetas, I. Could we predict the prognosis of the COVID-19 disease? J. Med. Virol. 2021, 93, 2420–2430. [Google Scholar] [CrossRef] [PubMed]
- Antunez Muiños, P.J.; López Otero, D.; Amat-Santos, I.J.; López País, J.; Aparisi, A.; Antonio, C.; Catalá, C.P.; Ferrero Cabezón, T.G.; Otero García, O.; Gil, J.F.; et al. The COVID-19 lab score: An accurate dynamic tool to predict in-hospital outcomes in COVID-19 patients. Sci. Rep. 2021, 11, 9361. [Google Scholar] [CrossRef]
- Özyılmaz, S.; Ergün Alış, E.; Ermiş, E.; Allahverdiyev, S.; Uçar, H. Assessment of the relationship between mortality and troponin I levels in hospitalized patients with the novel coronavirus (COVID-19). Medicina 2020, 56, 693. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Cai, Y.; Liu, T.; Shen, B.; Yang, F.; Cao, S.; Liu, X.; Xiang, Y.; Zhao, X.; et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur. Heart J. 2020, 41, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef]

| Feature (Variable) | Statistics |
|---|---|
| Gender: | |
| Female, n (%) | 181 (40.6) |
| Male, n (%) | 265 (59.4) |
| Age (years of age): | |
| M ± SD | 71.7 ± 13.2 |
| Me [Q1; Q3] | 72 [64; 82] |
| Min–Max | 22–98 |
| Hospitalization time (days) | |
| M ± SD | 15.1 ± 11.6 |
| Me [Q1; Q3] | 12 [7; 20] |
| Min–Max | 0–78 |
| Discharge mode: | |
| Death, n (%) | 135 (30.3) |
| End of the therapeutic process, n (%) | 224 (50.2) |
| Referral for rehabilitation, n (%) | 87 (19.5) |
| Feature (Variable) | Number (%) |
|---|---|
| Comorbidities: | |
| Stroke | 68 (15.2) |
| Pulmonary embolism | 28 (6.3) |
| Atherosclerosis | 20 (4.5) |
| Atrial fibrillation and other arrhythmias | 170 (40) |
| Diabetes | 77 (17) |
| Hypertension | 131 (29) |
| Myocardial infarction | 29 (6.5) |
| Ischemic heart disease | 29 (6.5) |
| Embolism and arterial heart failure | 11 (2.5) |
| Heart failure | 68 (15.2) |
| Other | 233 (52.2) |
| Number of comorbidities: | |
| M ± SD | 2.0 ± 0.9 |
| Me [Q1; Q3] | 2 [1; 2] |
| Min–Max | 0–5 |
| Feature (Variable) | Statistics |
|---|---|
| HGB | |
| M ± SD | 13.1 ± 2.3 |
| Me [Q1; Q3] | 13.2 [11.9; 14.5] |
| Min–Max | 4.3–19.4 |
| WBC | |
| M ± SD | 9.8 ± 5.1 |
| Me [Q1; Q3] | 8.6 [6.3; 11.9] |
| Min–Max | 0.9–33.4 |
| PLT | |
| M ± SD | 239 ± 109 |
| Me [Q1; Q3] | 221 [164; 288] |
| Min–Max | 10–682 |
| CRP | |
| M ± SD | 75.0 ± 78.6 |
| Me [Q1; Q3] | 48.1 [11.9; 117.2] |
| Min–Max | 0.1–475 |
| Na+ | |
| M ± SD | 136 ± 5 |
| Me [Q1; Q3] | 137 [134; 140] |
| Min–Max | 108–154 |
| K+ | |
| M ± SD | 4.1 ± 0.7 |
| Me [Q1; Q3] | 4.0 [3.6; 4.5] |
| Min–Max | 1.7–7.0 |
| Creatinine | |
| M ± SD | 1.53 ± 1.24 |
| Me [Q1; Q3] | 1.21 [0.96; 1.63] |
| Min–Max | 0.44–15.13 |
| Troponin | |
| M ± SD | 1.77 ± 10.24 |
| Me [Q1; Q3] | 0.03 [0.01; 0.16] |
| Min–Max | 0.0–100.84 |
| D-dimers | |
| M ± SD | 5.72 ± 14.74 |
| Me [Q1; Q3] | 1.29 [0.71; 3.24] |
| Min–Max | 0.16–143.38 |
| Death Risk Factors | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| B | p | beta | p | OR [95% CI] | |
| HGB < 13 mg/dL | 0.567 | 0.008 | |||
| WBC > 9.1 × 109/L | 0.612 | 0.004 | |||
| PLT < 138 × 109/L | 0.945 | 0.001 | |||
| CRP > 89 mg/L | 0.943 | <0.001 | 0.822 | 0.008 | 2.28 [1.24; 4.18] |
| K+ > 4.47 mmol/L | 0.800 | <0.001 | |||
| Creatinine > 1.28 mg/dL | 1.249 | <0.001 | 1.059 | 0.001 | 2.88 [1.57; 5.30] |
| Troponin > 33 ng/mL | 0.965 | <0.001 | 0.713 | 0.024 | 2.04 [1.10; 3.79] |
| D-dimers > 0.97 g/L | 1.260 | <0.001 | 0.712 | 0.044 | 2.04 [1.02; 4.07] |
| Blood Laboratory Parameters [Cut-Off Point] | The Survival Rate of People with Values below vs. People with Values above the Cut-Off Point | The Survival Rate of People with Values above vs. People with Values below the Cut-Off Point |
|---|---|---|
| WBC [9.1 g/L] | 32.8% | - |
| PLT [138 g/L] | - | 43.3% |
| D-dimers [0.97 g/L] | 10% | - |
| CRP [89 mg/L] | 17.3% | - |
| K+ [4.47 mmol/L] | 11.8% | - |
| Creatinine [1.28 mg/dL] | 12.8% | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarzyńska, K.; Świątkowski, F.; Janc, J.; Zabierowski, J.; Jankowska-Polańska, B.; Chabowski, M. Analysis of Mortality in Unvaccinated Patients with COVID-19 and Cardiovascular Risk. J. Clin. Med. 2022, 11, 5004. https://doi.org/10.3390/jcm11175004
Sarzyńska K, Świątkowski F, Janc J, Zabierowski J, Jankowska-Polańska B, Chabowski M. Analysis of Mortality in Unvaccinated Patients with COVID-19 and Cardiovascular Risk. Journal of Clinical Medicine. 2022; 11(17):5004. https://doi.org/10.3390/jcm11175004
Chicago/Turabian StyleSarzyńska, Kathie, Filip Świątkowski, Jarosław Janc, Jan Zabierowski, Beata Jankowska-Polańska, and Mariusz Chabowski. 2022. "Analysis of Mortality in Unvaccinated Patients with COVID-19 and Cardiovascular Risk" Journal of Clinical Medicine 11, no. 17: 5004. https://doi.org/10.3390/jcm11175004
APA StyleSarzyńska, K., Świątkowski, F., Janc, J., Zabierowski, J., Jankowska-Polańska, B., & Chabowski, M. (2022). Analysis of Mortality in Unvaccinated Patients with COVID-19 and Cardiovascular Risk. Journal of Clinical Medicine, 11(17), 5004. https://doi.org/10.3390/jcm11175004






