Safe Performance of Track Dilation and Bile Aspiration with ERCP Catheter in EUS-Guided Hepaticogastrostomy with Plastic Stents: A Retrospective Multicenter Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
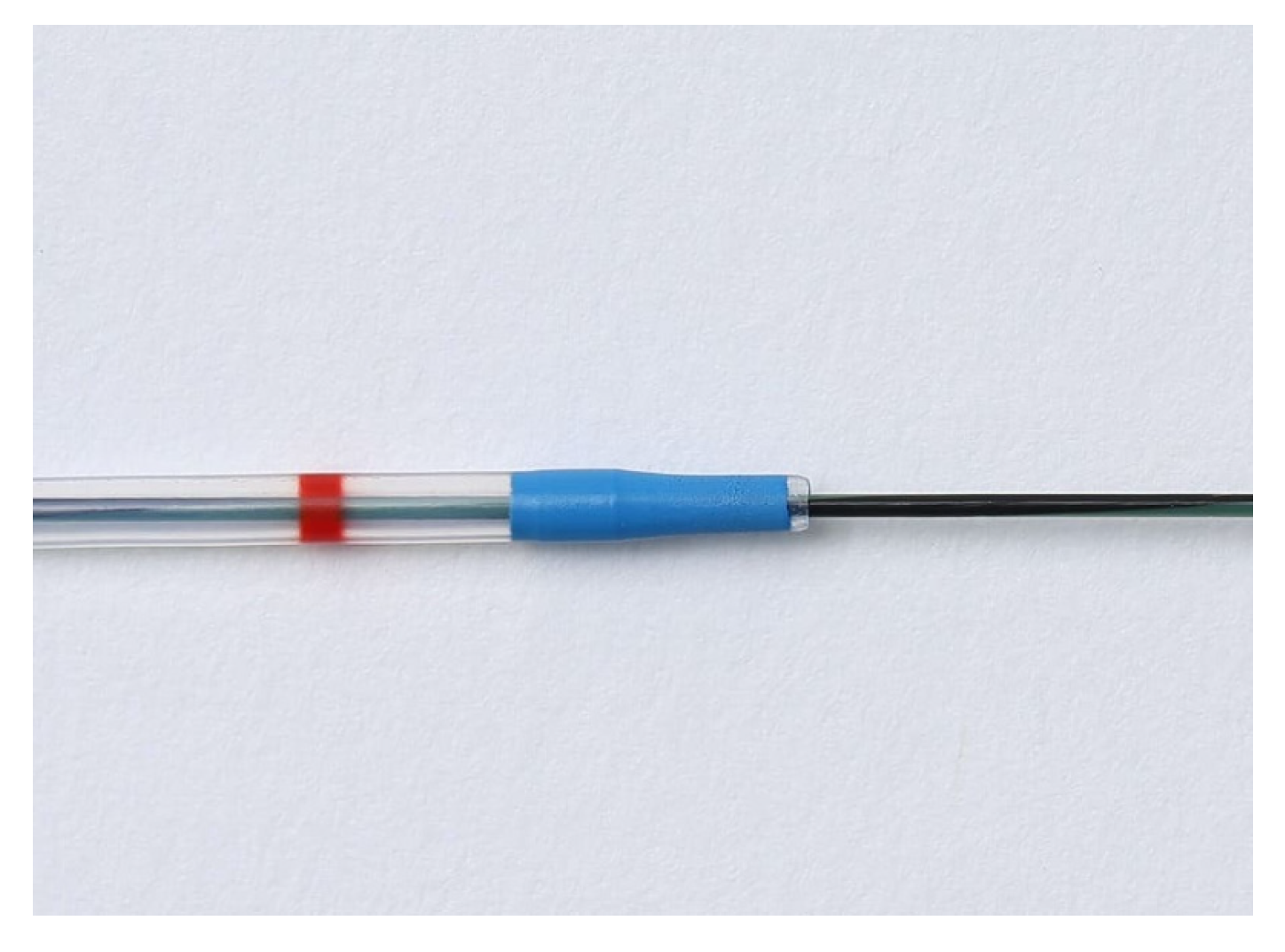
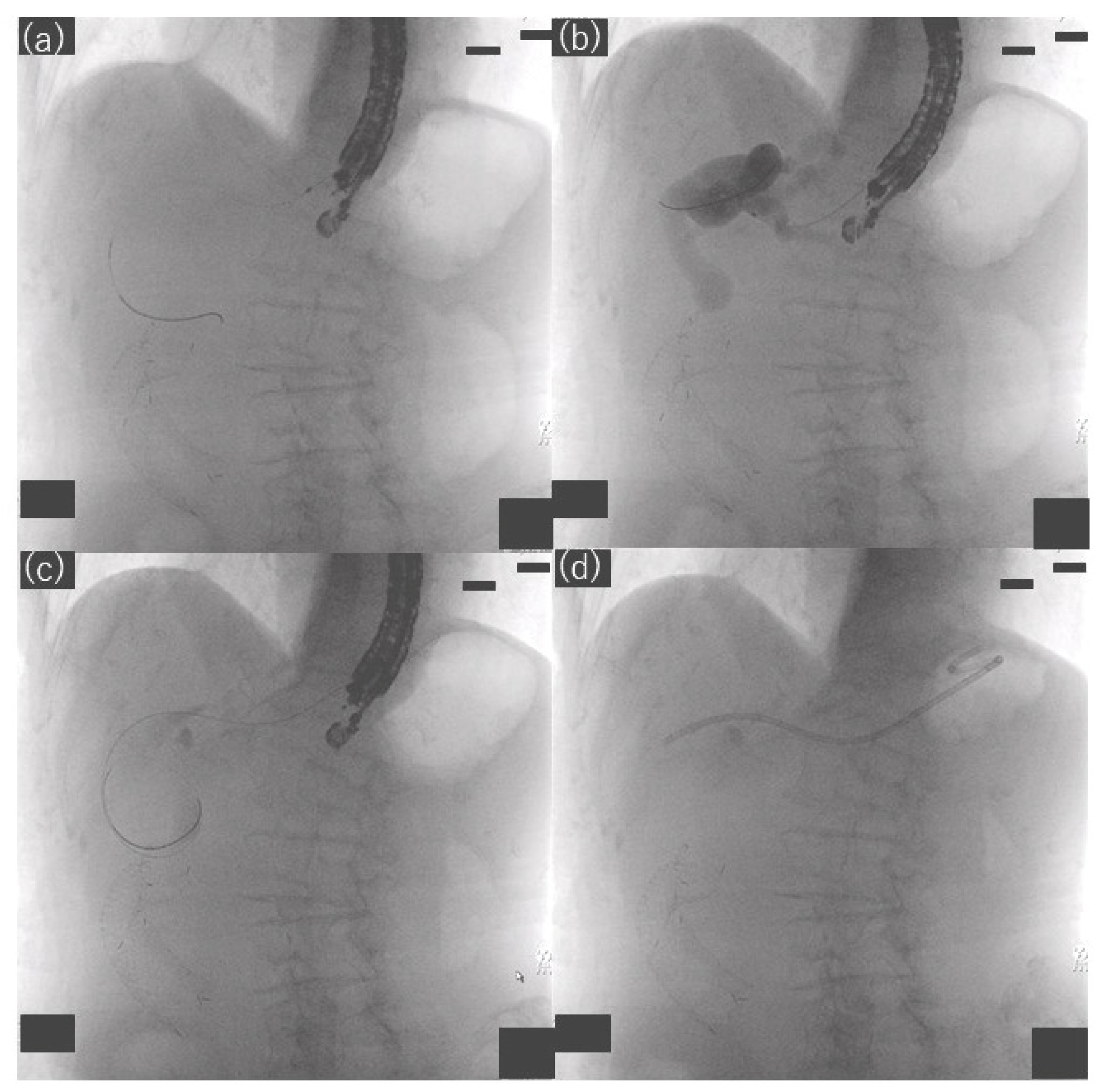
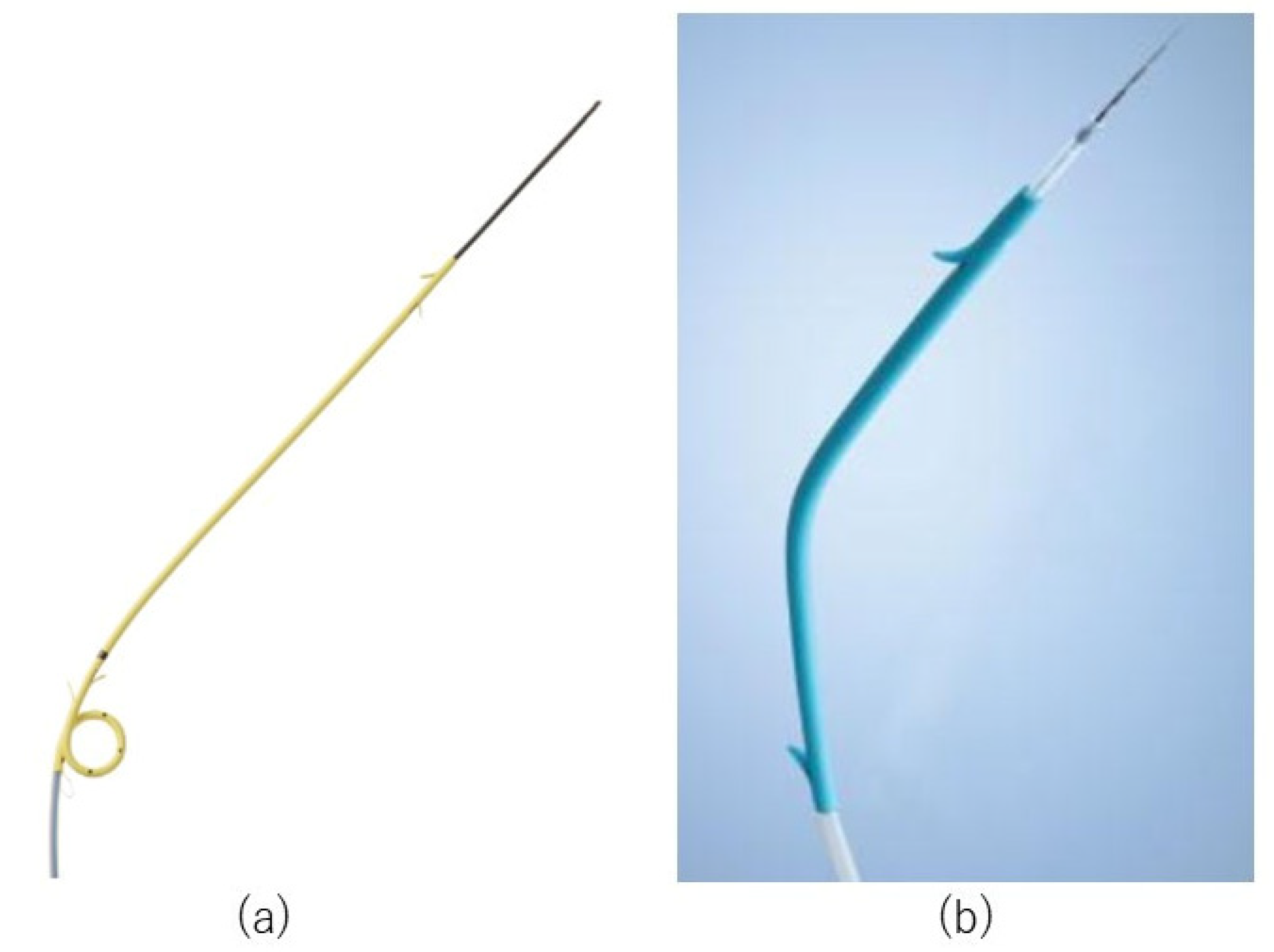
2.3. Procedures
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
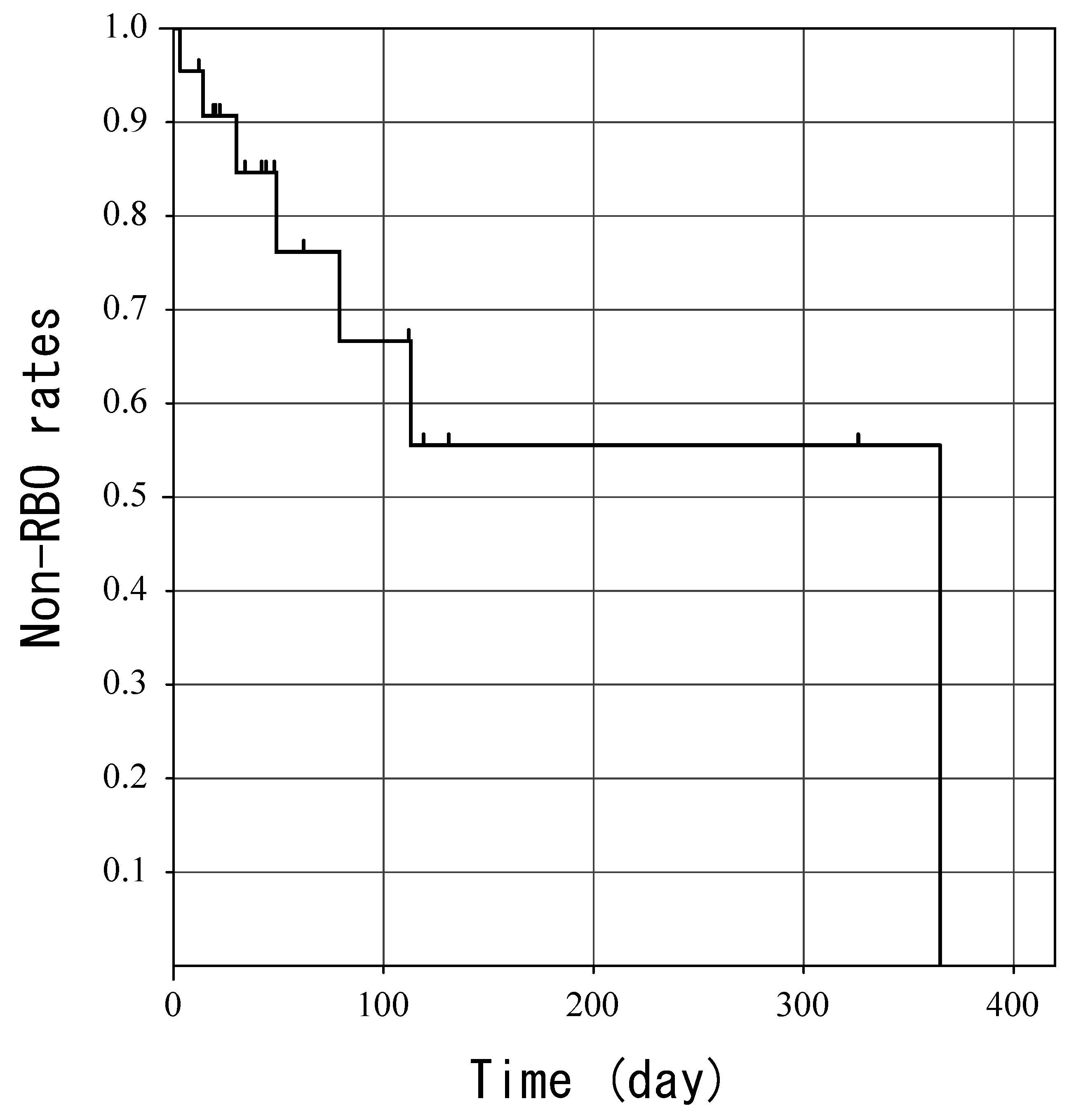
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isayama, H.; Nakai, Y.; Itoi, T.; Yasuda, I.; Kawakami, H.; Ryozawa, S.; Kitano, M.; Irisawa, A.; Katanuma, A.; Hara, K.; et al. Clinical practice guidelines for safe performance of endoscopic ultrasound/ultrasonography-Guided biliary drainage: 2018. J. Hepato-Biliary-Pancreat. Sci. 2019, 26, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Moutardier, V.; Pesenti, C.; Bories, E.; Lelong, B.; Delpero, J. Endoscopic ultrasound-Guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy 2001, 33, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Itoi, T.; Sofuni, A.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Umeda, J.; Moriyasu, F.; et al. Endoscopic ultrasonography-Guided biliary drainage. J. Hepato-Biliary-Pancreat. Sci. 2010, 17, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Baars, J.E.; Kaffes, A.J.; Saxena, P. EUS-Guided biliary drainage: A comprehensive review of the literature. Endosc. Ultrasound 2018, 7, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.; Dhindsa, B.; Mashiana, H.; Dhaliwal, A.; Mohan, B.; Jayaraj, M.; Sayles, H.; Singh, S.; Ohning, G.; Bhat, I. EUS-Guided biliary drainage: A systematic review and meta-Analysis. Endosc. Ultrasound 2020, 9, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Minaga, K.; Ogura, T.; Shiomi, H.; Imai, H.; Hoki, N.; Takenaka, M.; Nishikiori, H.; Yamashita, Y.; Hisa, T.; Kato, H.; et al. Comparison of the efficacy and safety of endoscopic ultrasound-Guided choledochoduodenostomy and hepaticogastrostomy for malignant distal biliary obstruction: Multicenter, randomized, clinical trial. Dig. Endosc. 2019, 31, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.D.B.; Nájera-Muñoz, R.; de la Serna-Higuera, C.; Fuentes-Valenzuela, E.; Fanjul, I.; Chavarría, C.; García-Alonso, F.J.; Sanchez-Ocana, R.; Carbajo, A.Y.; Bazaga, S.; et al. Lumen apposing metal stents versus tubular self-Expandable metal stents for endoscopic ultrasound-Guided choledochoduodenostomy in malignant biliary obstruction. Surg. Endosc. 2021, 35, 6754–6762. [Google Scholar] [CrossRef] [PubMed]
- Dhir, V.; Bale, A. EUS-Guided Hepatico-Gastrostomy: To Dilate or Not to Dilate? Dig. Dis. Sci. 2022. [Google Scholar] [CrossRef]
- Khashab, M.A.; Messallam, A.A.; Penas, I.; Nakai, Y.; Modayil, R.J.; De la Serna, C.; Hara, K.; El Zein, M.; Stavropoulos, S.N.; Perez-Miranda, M.; et al. International multicenter comparative trial of transluminal EUS-Guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc. Int. Open 2016, 4, E175–E181. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari, H.; Satoh, T.; Sato, J.; Kaneko, J.; Matsubayashi, H.; Yabuuchi, Y.; Kishida, Y.; Yoshida, M.; Ito, S.; Kawata, N.; et al. Bile aspiration during EUS-Guided hepaticogastrostomy is associated with lower risk of postprocedural adverse events: A retrospective single-Center study. Surg. Endosc. 2021, 35, 6836–6845. [Google Scholar] [CrossRef]
- Kanno, Y.; Ito, K.; Koshita, S.; Ogawa, T.; Masu, K.; Masaki, Y.; Noda, Y. Efficacy of a newly developed dilator for endoscopic ultrasound-Guided biliary drainage. World J. Gastrointest. Endosc. 2017, 9, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Vargo, J.J. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Umeda, J.; Itoi, T.; Tsuchiya, T.; Sofuni, A.; Itokawa, F.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Kamada, K.; Tanaka, R.; et al. A newly designed plastic stent for EUS-Guided hepaticogastrostomy: A prospective preliminary feasibility study (with videos). Gastrointest. Endosc. 2015, 82, 390–396.e392. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.P.; Rossini, L.G.; Ferrari, A.P. Migration of a covered metallic stent following endoscopic ultrasound-Guided hepaticogastrostomy: Fatal complication. Endoscopy 2010, 42, E126–E127. [Google Scholar] [CrossRef] [PubMed]
- Harai, S.; Hijioka, S.; Nagashio, Y.; Ohba, A.; Maruki, Y.; Sone, M.; Saito, Y.; Okusaka, T.; Fukasawa, M.; Enomoto, N. Usefulness of the laser-Cut, fully covered, self-Expandable metallic stent for endoscopic ultrasound-Guided hepaticogastrostomy. J. Hepato-Biliary-Pancreat. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Isayama, H.; Yamamoto, N.; Matsubara, S.; Ito, Y.; Sasahira, N.; Hakuta, R.; Umefune, G.; Takahara, N.; Hamada, T.; et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-Guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy 2016, 48, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Lee, T.H.; Paik, W.H.; Choi, J.-H.; Song, T.J.; Lee, S.S.; Seo, D.-W.; Lee, S.K.; Kim, M.-H. Feasibility and safety of a novel dedicated device for one-Step EUS-Guided biliary drainage: A randomized trial. J. Gastroenterol. Hepatol. 2015, 30, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Yamao, K.; Mizuno, N.; Hijioka, S.; Imaoka, H.; Tajika, M.; Tanaka, T.; Ishihara, M.; Okuno, N.; Hieda, N.; et al. Endoscopic ultrasonography-Guided biliary drainage: Who, when, which, and how? World J. Gastroenterol. 2016, 22, 1297–1303. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Value |
|---|---|
| Age, y | 72 (47–90) |
| Sex, male/female | 12/10 |
| Cause of biliary obstruction | 9 (40.9) |
| Gastric cancer | |
| Pancreatic cancer | 6 (27.3) |
| Bile duct cancer | 3 (13.6) |
| Duodenal cancer | 2 (9.1) |
| Intrahepatic gallstone | 1 (4.5) |
| Stenosis of choledochojejunostomy | 1 (4.5) |
| Performance Status | |
| 0 | 6 (27.3) |
| 1 | 9 (40.9) |
| 2 | 2 (9.1) |
| 3 | 5 (22.7) |
| Site of biliary obstruction | |
| Distal | 14 (63.6) |
| Hilar | 5 (22.7) |
| Anastomotic | 3 (13.6) |
| Indication of EUS-HGS | |
| Difficulty reaching the papilla | 12 (54.5) |
| Surgically altered anatomy | 7 (31.8) |
| Difficulty cannulating the bile duct | 3 (13.6) |
| Presence of cholangitis before EUS-HGS | 4 (18.2) |
| Value | |
|---|---|
| Procedure time (min) | 45.5 (15–90) |
| Puncture needle used | |
| 19-gauge | 21 (95.5) |
| 22-gauge | 1 (4.5) |
| Puncture site | |
| B2 | 15 (68.2) |
| B3 | 7 (31.8) |
| Types of plastic stents | |
| Through & Pass Type-IT | 19 (86.4) |
| Quick Place V | 3 (13.6) |
| Antegrade stent placement | 4 (18.2) |
| Bile aspiration whenever possible | |
| No | 11 (50) |
| Yes | 11 (50) |
| n (%) | |
|---|---|
| Functional success | 20 (90.9) |
| Recurrent biliary obstruction | 7 (31.8) |
| Occlusion | 6 (27.3) |
| Sludge formation | 4 (18.2) |
| Other | 2 (9.1) |
| Migration (toward the gastric side) | 1 (4.5) |
| Adverse events other than recurrent biliary obstruction | |
| Early adverse events | 7 (31.8) |
| Bile leakage | 3 (13.6) |
| Peritonitis | 3 (13.6) |
| Bleeding | 1 (4.5) |
| Late adverse events | 0 (0) |
| Bile Aspiration Whenever Possible | Yes (n = 11) | No (n = 11) |
|---|---|---|
| Preprocedural cholangitis (n) | 3 | 1 |
| Procedure time (min) | 47 (27–89) | 42 (15–90) |
| Time that the bile aspiration takes (min) | 13 (5–20) | |
| Antegrade stent placement (n) | 2 | 2 |
| Adverse events other than recurrent biliary obstruction (n) | 1 | 6 |
| Bile leakage (n) | 0 | 3 |
| Peritonitis (n) | 0 | 3 |
| Bleeding (n) | 1 | 0 |
| Univariate Analysis OR (95%CI) | p Value | |
|---|---|---|
| Age (years) | 0.29 | |
| <75 | 1 | |
| ≥75 | 2.86 (0.42–19.65) | |
| Performance status | 0.25 | |
| 0–1 | 1 | |
| 2–4 | 0.46 (0.02–2.67) | |
| Puncture site | 0.45 | |
| B2 | 1 | |
| B3 | 0.48(0.07–3.19) | |
| Preprocedural cholangitis | 0.75 | |
| Yes | 1.50 (0.13–17.67) | |
| No | 1 | |
| Bile aspiration whenever possible | 0.04 | |
| No | 12.0 (1.12–128.84) | |
| Yes | 1 | |
| Antegrade stent placement | 0.40 | |
| Yes | 2.60 (0.28–23.81) | |
| No | 1 | |
| Procedure time (min) | 0.65 | |
| <44.5 | 1 | |
| ≥44.5 | 1.52 (0.25–9.29) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobori, I.; Hashimoto, Y.; Shibuki, T.; Okumura, K.; Sekine, M.; Miyagaki, A.; Sasaki, Y.; Takano, Y.; Katayama, Y.; Kuwada, M.; et al. Safe Performance of Track Dilation and Bile Aspiration with ERCP Catheter in EUS-Guided Hepaticogastrostomy with Plastic Stents: A Retrospective Multicenter Study. J. Clin. Med. 2022, 11, 4986. https://doi.org/10.3390/jcm11174986
Kobori I, Hashimoto Y, Shibuki T, Okumura K, Sekine M, Miyagaki A, Sasaki Y, Takano Y, Katayama Y, Kuwada M, et al. Safe Performance of Track Dilation and Bile Aspiration with ERCP Catheter in EUS-Guided Hepaticogastrostomy with Plastic Stents: A Retrospective Multicenter Study. Journal of Clinical Medicine. 2022; 11(17):4986. https://doi.org/10.3390/jcm11174986
Chicago/Turabian StyleKobori, Ikuhiro, Yusuke Hashimoto, Taro Shibuki, Kei Okumura, Masanari Sekine, Aki Miyagaki, Yoshihiro Sasaki, Yuichi Takano, Yasumi Katayama, Masaru Kuwada, and et al. 2022. "Safe Performance of Track Dilation and Bile Aspiration with ERCP Catheter in EUS-Guided Hepaticogastrostomy with Plastic Stents: A Retrospective Multicenter Study" Journal of Clinical Medicine 11, no. 17: 4986. https://doi.org/10.3390/jcm11174986
APA StyleKobori, I., Hashimoto, Y., Shibuki, T., Okumura, K., Sekine, M., Miyagaki, A., Sasaki, Y., Takano, Y., Katayama, Y., Kuwada, M., Gyotoku, Y., Kusano, Y., & Tamano, M. (2022). Safe Performance of Track Dilation and Bile Aspiration with ERCP Catheter in EUS-Guided Hepaticogastrostomy with Plastic Stents: A Retrospective Multicenter Study. Journal of Clinical Medicine, 11(17), 4986. https://doi.org/10.3390/jcm11174986






