Interleukin 17 and Its Involvement in Renal Cell Carcinoma
Abstract
1. Introduction
2. Evidence Acquisition
3. RCC—A Brief Overview
4. IL-17—A Versatile Lymphokine
5. IL-17 in Kidney Diseases
6. IL-17 in Tumors
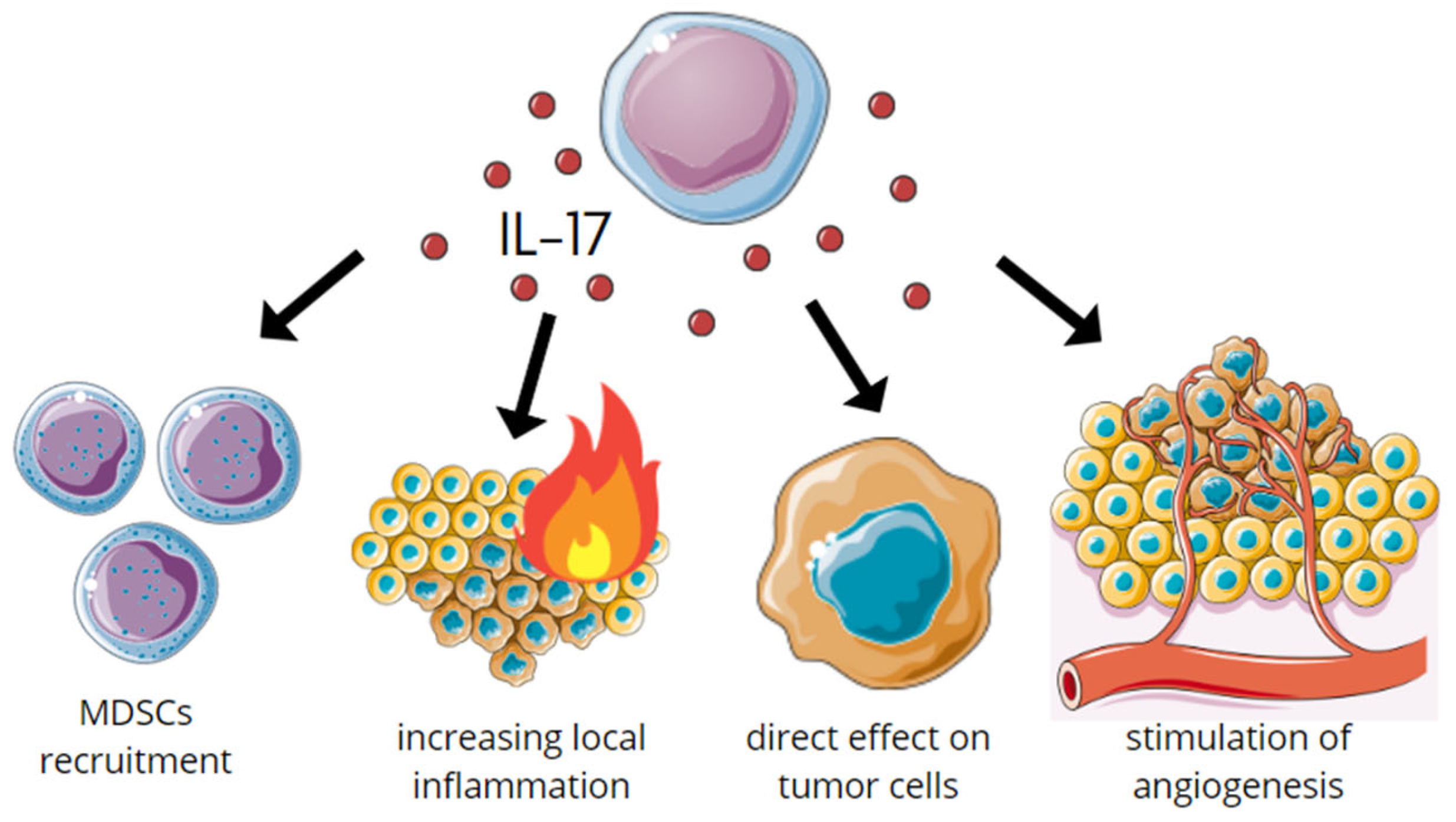
7. IL-17s Role in Carcinogenesis
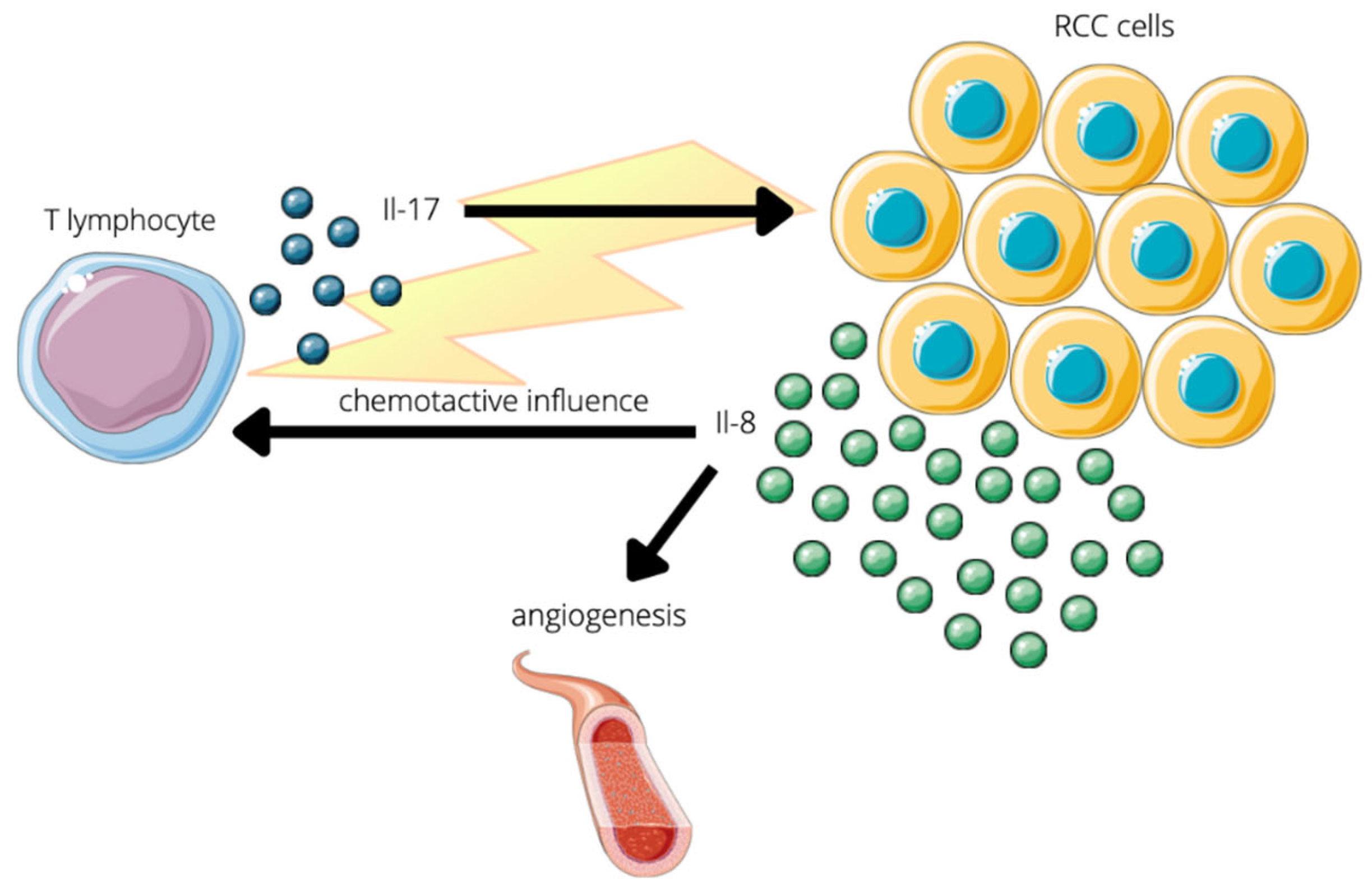
7.1. Protumor
7.2. Prometastatic
7.3. Antitumor
7.4. Antimetastatic
8. IL-17 in RCC Detection
9. RCC Treatment
10. Drugs Targeting IL-17 Axis
10.1. Secukinumab
10.2. Brodalumab
10.3. Ixekizumab
10.4. MSB0010841/ALX-0761
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, B.-S.; Park, Y.-J.; Chung, Y. Targeting IL-17 in Autoimmunity and Inflammation. Arch. Pharm. Res. 2016, 39, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Kuen, D.-S.; Kim, B.-S.; Chung, Y. IL-17-Producing Cells in Tumor Immunity: Friends or Foes? Immune Netw. 2020, 20, e6. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal Cell Carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Garfield, K.; LaGrange, C.A. Renal Cell Cancer; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- de Cássio Zequi, S.; Mourão, T.C.; de Oliveira, M.M.; Curado, M.P.; Gueglio, G.; da Costa, W.H.; Zuñiga, A.; Bengió, R.; Scorticati, C.; Rodriguez, F.; et al. Predictors of Survival Outcomes in Non-Metastatic Renal Cell Carcinoma in Latin America and Spain: A Multicentric Analysis. Kidney Cancer 2019, 3, 253–261. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 Immune Axis: From Mechanisms to Therapeutic Testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Rouvier, E.; Luciani, M.F.; Mattéi, M.G.; Denizot, F.; Golstein, P. CTLA-8, Cloned from an Activated T Cell, Bearing AU-Rich Messenger RNA Instability Sequences, and Homologous to a Herpesvirus Saimiri Gene. J. Immunol. 1993, 150, 5445–5456. [Google Scholar]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.-H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A Distinct Lineage of CD4 T Cells Regulates Tissue Inflammation by Producing Interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef]
- Murugaiyan, G.; Saha, B. Protumor vs Antitumor Functions of IL-17. J. Immunol. 2009, 183, 4169–4175. [Google Scholar] [CrossRef]
- Cua, D.J.; Tato, C.M. Innate IL-17-Producing Cells: The Sentinels of the Immune System. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional Specialization of Interleukin-17 Family Members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Ramani, K.; Garg, A.V.; Jawale, C.V.; Conti, H.R.; Whibley, N.; Jackson, E.K.; Shiva, S.S.; Horne, W.; Kolls, J.K.; Gaffen, S.L.; et al. The Kallikrein-Kinin System: A Novel Mediator of IL-17-Driven Anti-Candida Immunity in the Kidney. PLoS Pathog. 2016, 12, e1005952. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Gaffen, S.L. Structure-Function Relationships in the IL-17 Receptor: Implications for Signal Transduction and Therapy. Cytokine 2008, 41, 92–104. [Google Scholar] [CrossRef]
- Xu, S.; Cao, X. Interleukin-17 and Its Expanding Biological Functions. Cell. Mol. Immunol. 2010, 7, 164–174. [Google Scholar] [CrossRef]
- Brevi, A.; Cogrossi, L.L.; Grazia, G.; Masciovecchio, D.; Impellizzieri, D.; Lacanfora, L.; Grioni, M.; Bellone, M. Much More Than IL-17A: Cytokines of the IL-17 Family Between Microbiota and Cancer. Front. Immunol. 2020, 11, 565470. [Google Scholar] [CrossRef]
- Curtis, M.M.; Way, S.S. Interleukin-17 in Host Defence against Bacterial, Mycobacterial and Fungal Pathogens. Immunology 2009, 126, 177–185. [Google Scholar] [CrossRef]
- Krebs, C.F.; Schmidt, T.; Riedel, J.-H.; Panzer, U. T Helper Type 17 Cells in Immune-Mediated Glomerular Disease. Nat. Rev. Nephrol. 2017, 13, 647–659. [Google Scholar] [CrossRef]
- Paquissi, F.C.; Abensur, H. The Th17/IL-17 Axis and Kidney Diseases, with Focus on Lupus Nephritis. Front. Med. 2021, 8, 654912. [Google Scholar] [CrossRef]
- Peng, X.; Xiao, Z.; Zhang, J.; Li, Y.; Dong, Y.; Du, J. IL-17A Produced by Both Γδ T and Th17 Cells Promotes Renal Fibrosis via RANTES-Mediated Leukocyte Infiltration after Renal Obstruction. J. Pathol. 2015, 235, 79–89. [Google Scholar] [CrossRef]
- Stengel, B. Chronic Kidney Disease and Cancer: A Troubling Connection. J. Nephrol. 2010, 23, 253–262. [Google Scholar] [PubMed]
- Vitiello, G.A.; Miller, G. Targeting the Interleukin-17 Immune Axis for Cancer Immunotherapy. J. Exp. Med. 2019, 217, e20190456. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kang, H.; Fung, A.; Zhao, H.; Wang, T.; Ma, D. The Role of Interleukin 17 in Tumour Proliferation, Angiogenesis, and Metastasis. Mediat. Inflamm. 2014, 2014, 623759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, X.; Herjan, T.; Li, X. The Role of Interleukin-17 in Tumor Development and Progression. J. Exp. Med. 2020, 217, e20190297. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Liu, Z.; Zhang, J.; Jin, X. Myeloid-Derived Suppressor Cell Accumulation in Renal Cell Carcinoma Is Correlated with CCL2, IL-17 and IL-18 Expression in Blood and Tumors. Adv. Clin. Exp. Med. 2018, 27, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Numasaki, M.; Fukushi, J.; Ono, M.; Narula, S.K.; Zavodny, P.J.; Kudo, T.; Robbins, P.D.; Tahara, H.; Lotze, M.T. Interleukin-17 Promotes Angiogenesis and Tumor Growth. Blood 2003, 101, 2620–2627. [Google Scholar] [CrossRef]
- Benchetrit, F.; Ciree, A.; Vives, V.; Warnier, G.; Gey, A.; Sautès-Fridman, C.; Fossiez, F.; Haicheur, N.; Fridman, W.H.; Tartour, E. Interleukin-17 Inhibits Tumor Cell Growth by Means of a T-Cell-Dependent Mechanism. Blood 2002, 99, 2114–2121. [Google Scholar] [CrossRef]
- Shang, Z.-J.; Li, J.-R.; Li, Z.-B. Effects of Exogenous Nitric Oxide on Oral Squamous Cell Carcinoma: An in Vitro Study. J. Oral Maxillofac. Surg. 2002, 60, 901–905. [Google Scholar] [CrossRef]
- Benatar, T.; Cao, M.Y.; Lee, Y.; Lightfoot, J.; Feng, N.; Gu, X.; Lee, V.; Jin, H.; Wang, M.; Wright, J.A.; et al. IL-17E, a Proinflammatory Cytokine, Has Antitumor Efficacy against Several Tumor Types In Vivo. Cancer Immunol. Immunother. 2010, 59, 805–817. [Google Scholar] [CrossRef]
- Peng, Z.; Hu, Y.; Ren, J.; Yu, N.; Li, Z.; Xu, Z. Circulating Th22 Cells, as Well as Th17 Cells, Are Elevated in Patients with Renal Cell Carcinoma. Int. J. Med. Sci. 2021, 18, 99–108. [Google Scholar] [CrossRef]
- Veldhoen, M.; Hirota, K.; Westendorf, A.M.; Buer, J.; Dumoutier, L.; Renauld, J.-C.; Stockinger, B. The Aryl Hydrocarbon Receptor Links TH17-Cell-Mediated Autoimmunity to Environmental Toxins. Nature 2008, 453, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Inozume, T.; Hanada, K.; Wang, Q.J.; Yang, J.C. IL-17 Secreted by Tumor Reactive T Cells Induces IL-8 Release by Human Renal Cancer Cells. J. Immunother. 2009, 32, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Witowski, J.; Książek, K.; Jörres, A. Interleukin-17: A Mediator of Inflammatory Responses. Cell. Mol. Life Sci. 2004, 61, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Kehlen, A.; Thiele, K.; Riemann, D.; Rainov, N.; Langner, J. Interleukin-17 Stimulates the Expression of IkappaB Alpha MRNA and the Secretion of IL-6 and IL-8 in Glioblastoma Cell Lines. J. Neuroimmunol. 1999, 101, 1–6. [Google Scholar] [CrossRef]
- Yazawa, T.; Shibata, M.; Gonda, K.; Machida, T.; Suzuki, S.; Kenjo, A.; Nakamura, I.; Tsuchiya, T.; Koyama, Y.; Sakurai, K.; et al. Increased IL-17 Production Correlates with Immunosuppression Involving Myeloid-Derived Suppressor Cells and Nutritional Impairment in Patients with Various Gastrointestinal Cancers. Mol. Clin. Oncol. 2013, 1, 675–679. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The Role of Myeloid Cells in the Promotion of Tumour Angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631. [Google Scholar] [CrossRef]
- Cochaud, S.; Giustiniani, J.; Thomas, C.; Laprevotte, E.; Garbar, C.; Savoye, A.-M.; Curé, H.; Mascaux, C.; Alberici, G.; Bonnefoy, N.; et al. IL-17A Is Produced by Breast Cancer TILs and Promotes Chemoresistance and Proliferation through ERK1/2. Sci. Rep. 2013, 3, 3456. [Google Scholar] [CrossRef]
- Do Thi, V.A.; Park, S.M.; Lee, H.; Kim, Y.S. The Membrane-Bound Form of IL-17A Promotes the Growth and Tumorigenicity of Colon Cancer Cells. Mol. Cells 2016, 39, 536–542. [Google Scholar] [CrossRef]
- Ngiow, S.F.; Smyth, M.J.; Teng, M.W.L. Does IL-17 Suppress Tumor Growth? Blood 2010, 115, 2554–2557. [Google Scholar] [CrossRef][Green Version]
- Numasaki, M.; Lotze, M.T.; Sasaki, H. Interleukin-17 Augments Tumor Necrosis Factor-Alpha-Induced Elaboration of Proangiogenic Factors from Fibroblasts. Immunol. Lett. 2004, 93, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, L.; Ren, T.; Xu, L.; Wen, Z. IL-17A/IL-17RA Interaction Promoted Metastasis of Osteosarcoma Cells. Cancer Biol. Ther. 2013, 14, 155–163. [Google Scholar] [CrossRef]
- Sakurai, T.; Yoshiga, D.; Ariyoshi, W.; Okinaga, T.; Kiyomiya, H.; Furuta, J.; Yoshioka, I.; Tominaga, K.; Nishihara, T. Essential Role of Mitogen-Activated Protein Kinases in IL-17A-Induced MMP-3 Expression in Human Synovial Sarcoma Cells. BMC Res. Notes 2016, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Honorati, M.C.; Cattini, L.; Facchini, A. Possible Prognostic Role of IL-17R in Osteosarcoma. J. Cancer Res. Clin. Oncol. 2007, 133, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, J.; Sharma, M.; Sreenivas, V.; Kumar, R.; Gamnagatti, S.; Khan, S.A.; Rastogi, S.; Malhotra, A.; Bakhshi, S. VEGF Expression as a Prognostic Marker in Osteosarcoma. Pediatr. Blood Cancer 2009, 53, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kortylewski, M.; Pardoll, D. Crosstalk between Cancer and Immune Cells: Role of STAT3 in the Tumour Microenvironment. Nat. Rev. Immunol. 2007, 7, 41–51. [Google Scholar] [CrossRef]
- Lavecchia, A.; Di Giovanni, C.; Novellino, E. STAT-3 Inhibitors: State of the Art and New Horizons for Cancer Treatment. Curr. Med. Chem. 2011, 18, 2359–2375. [Google Scholar] [CrossRef]
- Ren, T.; Wen, Z.-K.; Liu, Z.-M.; Liang, Y.-J.; Guo, Z.-L.; Xu, L. Functional Expression of TLR9 Is Associated to the Metastatic Potential of Human Lung Cancer Cell: Functional Active Role of TLR9 on Tumor Metastasis. Cancer Biol. Ther. 2007, 6, 1704–1709. [Google Scholar] [CrossRef]
- Woessner, J.F.J. Matrix Metalloproteinases and Their Inhibitors in Connective Tissue Remodeling. FASEB J. 1991, 5, 2145–2154. [Google Scholar] [CrossRef]
- Ye, S.; Eriksson, P.; Hamsten, A.; Kurkinen, M.; Humphries, S.E.; Henney, A.M. Progression of Coronary Atherosclerosis Is Associated with a Common Genetic Variant of the Human Stromelysin-1 Promoter Which Results in Reduced Gene Expression. J. Biol. Chem. 1996, 271, 13055–13060. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Jia, P.; Li, X.; Pei, G.; Wang, C.; Fang, X.; Zhao, Z.; Cai, Z.; Yi, X.; et al. Clonal Architectures Predict Clinical Outcome in Clear Cell Renal Cell Carcinoma. Nat. Commun. 2019, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. Immunosuppression Associated with Chronic Inflammation in the Tumor Microenvironment. Carcinogenesis 2015, 36, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xia, J.; Liang, X.; Pan, K.; Wang, W.; Lv, L.; Zhao, J.; Wang, Q.; Li, Y.; Chen, S.; et al. Intratumoral Expression of IL-17 and Its Prognostic Role in Gastric Adenocarcinoma Patients. Int. J. Biol. Sci. 2011, 7, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Punt, S.; van Vliet, M.E.; Spaans, V.M.; de Kroon, C.D.; Fleuren, G.J.; Gorter, A.; Jordanova, E.S. FoxP3(+) and IL-17(+) Cells Are Correlated with Improved Prognosis in Cervical Adenocarcinoma. Cancer Immunol. Immunother. 2015, 64, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Javdan, M.; Feger, F.K.; Chiu, P.Y.; Sison, C.; Damle, R.N.; Bhuiya, T.A.; Sen, F.; Abruzzo, L.V.; Burger, J.A.; et al. Th17 and Non-Th17 Interleukin-17-Expressing Cells in Chronic Lymphocytic Leukemia: Delineation, Distribution, and Clinical Relevance. Haematologica 2012, 97, 599–607. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Ugai, S.-I.; Shimozato, O.; Yu, L.; Kawamura, K.; Yamamoto, H.; Yamaguchi, T.; Saisho, H.; Tagawa, M. Induction of Systemic Immunity by Expression of Interleukin-23 in Murine Colon Carcinoma Cells. Int. J. Cancer 2003, 105, 820–824. [Google Scholar] [CrossRef]
- Shimozato, O.; Ugai, S.; Chiyo, M.; Takenobu, H.; Nagakawa, H.; Wada, A.; Kawamura, K.; Yamamoto, H.; Tagawa, M. The Secreted Form of the P40 Subunit of Interleukin (IL)-12 Inhibits IL-23 Functions and Abrogates IL-23-Mediated Antitumour Effects. Immunology 2006, 117, 22–28. [Google Scholar] [CrossRef]
- Shan, B.E.; Hao, J.S.; Li, Q.X.; Tagawa, M. Antitumor Activity and Immune Enhancement of Murine Interleukin-23 Expressed in Murine Colon Carcinoma Cells. Cell. Mol. Immunol. 2006, 3, 47–52. [Google Scholar]
- Lo, C.-H.; Lee, S.-C.; Wu, P.-Y.; Pan, W.-Y.; Su, J.; Cheng, C.-W.; Roffler, S.R.; Chiang, B.-L.; Lee, C.-N.; Wu, C.-W.; et al. Antitumor and Antimetastatic Activity of IL-23. J. Immunol. 2003, 171, 600–607. [Google Scholar] [CrossRef]
- Kapoor, J.; Claps, F.; Mir, M.; Ischia, J. Promising Biomarkers in Renal Cell Carcinoma. Société Int. D’urologie J. 2021, 2, 43–52. [Google Scholar] [CrossRef]
- Claps, F.; Mir, M.C. Novel Expanding Renal Cell Carcinoma Biomarkers. Société Int. D’urologie J. 2021, 2, 32–42. [Google Scholar] [CrossRef]
- Chowdhury, N.; Drake, C.G. Kidney Cancer: An Overview of Current Therapeutic Approaches. Urol. Clin. N. Am. 2020, 47, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.B.; Georgiades, C.S. Kidney Cancer. Cancer J. 2016, 22, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Barata, P.C.; Rini, B.I. Treatment of Renal Cell Carcinoma: Current Status and Future Directions. CA Cancer J. Clin. 2017, 67, 507–524. [Google Scholar] [CrossRef]
- Krabbe, L.-M.; Bagrodia, A.; Margulis, V.; Wood, C.G. Surgical Management of Renal Cell Carcinoma. Semin. Interv. Radiol. 2014, 31, 27–32. [Google Scholar] [CrossRef]
- Mickisch, G.H.; Garin, A.; van Poppel, H.; de Prijck, L.; Sylvester, R. Radical Nephrectomy plus Interferon-Alfa-Based Immunotherapy Compared with Interferon Alfa Alone in Metastatic Renal-Cell Carcinoma: A Randomised Trial. Lancet 2001, 358, 966–970. [Google Scholar] [CrossRef]
- Marco, C.; Andrea, M.; Alberto, L.; Lorenzo, M.; Sergio, S. Simple Enucleation for the Treatment of Renal Cell Carcinoma Between 4 and 7 Cm in Greatest Dimension: Progression and Long-Term Survival. J. Urol. 2006, 175, 2022–2026. [Google Scholar] [CrossRef]
- Mari, A.; Di Maida, F.; Tellini, R.; Campi, R.; Sforza, S.; Cocci, A.; Siena, G.; Vittori, G.; Tuccio, A.; Masieri, L.; et al. Oncologic Outcomes in Patients Treated with Endoscopic Robot Assisted Simple Enucleation (ERASE) for Renal Cell Carcinoma: Results from a Tertiary Referral Center. Eur. J. Surg. Oncol. 2019, 45, 1977–1982. [Google Scholar] [CrossRef]
- Abel, E.J.; Margulis, V.; Bauman, T.M.; Karam, J.A.; Christensen, W.P.; Krabbe, L.-M.; Haddad, A.; Golla, V.; Wood, C.G. Risk Factors for Recurrence after Surgery in Non-Metastatic RCC with Thrombus: A Contemporary Multicentre Analysis. BJU Int. 2016, 117, E87–E94. [Google Scholar] [CrossRef]
- Campi, R.; Tellini, R.; Sessa, F.; Mari, A.; Cocci, A.; Greco, F.; Crestani, A.; Gomez Rivas, J.; Fiori, C.; Lapini, A.; et al. Techniques and Outcomes of Minimally-Invasive Surgery for Nonmetastatic Renal Cell Carcinoma with Inferior Vena Cava Thrombosis: A Systematic Review of the Literature. Minerva Urol. Nefrol. 2019, 71, 339–358. [Google Scholar] [CrossRef]
- Mennitto, A.; Verzoni, E.; Grassi, P.; Ratta, R.; Fucà, G.; Procopio, G. Multimodal Treatment of Advanced Renal Cancer in 2017. Expert Rev. Clin. Pharmacol. 2017, 10, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- El Rassy, E.; Khoury Abboud, R.M.; Ibrahim, N.; Assi, T.; Aoun, F.; Kattan, J. The Current State of Immune Checkpoint Inhibitors in the First-Line Treatment of Renal Cancer. Immunotherapy 2018, 10, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Sieper, J.; Braun, J.; Baraliakos, X.; Dougados, M.; Emery, P.; Deodhar, A.; Porter, B.; Martin, R.; Andersson, M.; et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 2015, 373, 2534–2548. [Google Scholar] [CrossRef]
- Aboobacker, S.; Kurn, H.; Al Aboud, A.M. Secukinumab; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bilal, J.; Berlinberg, A.; Riaz, I.B.; Faridi, W.; Bhattacharjee, S.; Ortega, G.; Murad, M.H.; Wang, Z.; Prokop, L.J.; Alhifany, A.A.; et al. Risk of Infections and Cancer in Patients with Rheumatologic Diseases Receiving Interleukin Inhibitors: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2019, 2, e1913102. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Soliman, A.M.; Betts, K.A.; Wang, Y.; Gao, Y.; Stakias, V.; Puig, L. Long-Term Benefit–Risk Profiles of Treatments for Moderate-to-Severe Plaque Psoriasis: A Network Meta-Analysis. Dermatol. Ther. 2022, 12, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, A.C.; Warren, R.B. Brodalumab in Psoriasis: Evidence to Date and Clinical Potential. Drugs Context 2019, 8, 212570. [Google Scholar] [CrossRef]
- Galluzzo, M.; Caldarola, G.; De Simone, C.; Bernardini, N.; Moretta, G.; Pallotta, S.; Botti, E.; Campione, E.; Pirro, F.; Potenza, C.; et al. Use of Brodalumab for the Treatment of Chronic Plaque Psoriasis: A One-Year Real-Life Study in the Lazio Region, Italy. Expert Opin. Biol. Ther. 2021, 21, 1299–1310. [Google Scholar] [CrossRef]
- Gottlieb, A.; Lebwohl, M.; Liu, C.; Israel, R.J.; Jacobson, A. Malignancy Rates in Brodalumab Clinical Studies for Psoriasis. Am. J. Clin. Dermatol. 2020, 21, 421–430. [Google Scholar] [CrossRef]
- Huang, J.-X.; Lee, Y.-H.; Wei, J.C.-C. Ixekizumab for the Treatment of Ankylosing Spondylitis. Expert Rev. Clin. Immunol. 2020, 16, 745–750. [Google Scholar] [CrossRef]
- Craig, S.; Warren, R.B. Ixekizumab for the Treatment of Psoriasis: Up to Date. Expert Opin. Biol. Ther. 2020, 20, 549–557. [Google Scholar] [CrossRef]
- Porcar Saura, S.; Martínez Casimiro, L.; García Vázquez, A.; Pons Benavent, M.; Guillén-Climent, S.; Montesinos Villaescusa, E. Successful Outcome of Psoriasis in a Laryngeal Cancer Patient Treated with Ixekizumab, a Possible Beneficial Treatment for Both Pathologies. Dermatol. Online J. 2021, 27, 21. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, M.; Zhang, H.; Sun, Y.; Tao, Y.; Li, H.; Zhang, J.; Li, Y.; Yang, J. IL-17 Affects the Progression, Metastasis, and Recurrence of Laryngeal Cancer via the Inhibition of Apoptosis through Activation of the PI3K/AKT/FAS/FASL Pathways. J. Immunol. Res. 2020, 2020, 2953191. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; De Simone, C.; Fossati, B.; Peris, K. Emerging Treatment Options for the Treatment of Moderate to Severe Plaque Psoriasis and Psoriatic Arthritis: Evaluating Bimekizumab and Its Therapeutic Potential. Psoriasis 2019, 9, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Jovčevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef]

| Drug | Mechanism of Action | Application |
|---|---|---|
| Secukinumab | Anti-interleukin-17A IgG1 monoclonal antibody | Severe psoriasis, ankylosing spondylitis, SLE, RA |
| Brodalumab | Anti-interleukin-17A receptor human IgG2 antibody | Chronic plaque psoriasis |
| Ixekizumab | Anti-interleukin-17A humanized IgG4 antibody | Ankylosing spondylitis, plaque psoriasis |
| MSB0010841/ALX-0761 | Anti-interleukin-17A and IL-17F trivalent nanobody | Clinical trials in psoriasis and refractory/relapsed B-cell lymphoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarocki, M.; Karska, J.; Kowalski, S.; Kiełb, P.; Nowak, Ł.; Krajewski, W.; Saczko, J.; Kulbacka, J.; Szydełko, T.; Małkiewicz, B. Interleukin 17 and Its Involvement in Renal Cell Carcinoma. J. Clin. Med. 2022, 11, 4973. https://doi.org/10.3390/jcm11174973
Jarocki M, Karska J, Kowalski S, Kiełb P, Nowak Ł, Krajewski W, Saczko J, Kulbacka J, Szydełko T, Małkiewicz B. Interleukin 17 and Its Involvement in Renal Cell Carcinoma. Journal of Clinical Medicine. 2022; 11(17):4973. https://doi.org/10.3390/jcm11174973
Chicago/Turabian StyleJarocki, Michał, Julia Karska, Szymon Kowalski, Paweł Kiełb, Łukasz Nowak, Wojciech Krajewski, Jolanta Saczko, Julita Kulbacka, Tomasz Szydełko, and Bartosz Małkiewicz. 2022. "Interleukin 17 and Its Involvement in Renal Cell Carcinoma" Journal of Clinical Medicine 11, no. 17: 4973. https://doi.org/10.3390/jcm11174973
APA StyleJarocki, M., Karska, J., Kowalski, S., Kiełb, P., Nowak, Ł., Krajewski, W., Saczko, J., Kulbacka, J., Szydełko, T., & Małkiewicz, B. (2022). Interleukin 17 and Its Involvement in Renal Cell Carcinoma. Journal of Clinical Medicine, 11(17), 4973. https://doi.org/10.3390/jcm11174973










