Empiric Anticoagulation Therapy in Hospitalized COVID-19 Patients: An Evaluation of Bleeding Risk Scores Performances in Predicting Bleeding Events
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Bleeding Risk Scores and Outcome Measures
2.3. Statistical Analysis
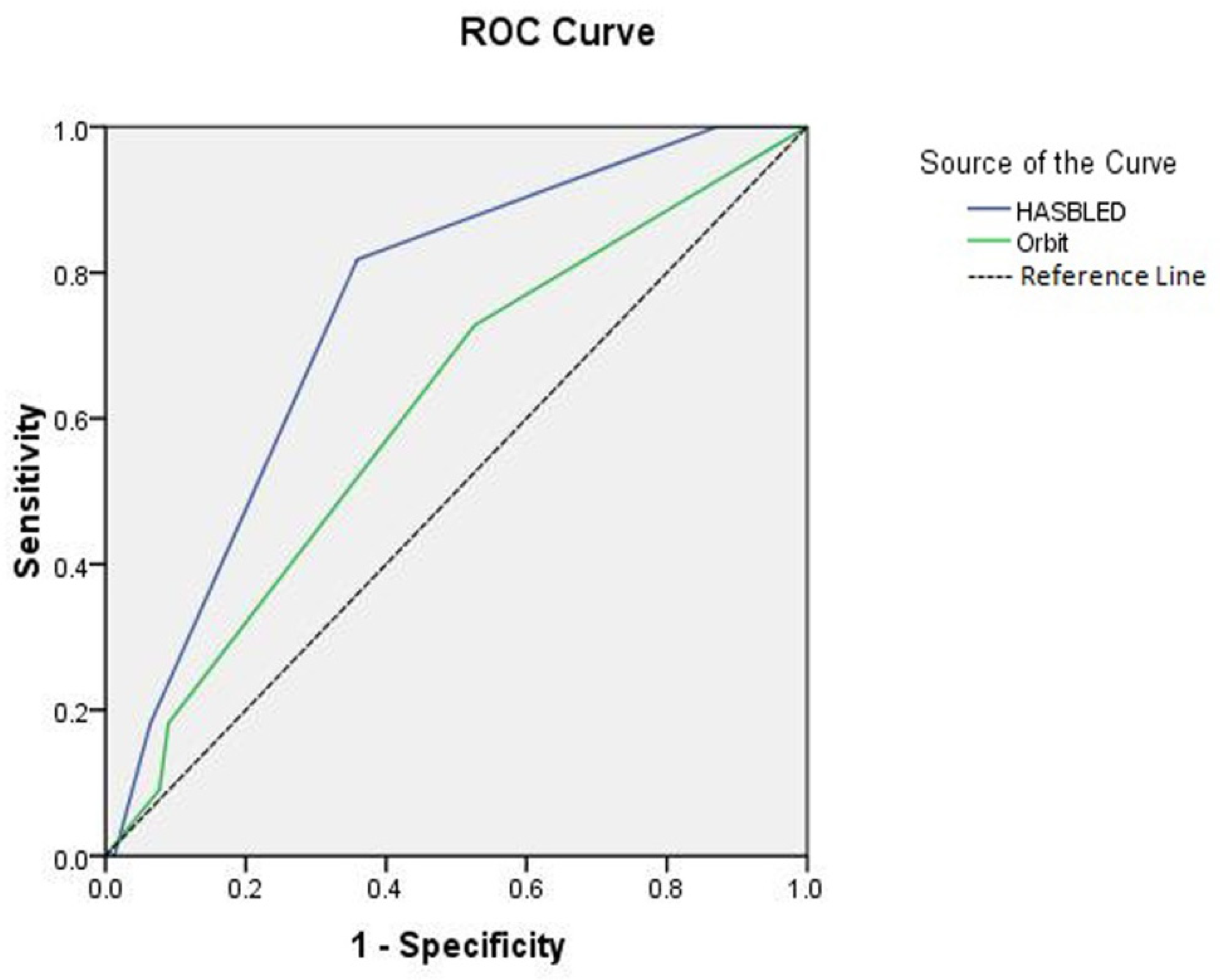
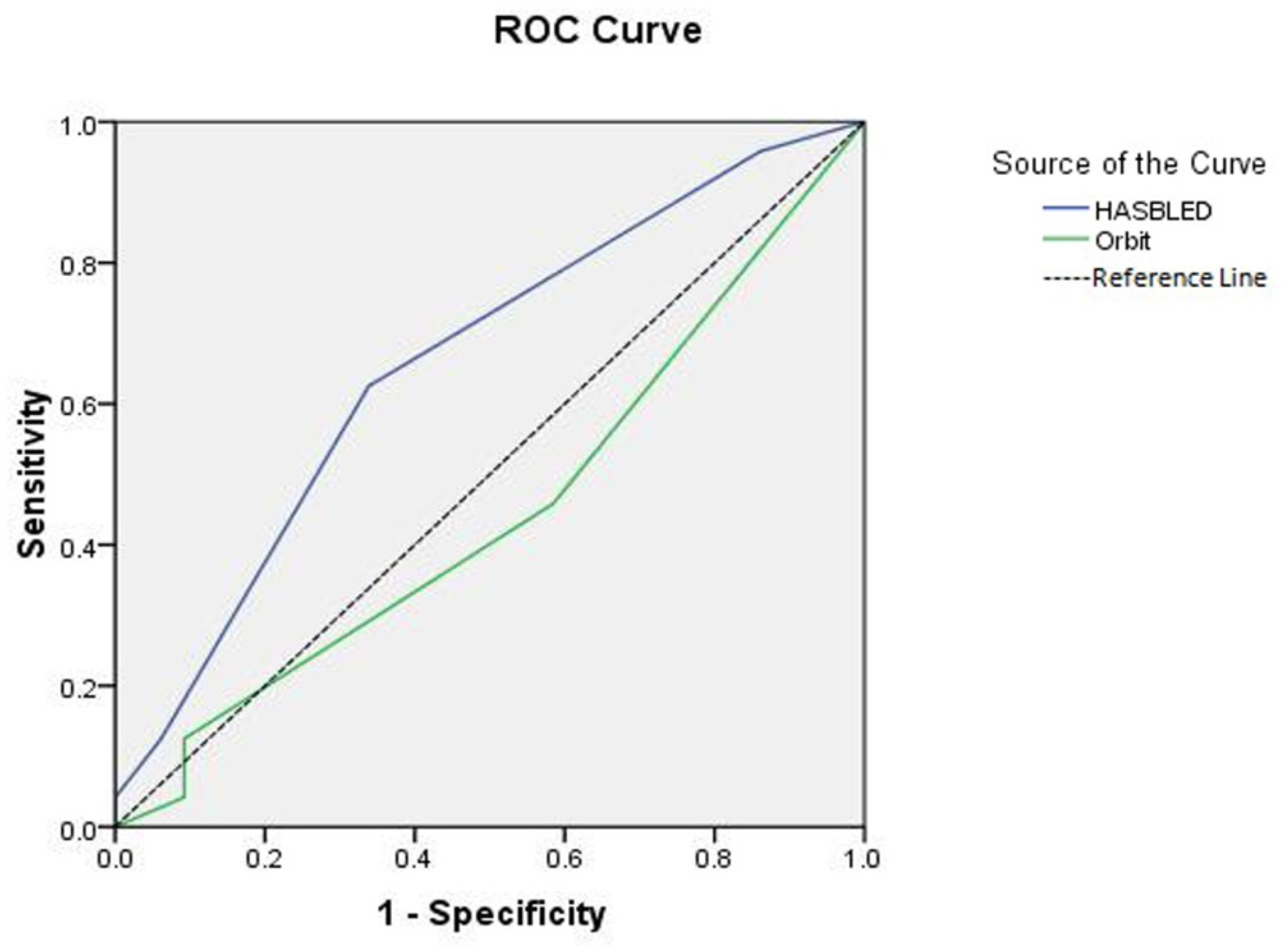
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lang, C.N.; Dettinger, J.S.; Berchtold-Herz, M.; Utzolino, S.; Bemtgen, X.; Zotzmann, V.; Schmid, B.; Biever, P.M.; Bode, C.; Müller-Peltzer, K.; et al. Intracerebral Hemorrhage in COVID-19 Patients with Pulmonary Failure: A Propensity Score-Matched Registry Study. Neurocritical Care 2021, 34, 739–747. [Google Scholar] [CrossRef]
- Elmelhat, A.; Elbourai, E.; Dewedar, H.; Elgergawi, T.; Alkhanbouli, M.; Ahmed, S.; Malik, Z.; Nugud, A.; Mohammed, S.; Mohammad, H.; et al. Comparison between Prophylactic versus Therapeutic Doses of Low-Molecular-Weight Heparin in Severely Ill Coronavirus Disease 2019 Patients in Relation to Disease Progression and Outcome. Dubai Med. J. 2020, 3, 162–169. [Google Scholar] [CrossRef]
- Barton, L.M.; Duval, E.J.; Stroberg, E.; Ghosh, S.; Mukhopadhyay, S. COVID-19 autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020, 153, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Z.; Li, C.; Chen, Y.; Gerotziafas, G.; Zhang, Z.; Wan, J.; Liu, P.; Elalamy, I.; Wang, C.; On behalf of the Prevention Treatment of VTE Associated with COVID-19 Infection Consensus Statement Group; et al. Prevention and Treatment of Venous Thromboembolism Associated with Coronavirus Disease 2019 Infection: A Consensus Statement before Guidelines. Thromb. Haemost. 2020, 120, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.D.; Moore, H.B.; Yaffe, M.B.; Moore, E.E. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: A comment. J. Thromb. Haemost. 2020, 18, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Musoke, N.; Lo, K.B.; Albano, J.; Peterson, E.; Bhargav, R.; Gul, F.; DeJoy, R.; Salacup, G.; Pelayo, J.; Tipparaju, P.; et al. Anticoagulation and bleeding risk in patients with COVID-19. Thromb. Res. 2020, 196, 227–230. [Google Scholar] [CrossRef]
- Hoogenboom, W.S.; Lu, J.Q.; Musheyev, B.; Borg, L.; Janowicz, R.; Pamlayne, S.; Hou, W.; Duong, T.Q. Prophylactic versus therapeutic dose anticoagulation effects on survival among critically ill patients with COVID-19. PLoS ONE 2022, 17, e0262811. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.G.M.; Lip, G.Y.H. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibril-lation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Senoo, K.; Lip, G.Y. Predictive abilities of the HAS-BLED and ORBIT bleeding risk scores in non-warfarin anticoagulated atrial fibrillation patients: An ancillary analysis from the AMADEUS trial. Int. J. Cardiol. 2016, 221, 379–382. [Google Scholar] [CrossRef]
- Lip, G.Y.; Skjøth, F.; Nielsen, P.B.; Kjældgaard, J.N.; Larsen, T.B. The HAS-BLED, ATRIA, and ORBIT bleeding scores in atrial fibrillation patients using non-vitamin K an-tagonist oral anticoagulants. Am. J. Med. 2018, 131, 574.e13–574.e27. [Google Scholar] [CrossRef] [Green Version]
- Senoo, K.; Proietti, M.; Lane, D.A.; Lip, G.Y. Evaluation of the HAS-BLED, ATRIA, and ORBIT Bleeding Risk Scores in Patients with Atrial Fibrillation Taking Warfarin. Am. J. Med. 2016, 129, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulman, S.; Kearon, C.; The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Lillicrap, D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J. Thromb. Haemost. 2020, 18, 786–787. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef]
- Yin, S.; Huang, M.; Li, D.; Tang, N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis 2021, 51, 1107–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Mumoli, N.; Conte, G.; Cei, M.; Vitale, J.; Capra, R.; Rotiroti, G.; Porta, C.; Monolo, D.; Colombo, A.; Mazzone, A.; et al. In-hospital fatality and venous thromboembolism during the first and second COVID-19 waves at a center opting for standard-dose thromboprophylaxis. Thromb. Res. 2021, 203, 82–84. [Google Scholar] [CrossRef]
- Peterson, E.; Lo, K.B.; DeJoy, R.; Salacup, G.; Pelayo, J.; Bhargav, R.; Gul, F.; Albano, J.; Azmaiparashvili, Z.; Amanullah, A.; et al. The relationship between coronary artery disease and clinical outcomes in COVID-19: A single-center ret-rospective analysis. Coron. Artery Dis. 2020, 32, 367–371. [Google Scholar] [CrossRef]
- Schiavone, M.; Gasperetti, A.; Mancone, M.; Kaplan, A.V.; Gobbi, C.; Mascioli, G.; Busana, M.; Saguner, A.M.; Mitacchione, G.; Giacomelli, A.; et al. Redefining the Prognostic Value of High-Sensitivity Troponin in COVID-19 Patients: The Importance of Concomitant Coronary Artery Disease. J. Clin. Med. 2020, 9, 3263. [Google Scholar] [CrossRef]
- Barman, H.A.; Atici, A.; Sahin, I.; Alici, G.; Tekin, E.A.; Baycan, Ö.F.; Ozturk, F.; Oflar, E.; Tugrul, S.; Yavuz, M.B.; et al. Prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron. Artery Dis. 2021, 32, 359–366. [Google Scholar] [CrossRef]
- Schiavone, M.; Gasperetti, A.; Mancone, M.; Curnis, A.; Mascioli, G.; Mitacchione, G.; Busana, M.; Sabato, F.; Gobbi, C.; Antinori, S.; et al. Oral anticoagulation and clinical outcomes in COVID-19: An Italian multicenter experience. Int. J. Cardiol. 2021, 323, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Lippi, G.; Favaloro, E.J. D-dimer is associated with severity of coronavirus disease 2019: A pooled analysis. Thromb. Haemost. 2020, 120, 876–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busana, M.; Gasperetti, A.; Giosa, L.; Forleo, G.B.; Schiavone, M.; Mitacchione, G.; Bonino, C.; Villa, P.; Galli, M.; Tondo, C.; et al. Prevalence and outcome of silent hypoxemia in COVID-19. Minerva Anestesiol. 2021, 87, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Schuliga, M. The inflammatory actions of coagulant and fibrinolytic proteases in disease. Mediat. Inflamm. 2015, 2015, 437695. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Madoiwa, S. Recent advances in disseminated intravascular coagulation: Endothelial cells and fibrinolysis in sepsis-induced DIC. J. Intensive Care 2015, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Jiang, L.; Chen, T.; Wang, Y.; Zhang, B.; Hong, Y.; Wang, J.; Yan, F. Continuously available ratio of SpO2/FiO2 serves as a noninvasive prognostic marker for intensive care patients with COVID-19. Respir. Res. 2020, 21, 194. [Google Scholar] [CrossRef]
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.J.; Glicksberg, B.S.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124. [Google Scholar] [CrossRef]
- Lemos, A.C.B.; do Espirito Santo, D.A.; Salvetti, M.C.; Gilio, R.N.; Agra, L.B.; Pazin-Filho, A.; Miranda, C.H. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb. Res. 2020, 196, 359–366. [Google Scholar] [CrossRef] [PubMed]
- REMAP-CAP Investigators. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 385, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhatriwalla, A.K.; Amin, A.P.; Kennedy, K.F.; House, J.A.; Cohen, D.J.; Rao, S.V.; Messenger, J.C.; Marso, S.P.; National Cardiovascular Data Registry. Association between bleeding events and in-hospital mortality after percutaneous coronary intervention. JAMA 2013, 309, 1022–1029. [Google Scholar] [CrossRef] [Green Version]
- Bassand, J.-P.; Virdone, S.; Badoz, M.; Verheugt, F.W.A.; Camm, A.J.; Cools, F.; Fox, K.A.A.; Goldhaber, S.Z.; Goto, S.; Haas, S.; et al. Bleeding and related mortality with NOACs and VKAs in newly diagnosed atrial fibrillation: Results from the GARFIELD-AF registry. Blood Adv. 2021, 5, 1081–1091. [Google Scholar] [CrossRef]
- Lip, G.; Lane, D.A. Bleeding risk assessment in atrial fibrillation: Observations on the use and misuse of bleeding risk scores. J. Thromb. Haemost. 2016, 14, 1711–1714. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Total | Prophylactic AC | Therapeutic AC | p-Value |
|---|---|---|---|---|
| n = 921 | n = 446 | n = 475 | ||
| Age (mean ± SD) | 58.1 ± 13.2 | 57.8 ± 13.1 | 58.5 ± 13.4 | 0.441 |
| ≤60 years, n (%) | 489 (53.1) | 247 (55.4) | 242 (50.9) | |
| >60 years, n (%) | 432 (46.9) | 199 (44.6) | 233 (49.1) | |
| Gender, n (%) | ||||
| Male | 440 (47.8) | 201 (45.1) | 239 (50.3) | 0.112 |
| Female | 481 (52.2) | 245 (54.9) | 236 (49.7) | |
| Body weight | 70.47 ± 12.82 | 69.64 ± 11.67 | 71.25 ±13.79 | 0.057 |
| Comorbidities, n (%) | ||||
| Diabetes | 127 (13.8) | 54 (12.1) | 73 (15.4) | 0.091 |
| Hypertension | 339 (36.8) | 161 (36.1) | 187 (39.4) | 0.173 |
| Chronic kidney disease | 81 (9.12) | 32 (7.2) | 49 (10.3) | 0.052 |
| Chronic liver disease | 79 (8.6) | 35 (7.8) | 44 (9.3) | 0.223 |
| Congestive heart failure | 56 (6.1) | 22 (4.9) | 34 (7.16) | 0.082 |
| Cancer | 24 (2.6) | 13 (2.9) | 11 (2.3) | 0.283 |
| Concurrent medications, n (%) | ||||
| Antiplatelet | 148 (16.1) | 71 (15.9) | 77 (16.2) | 0.451 |
| NSAIDs | 89 (9.7) | 38 (8.5) | 51 (10.7) | 0.129 |
| Systemic steroid therapy | 36 (3.9) | 14 (3.14) | 22 (4.63) | 0.119 |
| Laboratory measurements (mean ± SD) Hemoglobin, g/dL C-reactive protein, mg/dL d-dimer, ng/mL Platelet count, 109/L | ||||
| 11.78 ± 5.49 | 12.29 ± 5.73 | 11.32 ± 5.21 | 0.007 * | |
| 91.4 ± 39.6 | 66.1 ± 24.8 | 115.2 ± 36.1 | <0.001 * | |
| 773.1 ± 522.7 | 360 ± 142.6 | 1161 ± 452.1 | <0.001 * | |
| 251.03 ± 77.5 | 254.7 ± 84.3 | 247.6 ± 70.5 | 0.165 | |
| Risk scores | ||||
| HAS-BLED risk score (mean ± SD) | 2.53 ± 0.93 | 2.49 ± 0.97 | 2.56 ± 0.89 | 0.253 |
| ORBIT risk score (mean ± SD) | 2.26 ± 1.29 | 2.18 ± 1.3 | 2.33 ± 1.27 | 0.077 |
| Clinical outcomes | ||||
| Invasive mechanical ventilation, n (%) | 79 (8.6) | 32 (7.17) | 47 (9.89) | 0.158 |
| Duration of mechanical ventilation, days median (IQR) | 5.5 (5–8) | 5 (4–6) | 8 (5–10) | 0.011 * |
| Major bleeding events, n (%) | 31 (3.4) | 12 (2.7) | 19 (4) | 0.137 |
| Clinically relevant non-major bleeding, n (%) | 39 (4.2) | 13 (2.9) | 26 (5.5) | 0.025 * |
| Duration of hospital stay, days | 13.35 ± 3.9 | 11.4 ± 3.3 | 15.2 ± 3.7 | <0.001 * |
| All-cause mortality, n (%) | 85 (9.2) | 34 (7.6) | 51 (10.7) | 0.052 |
| Risk Scores | Total | Clinically Relevant Non-Major Bleeding | Major Bleeding | All-Cause Mortality |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| HAS-BLED | ||||
| Low (0–1) | 204 (22.15) | 10 (1.1) | 6 (0.65) | 17 (1.8) |
| Moderate (2) | 533 (57.87) | 12 (1.3) | 10 (1.1) | 28 (3.04) |
| High (≥3) | 184 (19.98) | 17 (1.8) | 15 (1.6) | 40 (4.3) |
| ORBIT | ||||
| Low (0–2) | 439 (47.66) | 14 (1.5) | 8 (0.86) | 29 (3.1) |
| Moderate (3) | 388 (42.13) | 13 (1.4) | 12 (1.3) | 25 (2.7) |
| High (≥4) | 94 (10.21) | 12 (1.3) | 11 (1.2) | 31 (3.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, M.A.; Ahmed, A.; Alanazi, A.S.; Osama, H. Empiric Anticoagulation Therapy in Hospitalized COVID-19 Patients: An Evaluation of Bleeding Risk Scores Performances in Predicting Bleeding Events. J. Clin. Med. 2022, 11, 4965. https://doi.org/10.3390/jcm11174965
Abdelrahman MA, Ahmed A, Alanazi AS, Osama H. Empiric Anticoagulation Therapy in Hospitalized COVID-19 Patients: An Evaluation of Bleeding Risk Scores Performances in Predicting Bleeding Events. Journal of Clinical Medicine. 2022; 11(17):4965. https://doi.org/10.3390/jcm11174965
Chicago/Turabian StyleAbdelrahman, Mona A., Aya Ahmed, Abdullah S. Alanazi, and Hasnaa Osama. 2022. "Empiric Anticoagulation Therapy in Hospitalized COVID-19 Patients: An Evaluation of Bleeding Risk Scores Performances in Predicting Bleeding Events" Journal of Clinical Medicine 11, no. 17: 4965. https://doi.org/10.3390/jcm11174965
APA StyleAbdelrahman, M. A., Ahmed, A., Alanazi, A. S., & Osama, H. (2022). Empiric Anticoagulation Therapy in Hospitalized COVID-19 Patients: An Evaluation of Bleeding Risk Scores Performances in Predicting Bleeding Events. Journal of Clinical Medicine, 11(17), 4965. https://doi.org/10.3390/jcm11174965






