Utility of Initial Arterial Blood Gas in Neuromuscular versus Non-Neuromuscular Acute Respiratory Failure in Intensive Care Unit Patients
Abstract
:1. Introduction
2. Methods
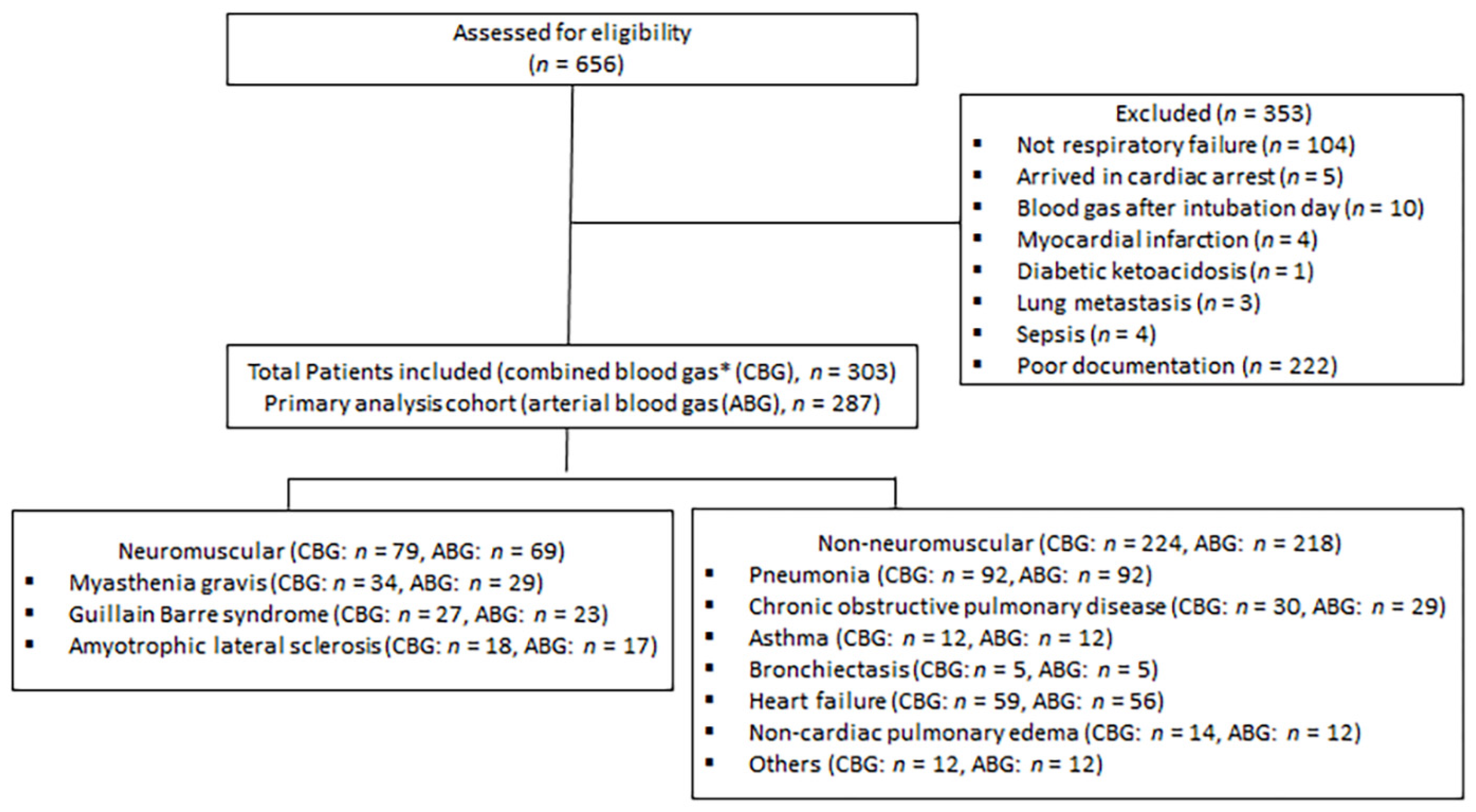
2.1. Study Design and Participants
2.2. Study Groups and Definition of Variables
- (A)
- Guillain–Barré syndrome (GBS): Patients presenting with acute-onset generalized weakness and areflexia that reaches the maximum within four weeks; objective evidence of a diagnosis is from an electrodiagnostic test and/or cerebrospinal fluid.
- (B)
- Myasthenia gravis (MG): this diagnosis is based on clinical presentation with objective evidence of diagnosis either through positive serology (acetylcholine receptor antibody or anti-muscle-specific kinase antibody) or a positive decrement in response to repetitive nerve stimulation.
- (C)
- Amyotrophic lateral sclerosis: indicated by the presence of progressive weakness with upper and lower motor neuron signs and objective evidence of diagnosis in an electrodiagnostic test performed by a neuromuscular specialist.
- (A)
- Pneumonia with X-ray or culture confirmation;
- (B)
- Known cases of COPD and use of bronchodilators prior to admission;
- (C)
- Known cases of asthma and use of bronchodilators prior to admission;
- (D)
- Heart failure with objective evidence on X-ray or echocardiogram;
- (E)
- Bronchiectasis confirmed by chest CT;
- (F)
- Noncardiac pulmonary edema;
- (G)
- Others causes of ARF, including pulmonary embolism based on CT angiogram, pulmonary fibrosis, cystic fibrosis, and combined etiologies from the causes mentioned above.
2.3. Study Measures
2.3.1. Primary Measures
2.3.2. Secondary Measures
- (1)
- We compared the ranges of ABG parameters between NMRF and the following three categories of non-NMRF:
- a.
- PPD (asthma, COPD, and bronchiectasis). This category represents the ventilation perfusion mismatch mechanism;
- b.
- Pneumonia: this category represents the acute shunting mechanism; and
- c.
- Pulmonary edema: heart failure and non-cardiac pulmonary edema; this category represents the chronic shunting mechanism.
- (2)
- We compared the prevalence of acidosis (pH < 7.35), hypercarbia (PaCO2 > 50 mmHg), hypoxia (<80 mmHg), and high bicarbonate levels (HCO3 > 22 mmol/L) between NMRF and non-NMRF and between NMRF and the three categories of non-NMRF mentioned above.
- (3)
- We defined the ranges and prevalence of ABG parameters for each of the diseases included in our criteria separately.
- (4)
- We compared the proportion of patients with NMRF and non-NMRF who fulfilled the definition of type II respiratory failure (PaCO2 > 50 mmHg).
- (5)
- We compared the proportion of patients with NMRF and non-NMRF who had both hypercarbia and hypoxia.
- (6)
- We compared the proportion of patients with NMRF and non-NMRF who presented with isolated high bicarbonate levels (defined as >22 mmol/L).
- (7)
- We compared the proportion of patients with NMRF and non-NMRF who had either hypercarbia or hypoxia.
2.4. Sensitivity Analysis
- (1)
- We defined and compare the ranges of ABG parameters between NMRF and non-NMRF patients in severe ARF cases (defined as requiring intubation for ≥5 days or death due to ARF within 5 days).
- (2)
- We defined and compare the ranges of combined BG (CBG) parameters (which includes venous BG when ABG prior to intubation was not available) between NMRF and non-NMRF.
- (3)
- We compared data provided by King Abdulaziz University hospital with those provided by other centers for NMRF cases.
2.5. Statistical Analysis
3. Results
3.1. Primary Measures
3.2. Secondary Measures
| pH | p Value * | PaCO2 | p Value * | PaO2 | p Value * | HCO3 | p Value * | |
|---|---|---|---|---|---|---|---|---|
| Neuromuscular respiratory failure, median (IQR) | 7.39 (7.32–7.43) | 41.5 (35.3–49.6) | 86.9 (71.4–123) | 24.8 (22.9–27.8) | ||||
| Non-neuromuscular respiratory failure, median (IQR) | 7.33 (7.22–7.39) | <0.01 | 43.9 (35.9–62) | 0.13 | 79.6 (64.6–99.1) | 0.02 | 23.4 (19.4–26.8) | <0.01 |
| Primary pulmonary disease (COPD, asthma, and bronchiectasis) | 7.29 (7.21–7.36) | <0.01 | 63.2 (46–77.3) | <0.01 | 79.9 (68.7–97.2) | 0.12 | 27.5 (22.8–29.8) | 0.33 |
| Pneumonia, median (IQR) | 7.355 (7.27–7.4) | 0.01 | 41 (35.6–55.9) | 0.84 | 74.3 (61.1–94.5) | <0.01 | 23.4 (19.6–25.5) | <0.01 |
| Pulmonary edema (cardiac and non-cardiac), median (IQR) | 7.33 (7.21–7.39) | <0.01 | 42.4 (33.4–53.9) | 0.85 | 86.6 (65.2–101) | 0.32 | 20.9 (17.4–25.5) | <0.01 |
| Others, median (IQR) | 7.34 (7.31–7.39) | 0.19 | 49.5 (36.5–64.1) | 0.29 | 83.5 (76.7–101.1) | 0.66 | 24.1 22.5–26.1) | 0.48 |
3.3. Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabinstein, A.A. Acute Neuromuscular Respiratory Failure. Continuum 2015, 21, 1324–1345. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.D.; Wijdicks, E.F.M. Neuromuscular Respiratory Failure. Neurol. Clin. 2021, 39, 333–353. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Hur, J.; Lee, T.W.; Ju, S.; Lee, S.H.; Park, K.J.; Cho, Y.J.; Jeong, Y.Y.; Lee, J.D.; Kim, H.C. Myasthenia gravis presenting initially as acute respiratory failure. Respir. Care 2015, 60, e14–e16. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, H. Case of the month: Unusual presentation of myasthenia gravis with acute respiratory failure in the emergency room. Emerg. Med. J. 2006, 23, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Kim, J.H.; Kim, E.K.; Yun, S.P.; Kim, K.K.; Kim, W.C.; Jeong, H.C. Myasthenia gravis presenting as isolated respiratory failure: A case report. Korean J. Intern. Med. 2010, 25, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Cabrera Serrano, M.; Rabinstein, A.A. Usefulness of pulmonary function tests and blood gases in acute neuromuscular respiratory failure. Eur. J. Neurol. 2012, 19, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Shebl, E.; Burns, B. Respiratory Failure; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Epstein, S.K.; Singh, N. Respiratory acidosis. Respir. Care 2001, 46, 366–383. [Google Scholar] [PubMed]
- Roussos, C.; Koutsoukou, A. Respiratory failure. Eur. Respir. J. Suppl. 2003, 47, 3s–14s. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Sweberg, T. Acute respiratory failure. Crit. Care Clin. 2013, 29, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, J.; Mandrekar, J.; Wijdicks, E.F.; Rabinstein, A.A. Predictors of extubation failure in myasthenic crisis. Arch. Neurol. 2008, 65, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.I.; Fernández-Pérez, E.R.; Pendem, S.; Brown, D.R.; Wijdicks, E.F.; Gajic, O. Mechanical ventilation in patients with Guillain-Barré syndrome. Respir. Care 2006, 51, 1403–1407. [Google Scholar] [PubMed]
- Kalita, J.; Kumar, M.; Misra, U.K. Serial single breath count is a reliable tool for monitoring respiratory functions in Guillain-Barré Syndrome. J. Clin. Neurosci. 2020, 72, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Rabinstein, A.A.; Wijdicks, E.F. Warning signs of imminent respiratory failure in neurological patients. Semin. Neurol. 2003, 23, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Vitacca, M.; Clini, E.; Facchetti, D.; Pagani, M.; Poloni, M.; Porta, R.; Ambrosino, N. Breathing pattern and respiratory mechanics in patients with amyotrophic lateral sclerosis. Eur. Respir. J. 1997, 10, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.K.C.; Lee, P.C.S.; Ansary, S.; Asha, S.; Wong, K.K.H.; Yee, B.J.; Ng, A.T. Role of venous blood gases in hypercapnic respiratory failure chronic obstructive pulmonary disease patients presenting to the emergency department. Intern. Med. J. 2019, 49, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.M.; Leicht, R.V.; Carlsson, C.J.; Elvekjaer, M.; Porsbjerg, C.; Aasvang, E.K.; Meyhoff, C.S. Agreement Between Transcutaneous Monitoring and Arterial Blood Gases During COPD Exacerbation. Respir. Care 2021, 66, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Soler-Cataluña, J.J.; Martínez-García, M.A.; Román Sánchez, P.; Salcedo, E.; Navarro, M.; Ochando, R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005, 60, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Hadjikoutis, S.; Wiles, C.M. Venous serum chloride and bicarbonate measurements in the evaluation of respiratory function in motor neuron disease. QJM 2001, 94, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Mayer, S.A.; Gungor, Y.; Swarup, R.; Webster, E.A.; Chang, I.; Brannagan, T.H.; Fink, M.E.; Rowland, L.P. Myasthenic crisis: Clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology 1997, 48, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Kuo, P.H.; Fan, P.C.; Wu, H.D.; Shih, F.Y.; Yang, P.C. The role of non-invasive ventilation and factors predicting extubation outcome in myasthenic crisis. Neurocrit. Care 2009, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]

| Neuromuscular Respiratory Failure Cases (n = 69) | Non-Neuromuscular Respiratory Failure Cases (n = 218) | p Value * | |
|---|---|---|---|
| Age, median (IQR) | 51 (34–62) | 63 (50–74) | <0.01 |
| Male, n (%) | 39 (56.5) | 120 (55.1) | 0.89 |
| BMI, median (IQR) | 24.8 (20.5–29) | 27 (23.4–31.2) | 0.06 |
| Diabetes, n (%) | 17 (24.6) | 123 (56.4) | <0.01 |
| Hypertension, n (%) | 26 (37.6) | 127 (58.2) | <0.01 |
| Cardiac diseases, n (%) | 6 (8.7) | 88 (40.3) | <0.01 |
| Pulmonary diseases, n (%) | 8 (11.5) | 68 (31.1) | <0.01 |
| Creatine kinase level, median (IQR) | 79 (43–143.5) | 159 (79–375) | <0.01 |
| Days from ABG to ventilation, median (IQR) | 0 (0–1) | 0 (0–1) | 0.12 |
| Respiratory rate, median (IQR) | 23 (20–30) | 30 (24.5–40) | <0.01 |
| FiO2 §, median (IQR) | 28% (20–40%) | 40% (30–60%) | <0.01 |
| Ventilation needed at any time during ICU stay, n (%) | 66 (95.6) | 209 (95.8) | 1.00 |
| Invasive mechanical ventilation needed at any time during ICU stay, n (%) | 60 (86.9) | 137 (62.8) | <0.01 |
| Only non-invasive ventilation needed during ICU stay, n (%) | 6 (8.7) | 72 (33) | |
| Death, n (%) | 6 (8.7) | 68 (31.1) | <0.01 |
| pH | PaCO2 | PaO2 | HCO3 | |
|---|---|---|---|---|
| Myasthenia gravis, median (IQR) | 7.39 (7.32–7.4) | 39 (34.5–43.8) | 88.1 (76.4–127.4) | 23.7 (19.3–26.3) |
| Guillain–Barré syndrome, median (IQR) | 7.39 (7.34–7.43) | 42 (37–51) | 86.3 (72–106) | 25 (23–26.9) |
| Amyotrophic lateral sclerosis, median (IQR) | 7.39 (7.3–7.45) | 47.8 (35.3–77.9) | 81.1 (58.2–141) | 27.8 (24.1–32.5) |
| Pneumonia, median (IQR) | 7.36 (7.27–7.4) | 41 (35.6–55.9) | 74.3 (61.1–94.5) | 23.4 (19.6–25.5) |
| COPD, median (IQR) | 7.29 (7.21–7.36) | 67.7 (50.1–78.1) | 76 (68.4–90.1) | 28.2 (24–29.7) |
| Asthma, median (IQR) | 7.28 (7.21–7.36) | 45.8 (36.4–57.4) | 89.6 (79.9–128) | 20.7 (16.6–23.4) |
| Bronchiectasis, median (IQR) | 7.32 (7.28–7.33) | 69 (65.6–76.4) | 65 (64.9–83.9) | 32.8 (32–36) |
| Heart failure, median (IQR) | 7.34 (7.21–7.39) | 42.4 (33.4–55.8) | 85.7 (62.8–98.7) | 21.25 (17.4–25.6) |
| Non cardiac pulmonary edema, median (IQR) | 7.32 (7.21–7.36) | 42.45 (35.3–48.3) | 99.15 (81.65–111) | 19.55 (17.4–22.7) |
| Others, median (IQR) | 7.34 (7.31–7.39) | 49.5 (36.5–64.1) | 83.55 (76.7–101.1) | 24.2 (22.5–26.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuzinadah, A.R.; Almalki, A.K.; Almuteeri, R.Z.; Althalabi, R.H.; Sahli, H.A.; Hayash, F.A.; Alrayiqi, R.H.; Makkawi, S.; Maglan, A.; Alamoudi, L.O.; et al. Utility of Initial Arterial Blood Gas in Neuromuscular versus Non-Neuromuscular Acute Respiratory Failure in Intensive Care Unit Patients. J. Clin. Med. 2022, 11, 4926. https://doi.org/10.3390/jcm11164926
Abuzinadah AR, Almalki AK, Almuteeri RZ, Althalabi RH, Sahli HA, Hayash FA, Alrayiqi RH, Makkawi S, Maglan A, Alamoudi LO, et al. Utility of Initial Arterial Blood Gas in Neuromuscular versus Non-Neuromuscular Acute Respiratory Failure in Intensive Care Unit Patients. Journal of Clinical Medicine. 2022; 11(16):4926. https://doi.org/10.3390/jcm11164926
Chicago/Turabian StyleAbuzinadah, Ahmad R., Asma Khaled Almalki, Rinad Zuwaimel Almuteeri, Rahaf Hassan Althalabi, Hanin Abdullah Sahli, Fatima Abdulrahman Hayash, Rahaf Hamed Alrayiqi, Seraj Makkawi, Alaa Maglan, Loujen O. Alamoudi, and et al. 2022. "Utility of Initial Arterial Blood Gas in Neuromuscular versus Non-Neuromuscular Acute Respiratory Failure in Intensive Care Unit Patients" Journal of Clinical Medicine 11, no. 16: 4926. https://doi.org/10.3390/jcm11164926
APA StyleAbuzinadah, A. R., Almalki, A. K., Almuteeri, R. Z., Althalabi, R. H., Sahli, H. A., Hayash, F. A., Alrayiqi, R. H., Makkawi, S., Maglan, A., Alamoudi, L. O., Alamri, N. M., Alsaati, M. H., Alshareef, A. A., Aljereish, S. S., Bamaga, A. K., Alhejaili, F., Abulaban, A. A., & Alanazy, M. H. (2022). Utility of Initial Arterial Blood Gas in Neuromuscular versus Non-Neuromuscular Acute Respiratory Failure in Intensive Care Unit Patients. Journal of Clinical Medicine, 11(16), 4926. https://doi.org/10.3390/jcm11164926






