Abstract
Background: The arterial blood gas (ABG) parameters of patients admitted to intensive care units (ICUs) with acute neuromuscular respiratory failure (NMRF) and non-NMRF have not been defined or compared in the literature. Methods: We retrospectively collected the initial ABG parameters (pH, PaCO2, PaO2, and HCO3) of patients admitted to ICUs with acute respiratory failure. We compared ABG parameter ranges and the prevalence of abnormalities in NMRF versus non-NMRF and its categories, including primary pulmonary disease (PPD) (chronic obstructive pulmonary disease, asthma, and bronchiectasis), pneumonia, and pulmonary edema. Results: We included 287 patients (NMRF, n = 69; non-NMRF, n = 218). The difference between NMRF and non-NMRF included the median (interquartile range (IQR)) of pH (7.39 (7.32–7.43), 7.33 (7.22–7.39), p < 0.001), PaO2 (86.9 (71.4–123), 79.6 (64.6–99.1) mmHg, p = 0.02), and HCO3 (24.85 (22.9–27.8), 23.4 (19.4–26.8) mmol/L, p = 0.006). We found differences in the median of PaCO2 in NMRF (41.5 mmHg) versus PPD (63.3 mmHg), PaO2 in NMRF (86.9 mmHg) versus pneumonia (74.3 mmHg), and HCO3 in NMRF (24.8 mmol/L) versus pulmonary edema (20.9 mmol/L) (all p < 0.01). NMRF compared to non-NMRF patients had a lower frequency of hypercarbia (24.6% versus 39.9%) and hypoxia (33.8% versus 50.5%) (all p < 0.05). NMRF compared to PPD patients had lower frequency of combined hypoxia and hypercarbia (13.2% versus 37.8%) but more frequently isolated high bicarbonate (33.8% versus 8.9%) (all p < 0.001). Conclusions: The ranges of ABG changes in NMRF patients differed from those of non-NMRF patients, with a greater reduction in PaO2 in non-NMRF than in NMRF patients. Combined hypoxemia and hypercarbia were most frequent in PPD patients, whereas isolated high bicarbonate was most frequent in NMRF patients.
1. Introduction
Acute neuromuscular respiratory failure due to Guillain–Barré syndrome (GBS), myasthenia gravis (MG), or amyotrophic lateral sclerosis (ALS) is a common reason for admission to the intensive care unit (ICU) and ventilation [1,2]. Several reports have noted respiratory failure as an acute initial and isolated presentation of neuromuscular disorders [3,4,5]. Such presentation of isolated respiratory failure in neuromuscular conditions represents a diagnostic challenge at times. More than half of patients who present to ICU with acute neuromuscular respiratory failure lack initial diagnosis at presentation [6]. Hence, there is a need for diagnostic tools to differentiate between patients presenting with neuromuscular respiratory failure (NMRF) and non-neuromuscular respiratory failure (non-NMRF), such as respiratory or cardiac illnesses [6]. Current assessment methods to recognize NMRF involve clinical assessment and a pulmonary function test [1]. However, these methods require patient cooperation, which, in many contexts, is not feasible, with patients presenting to the emergency department due to severe weakness.
Respiratory failure (RF) occurs when the respiratory system fails to maintain gas exchange and is classified into types 1 and 2 according to blood gas (BG) abnormalities. In type 1 (hypoxemic) respiratory failure, the partial pressure of arterial oxygen (PaO2) is less than 60 mm of mercury (mmHg), and the partial pressure of arterial carbon dioxide (PaCO2) may be either normal or low. Type 1 respiratory failure occurs mainly due to ventilation perfusion mismatch, such as increased dead space in chronic obstructive pulmonary disease (COPD), causing ventilation without perfusion, or due to a shunt whereby the alveoli are perfused but not ventilated, as in pneumonia and pulmonary edema. In type 2 (hypercapnic) respiratory failure, PaCO2 is greater than 50 mmHg, and PaO2 may be normal or low; this occurs mainly due to hypoventilation [7,8,9]. Patients with NMRF due to MG, GBS, and ALS are thought to present with type 2 respiratory failure [9]. From a practical perspective, no prior study has compared the ABG parameters between NMRF and non-NMRF patients. However, few studies have looked into the utility of BG parameters of patients admitted to ICU who present with respiratory failure and whether BG parameters differ depending on the cause respiratory failure. Early diagnosis of such patients could considerably improve their management and ICU course.
Because the mechanism of respiratory failure differs between NMRF and non-NMRF cases, we hypothesized that the ranges of ABG parameters differ between NMRF and non-NMRF patients. The aim of this study is to define and compare the arterial BG (ABG) parameter ranges and prevalence of abnormalities in NMRF vs. non-NMRF patients. We aim to describe the ABG parameters for different diseases separately with regard to ABG parameter ranges and prevalence of ABG abnormalities.
2. Methods
2.1. Study Design and Participants
We conducted a retrospective, cross-sectional study between January 2015 and May 2021 at 4 tertiary centers in Saudi Arabia, (King Abdulaziz University Hospital (KAUH) in Jeddah, National Guard Hospital in Riyadh, King Saud University Medical City in Riyadh, and National Guard Hospital in Jeddah). The institutional review boards approved the protocol in each institution. All centers recruited NMRF cases, whereas the non-NMRF cases were recruited from King Abdulaziz University Hospital. We retrospectively collected data for patients who presented with acute respiratory failure (ARF) during the study period. Inclusion criteria included (1) age of 18–80 years; (2) patients admitted to ICU; (3) diagnosis of acute respiratory failure (ARF) as the indication for ICU admission (see below for ARF definition); and (4) patients with ARF due to one of the following diagnoses: (A) GBS; (B) MG; (C) amyotrophic lateral sclerosis; (D) pneumonia; (E) known cases of chronic obstructive pulmonary disease (COPD); (F) known cases of bronchial asthma; (G) known case of Bronchiectasis; (H) heart failure; (I) noncardiac pulmonary edema; and (J) other known causes of ARF, such as pulmonary embolism and pulmonary fibrosis. Our exclusion criteria were (1) intubation due to sepsis, (2) blood gas collected after the date of intubation, (3) cardiac arrest suffered at initial presentation, and (4) coexistent diabetic ketoacidosis (DKA).
2.2. Study Groups and Definition of Variables
Arterial blood gas (ABG) parameters: we refer to the first ABG taken from the patient before or at the date of admission to the ICU. We excluded ABG values that were taken after the date of ventilation. Parameters include pH, partial pressure of carbon dioxide (PaCO2) in mmHg, partial pressure of oxygen (PaO2) in mmHg, and bicarbonate level (HCO3) in mmol/L.
Combined blood gas (CBG) parameters: we refer to the first blood gas taken from the patient (including venous blood gas if ABG was not available) before or at the date of admission to ICU. Parameters included pH, PaCO2, PaO2, and HCO3.
Acute respiratory failure (ARF) refers to cases admitted to ICU due to respiratory distress caused by one of the diseases mentioned in the inclusion criteria (above) with no additional reason for ICU admission. Cases of ARF were identified by chart review, requiring at least two documentations from ICU and/or emergency physicians indicating the presence of ARF or respiratory distress as an indication for ICU admission. We also excluded cases with coexisting indication for ICU admission, such as sepsis (Figure 1: flow chart).
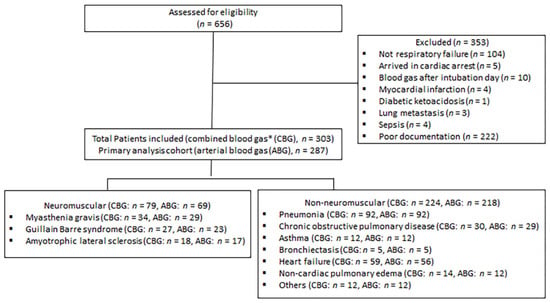
Figure 1.
Flow chart as per STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines. * Combined blood gas includes venous blood gas if the patient did not have arterial blood gas on initial presentation.
Group 1 was the NMRF group, which included patients with the following:
- (A)
- Guillain–Barré syndrome (GBS): Patients presenting with acute-onset generalized weakness and areflexia that reaches the maximum within four weeks; objective evidence of a diagnosis is from an electrodiagnostic test and/or cerebrospinal fluid.
- (B)
- Myasthenia gravis (MG): this diagnosis is based on clinical presentation with objective evidence of diagnosis either through positive serology (acetylcholine receptor antibody or anti-muscle-specific kinase antibody) or a positive decrement in response to repetitive nerve stimulation.
- (C)
- Amyotrophic lateral sclerosis: indicated by the presence of progressive weakness with upper and lower motor neuron signs and objective evidence of diagnosis in an electrodiagnostic test performed by a neuromuscular specialist.
Group 2 was the non-NMRF group, which included patients with the following:
- (A)
- Pneumonia with X-ray or culture confirmation;
- (B)
- Known cases of COPD and use of bronchodilators prior to admission;
- (C)
- Known cases of asthma and use of bronchodilators prior to admission;
- (D)
- Heart failure with objective evidence on X-ray or echocardiogram;
- (E)
- Bronchiectasis confirmed by chest CT;
- (F)
- Noncardiac pulmonary edema;
- (G)
- Others causes of ARF, including pulmonary embolism based on CT angiogram, pulmonary fibrosis, cystic fibrosis, and combined etiologies from the causes mentioned above.
2.3. Study Measures
2.3.1. Primary Measures
To define and compare the ranges of arterial blood gas parameters (pH, PaCO2, PaO2, HCO3) in patients presenting with ARF due to NMRF and non-NMRF.
2.3.2. Secondary Measures
- (1)
- We compared the ranges of ABG parameters between NMRF and the following three categories of non-NMRF:
- a.
- PPD (asthma, COPD, and bronchiectasis). This category represents the ventilation perfusion mismatch mechanism;
- b.
- Pneumonia: this category represents the acute shunting mechanism; and
- c.
- Pulmonary edema: heart failure and non-cardiac pulmonary edema; this category represents the chronic shunting mechanism.
- (2)
- We compared the prevalence of acidosis (pH < 7.35), hypercarbia (PaCO2 > 50 mmHg), hypoxia (<80 mmHg), and high bicarbonate levels (HCO3 > 22 mmol/L) between NMRF and non-NMRF and between NMRF and the three categories of non-NMRF mentioned above.
- (3)
- We defined the ranges and prevalence of ABG parameters for each of the diseases included in our criteria separately.
- (4)
- We compared the proportion of patients with NMRF and non-NMRF who fulfilled the definition of type II respiratory failure (PaCO2 > 50 mmHg).
- (5)
- We compared the proportion of patients with NMRF and non-NMRF who had both hypercarbia and hypoxia.
- (6)
- We compared the proportion of patients with NMRF and non-NMRF who presented with isolated high bicarbonate levels (defined as >22 mmol/L).
- (7)
- We compared the proportion of patients with NMRF and non-NMRF who had either hypercarbia or hypoxia.
2.4. Sensitivity Analysis
- (1)
- We defined and compare the ranges of ABG parameters between NMRF and non-NMRF patients in severe ARF cases (defined as requiring intubation for ≥5 days or death due to ARF within 5 days).
- (2)
- We defined and compare the ranges of combined BG (CBG) parameters (which includes venous BG when ABG prior to intubation was not available) between NMRF and non-NMRF.
- (3)
- We compared data provided by King Abdulaziz University hospital with those provided by other centers for NMRF cases.
2.5. Statistical Analysis
The characteristics of patients were analyzed using the median (IQR, interquartile range) and frequencies, as appropriate. Mann–Whitney U tests, χ2 tests, and Fisher exact tests were used to compare the data between patients with NMRF and non-NMRF, as appropriate. Because this was a retrospective study, we used a convenient sampling approach, including all available cases that satisfied the inclusion and exclusion criteria; therefore, the sample size was not calculated as priori. Statistical analysis was performed using STATA version 13 (Stata-Corp, College Station, TX, USA).
3. Results
The number of patients retrieved from the electronic medical record search was 656. A total of 303 patients with available CBG data were included; of them, 287 patients had ABG data available and were included in the primary analysis (Figure 1, flow chart). A total of 218 patients with non-NMRF and 69 patients with NMRF were included in the study. The former patients were older than those with NMRF; however, the two groups were similar in terms of gender distribution and body mass index (BMI) (Table 1). Additionally, respiratory rates and fractions of inspired oxygen (FiO2) were higher in the non-NMRF compared to NMRF group (Table 1).

Table 1.
Patient characteristics.
3.1. Primary Measures
The ranges of ABG parameters are shown in Table 2. NMRF patients had higher pH, PaO2, and HCO3 levels than non-NMRF patients; in contrast, there was no difference in PaCO2. We present the ABG parameters data for NMRF and non-NMRF cases in a scatter plot in Figure S1 (Supplementary Materials).
3.2. Secondary Measures
The ranges of ABG parameters for each category of the ARF mechanisms are presented in Table 2. NMRF patients presented with less acidosis when compared with non-NMRF patients and when compared with each one of the three major categories of non-NMRF. The highest levels of PaCO2 were observed in PPD patients. The lowest levels of PaO2 were observed in pneumonia patients. Pulmonary edema and pneumonia patients had the lowest HCO3 levels.

Table 2.
Comparing ABG parameters between NMRF and non-NMRF categories.
Table 2.
Comparing ABG parameters between NMRF and non-NMRF categories.
| pH | p Value * | PaCO2 | p Value * | PaO2 | p Value * | HCO3 | p Value * | |
|---|---|---|---|---|---|---|---|---|
| Neuromuscular respiratory failure, median (IQR) | 7.39 (7.32–7.43) | 41.5 (35.3–49.6) | 86.9 (71.4–123) | 24.8 (22.9–27.8) | ||||
| Non-neuromuscular respiratory failure, median (IQR) | 7.33 (7.22–7.39) | <0.01 | 43.9 (35.9–62) | 0.13 | 79.6 (64.6–99.1) | 0.02 | 23.4 (19.4–26.8) | <0.01 |
| Primary pulmonary disease (COPD, asthma, and bronchiectasis) | 7.29 (7.21–7.36) | <0.01 | 63.2 (46–77.3) | <0.01 | 79.9 (68.7–97.2) | 0.12 | 27.5 (22.8–29.8) | 0.33 |
| Pneumonia, median (IQR) | 7.355 (7.27–7.4) | 0.01 | 41 (35.6–55.9) | 0.84 | 74.3 (61.1–94.5) | <0.01 | 23.4 (19.6–25.5) | <0.01 |
| Pulmonary edema (cardiac and non-cardiac), median (IQR) | 7.33 (7.21–7.39) | <0.01 | 42.4 (33.4–53.9) | 0.85 | 86.6 (65.2–101) | 0.32 | 20.9 (17.4–25.5) | <0.01 |
| Others, median (IQR) | 7.34 (7.31–7.39) | 0.19 | 49.5 (36.5–64.1) | 0.29 | 83.5 (76.7–101.1) | 0.66 | 24.1 22.5–26.1) | 0.48 |
* p value compared to NMRF (neuromuscular respiratory failure), Wilcoxon rank sum (Mann–Whitney) test, IQR: interquartile range.
The prevalence of ABG abnormalities among the various categories of ARF is presented in Figure 2 and Table S1 (Supplementary Materials). Acidosis was less prevalent in NMRF (31.9%) compared to non-NMRF (55.1%) and PPD (67.4%) patients. Hypercarbia was more prevalent in non-NMRF (39.9%) and PPD (67.4%) patients compared to NMRF patients (24.6%) (p < 0.05). Hypoxia was more prevalent in non-NMRF (50.5%) and pneumonia (58.7%) patients compared to NMRF patients (33.8%) (p < 0.05). High normal or elevated bicarbonate levels were more prevalent in NMRF (76.8%) and PPD (73.9%) patients compared to pulmonary edema cases (44.1%).
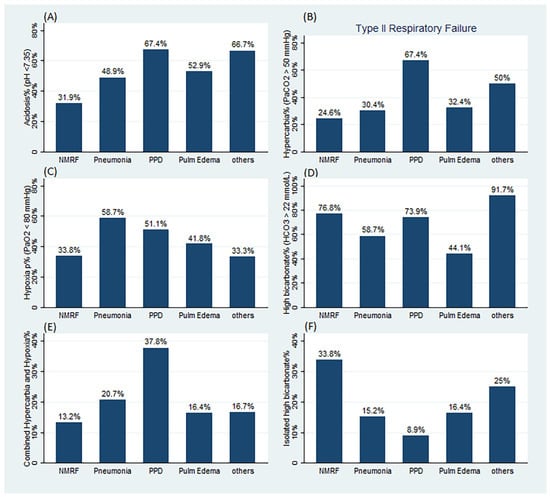
Figure 2.
Prevalence of ABG abnormalities per category of respiratory failure. (A) Prevalence of acidosis (pH < 7.35). (B) Prevalence of hypercarbia and type II respiratory failure (PaCO2 > 50 mmHg). (C) Prevalence of hypoxia (PaO2 < 80 mmHg). (D) Prevalence of high bicarbonate level (HCO3 > 22 mmol/L). (E) Prevalence of combined hypercarbia and hypoxia. (F) Prevalence of isolated high bicarbonate level. NMRF: neuromuscular respiratory failure, PPD: primary pulmonary diseases, Pulm Edem: pulmonary edema. For a comparison p values, refer to Table S1 in the Supplementary Materials.
The ranges of ABG parameters for each disease are presented in Table 3. The highest levels of PaCO2 were observed in bronchiectasis and COPD patients. The lowest levels of PaO2 were observed in pneumonia, COPD, and bronchiectasis patients. The highest levels of HCO3 were observed in bronchiectasis and COPD patients, followed by ALS. The prevalence of ABG abnormalities for each disease is presented in Table S2 (Supplementary Materials).

Table 3.
Arterial blood gas parameters according to disease.
We found that 17 (24.6%) and 87 (39.9%) patients with NMRF and non-NMRF, respectively, fulfilled type II respiratory failure criteria (p = 0.02). The proportion of patients with NMRF and non-NMRF who had combined hypercarbia and hypoxia was greater among PPD (37.8%) than NMRF (13.2%) patients (p < 0.01) (Figure 2 and Table S1, Supplementary Materials), whereas the proportion of patients who presented with isolated high bicarbonate levels was higher among NMRF (33.8%) compared to non-NMRF (14.8%), PPD (8.9%), pneumonia (15.2%), and pulmonary edema (16.4%) patients (all p < 0.01) (Figure 2 and Table S1, Supplementary Materials). The proportion of patients who presented with either hypoxia or hypercarbia was lower among NMRF (45.6%) compared to non-NMRF (67.1%) patients (p = 0.002) and compared to PPD (80%) patients (p < 0.001).
3.3. Sensitivity Analysis
When analysis was restricted to severe ARF, the results were similar to those of the primary endpoint. The ranges of ABG parameters for each category of the ARF mechanisms are presented in Table S3 (Supplementary Materials). The highest levels of PaCO2 were observed in PPD patients. The lowest levels of PaO2 were observed in pneumonia patients. Pulmonary edema and pneumonia patients had the lowest HCO3 levels. The ranges of CBG parameters are shown in Table S4 (Supplementary Materials); the results were similar to those of the primary endpoints, where NMRF patients higher PaO2 and HCO3 levels than non-NMRF patients; in contrast, there was no difference in PaCO2. A comparison of data between the study centers revealed no differences (Table S5, Supplementary Materials).
4. Discussion
In the present study, we established the ranges of initial ABG parameters in NMRF and non-NMRF patients admitted to intensive care units with acute respiratory failure. We found a lower degree of hypoxemia in patients with NMRF compared to those with non-NMRF. The levels of hypercarbia were similar between NMRF and non-NMRF patients; however, the proportion of patients who had hypercarbia and met the type 2 respiratory failure definition was greater in non-NMRF (39.9%) compared to NMRF (24.6%) patients. Combined hypoxemia and hypercarbia were most characteristic of PPD, whereas isolated high bicarbonate was characteristic of NMRF. A majority of NMRF patients in our cohort presented with either normal or mild hypoxemia and with normal PaCO2, which is similar to results reported in a cohort of 79 patients who presented with NMRF in ICU with PaCO2 of 48 mmHg and PaO2 of 92 mmHg [6]. This is expected, as neuromuscular disorders initially cause microatelectasis, particularly at the lung base, leading to shunting (more alveoli perfused and not ventilated), which causes mild hypoxemia; however, this stage is usually compensated by tachypnea, which leads to hypocarbia and normalization of PaO2 [1,2]. This is usually followed by fatigue in the respiratory muscle and leads to hypercarbia, which occurred in 24.6% of NMRF patients in our cohort. In fact, it is suggested that with tachypnea, patients should have hypocarbia, whereas cases of tachypnea with normocarbia indicate ineffective respiration and advanced respiratory failure [1]. The abnormalities in ABG in NMRF cases are due to hypoventilation; there are three factors that influence ventilation: (1) respiratory rate, (2) tidal volume, and (3) dead space (no gas exchange in the airway) [10]. As NMRF reduces the total tidal volume and does not affect dead space, the respiratory rate is the variable that can be modified physiologically to temporarily compensate for the reduced tidal volume in NMRF. Our study showed that patients with NMRF usually (54%) present without hypoxia or hypercarbia during this compensatory phase, at which time ABG parameters could be normal, in contrast with those in non-NMRF patients, who less frequently (32.9%) present without hypoxia or hypercarbia. Both groups (NMRF and non-NMRF) had elevated respiratory rates (RR); however, RR was higher among non-NMRF patients. The median of FiO2 was higher among non-NMRF compared to NMRF patients. This, along with the finding that PaO2 was higher among NMRF compared to non-NMRF patients, may indicate that with a normal gas diffusion and perfusion mechanism, such as in NMRF, respiratory and ABG parameters are expected to be corrected faster with oxygen supplementations than in diseases that affect gas diffusion and perfusion, such as non-NMRF.
When each neuromuscular disease was analyzed separately, we found that MG and GBS shared a common profile, with a low proportion of patients with hypercarbia (10.3% and 26.1%, respectively) and a low proportion with hypoxia (28.6% and 30.4%, respectively). This is in contrast to the ALS group, where the proportions of hypercarbia and hypoxia were higher (47.1% and 47.1%, respectively) than in the GBS and MG groups. A study examining MG exacerbation revealed PaCO2 levels ranging between 28 and 54 mmHg, which is similar to our data; however, the previous study reported a higher level of PaO2 than our data, with a mean of 101 mmHg [11]. Regarding GBS, the mean of PaCO2 was found to be normocarbic, at 37 mmHg (31–45 mmHg) [12]. Additionally, Kalita et al. found that GBS cases that required intubation had average PaCO2 levels of 41 mmHg (37–46 mmHg), which is consistent with our results, whereas average PaO2 levels were found to be 70 mmHg (61–93 mmHg) among intubated GBS cases, which is slightly lower than the PaO2 levels reported in our cohort [13]. This latter cohort probably included more severe cases, as the authors reported a single breath count of 3 [13]. In GBS and MG cases, hypoxia and hypercarbia occur late, and physicians should not wait for these changes to occur before they provide respiratory support [14]. However, we found that GBS and MG usually have isolated bicarbonate at the higher level. Prior studies have shown that hypercarbia does not develop in ALS unless loss of lung volume becomes severe with a forced vital capacity reduced down to at least 20% of the predicted value [15]. The same authors also found that hypoxia occurs in 50% of the cases that develop hypercarbia. There are no prior data on hypercarbia or hypoxia prevalence among ALS cases admitted to ICU; however, it was reported that at 6 months from ALS onset, 54% of ALS cases have hypercarbia and 27% have hypoxia [15].
Non-NMRF cases span various mechanistic categories. PPD, including COPD, asthma, and bronchiectasis, causes ABG abnormalities through ventilation perfusion mismatch, whereby there is an area in the lung ventilated without gas exchange. Our study showed that the levels and prevalence of hypercarbia are higher in PPD compared to NMRF (63.25 mmHg vs. 41.5 mmHg and 67.4% vs. 24% for PPD vs. NMRF, respectively). Hypoxia occurred more frequently in PPD (51.1%) compared to of NMRF (33.8%), with a median PaO2 of 79.9 vs. 86.9 mmHg, respectively. This finding is consistent with studies that have looked at COPD exacerbation cases and found an elevated mean of PaCO2 of 59 mmHg [16]. Another recent study looked at COPD cases admitted to ICU with respiratory failure and found the mean PaCO2 and PaO2 to be 54 mmHg and 63 mmHg, respectively [17]. This is in contrast with another study that showed that most patients with COPD do not have elevated PaCO2, whereas PaO2 levels vary between 60 and 80 mmHg [18]. Pneumonia and pulmonary edema cause respiratory failure due to shunting, whereby the perfused area in the lung is not ventilated [9]. This usually results in hypoxemia without hypercarbia, which is consistent with our finding. ABG in pneumonia differs from that in NMRF, mainly with a higher prevalence of hypoxemia, whereas the higher prevalence of hypoxemia in pulmonary edema compared to NMRF did not reach statistical significance in our cohort.
On average, arterial bicarbonate was higher among NMRF cases than non-NMRF cases. When NMRF was compared to different categories of non-NMRF, the difference in bicarbonate was more prominent versus pneumonia and pulmonary edema, whereas PPD had a similar level of bicarbonate. However, an important difference between NMRF and PPD is that patients with NMRF are more likely to present to ICU with isolated high bicarbonate (without hypoxia or hypercarbia) compared to PPD patients. Prior studies that looked at the serum bicarbonate level among patients with ALS have showed that 80% of those with elevated serum bicarbonate died within 5 months, whereas patients with normal serum bicarbonate levels remained alive at 15 months of follow-up [19]. Among MG patients, elevated serum bicarbonate (>30 mg/dL) was found to be a predictor for prolonged intubation [20]. The authors interpreted these results such that high serum bicarbonate levels are probably a more reliable indicator of chronic respiratory acidosis than pre-intubation carbon dioxide partial pressure, which may transiently normalize in some patients with increased respiratory effort. Another study among MG patients reported similar results of more successful noninvasive ventilation if the serum bicarbonate level was <30 mmol/L [21].
Our data can be used in conjunction with the clinical context but not as a stand-alone test. The clinical implications of our findings—in the appropriate clinical context—include helping physicians to identify the underlying cause of acute respiratory distress in cases presenting for the first time without a prior clinical history. This will guide further clinical testing and consultation with the relevant specialties to confirm clinical suspicion. In addition, our data may alert physicians to a coexisting disorder contributing to acute respiratory distress in patients known to have certain disease, particularly when the pattern of ABG results does not fit the pattern expected of the known disease. Our data may guide planning of future clinical studies that address respiratory distress in neuromuscular and non-neuromuscular diseases when it comes to using ABG in inclusion criteria or for calculations of effect size or sample size. Nonetheless, we emphasize the importance of taking the whole clinical picture into consideration when interpreting ABG results, rather than considering ABG results in isolation, which could limit the usefulness of our data. Finally, defining the magnitude of ABG changes in NMRF and non-NMRF patients in a single study with similar methodology may contribute to a deeper understanding of the identified concepts associated with acute respiratory failure.
Our study is subject to several limitations. The retrospective and observational nature of the study could contribute inherited bias. We did not incorporate oxygen saturation or respiratory rate data in our analysis. Another limitation of our study is that non-NMRF cases were included from only one center. However, because we collected the initial ABG upon presentation before therapeutic interventions, we believe that our data represent the disease course rather than a variation in the clinical practice in each center, although the later cannot be totally excluded (Figure 1: flow chart). Other measures of pulmonary function, such as vital capacity, were not collected due to difficulties associated with documentation. There were few cases included for some diseases, such as pulmonary embolism and interstitial pulmonary fibrosis. The influence of comorbidities, such as diabetes and hypertension, on the outcomes were not adjusted for. We did not evaluate the response to different treatment modalities, such as prednisone for myasthenic patients and bronchodilator for asthmatic and COPD patients.
In conclusion, our data provide the expected range of ABG changes among patients admitted to ICU, and we found that hypercarbia occurred in a quarter of patients with NMRF. When compared with NMRF, PPD patients presented more frequently with hypercarbia, whereas pneumonia patients presented more often with hypoxia, and pulmonary edema patients have less elevated bicarbonate levels. Combined hypoxemia and hypercarbia are characteristic of PPD, whereas isolated high bicarbonate is characteristic of NMRF.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11164926/s1, Table S1. Prevalence of ABG abnormalities per categories of Respiratory failure; Table S2. Prevalence of ABG abnormalities per disease; Table S3. Sensitivity analysis comparing severe NMRF versus severe non-NMRF and its categories; Table S4. Sensitivity analysis combined blood gases (arterial and venous), comparing NMRF versus non-NMRF and its categories; Table S5. Comparison of ABG data among the different centers in NMRF cases; Figure S1. Scatter plot for ABG parameters data for NMRF and non-NMRF cases.
Author Contributions
A.R.A.: study design, data acquisition and interpretation, statistical analysis, writing—original draft preparation. A.K.A.: study design, data acquisition, and writing—original draft preparation. R.Z.A.: study design, data acquisition, and writing—original draft preparation. R.H.A. (Rahaf Hassan Althalabi): study design, data acquisition, and writing—original draft preparation. H.A.S.: study design, data acquisition, and writing—original draft preparation. F.A.H.: study design, data acquisition, and writing—original draft preparation. R.H.A. (Rahaf Hamed Alrayiqi): study design, data acquisition, and writing—original draft preparation. S.M.: study design, data acquisition, and writing—review and editing. A.M.: study design, data, and writing—review and editing. L.O.A.: study design, data acquisition, and writing—review and editing. N.M.A.: study design, data acquisition, and writing—review and editing. M.H.A. (Maha H. Alsaati): reviewing the data and the manuscript. A.A.A. (Aysha A. Alshareef): Reviewing the manuscript. S.S.A.: study design, data acquisition, and writing—review and editing. A.K.B.: study design, data acquisition, and writing—original draft preparation. F.A.: data interpretation and writing—review and editing. A.A.A. (Ahmad Abdulaziz Abulaban): study design, data acquisition, and writing—original draft preparation. M.H.A. (Mohammed H. Alanazy): study design, data acquisition, and writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The institutional review boards approved the protocol in each participating institution. Approval code for King Abdulaziz University Hospital in Jeddah#656-20), King Saud University Medical City in Riyadh (E-21-5884), and National Guard Hospital in Jeddah and in Riyadh (ERJ21J/004/04).
Informed Consent Statement
This is a chart review retrospective study, so informed consent was waived by institutional review boards.
Data Availability Statement
All data are available upon request from the corresponding author.
Acknowledgments
We thank the medical records department at King Abdulaziz University Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rabinstein, A.A. Acute Neuromuscular Respiratory Failure. Continuum 2015, 21, 1324–1345. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.D.; Wijdicks, E.F.M. Neuromuscular Respiratory Failure. Neurol. Clin. 2021, 39, 333–353. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Hur, J.; Lee, T.W.; Ju, S.; Lee, S.H.; Park, K.J.; Cho, Y.J.; Jeong, Y.Y.; Lee, J.D.; Kim, H.C. Myasthenia gravis presenting initially as acute respiratory failure. Respir. Care 2015, 60, e14–e16. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, H. Case of the month: Unusual presentation of myasthenia gravis with acute respiratory failure in the emergency room. Emerg. Med. J. 2006, 23, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Kim, J.H.; Kim, E.K.; Yun, S.P.; Kim, K.K.; Kim, W.C.; Jeong, H.C. Myasthenia gravis presenting as isolated respiratory failure: A case report. Korean J. Intern. Med. 2010, 25, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Cabrera Serrano, M.; Rabinstein, A.A. Usefulness of pulmonary function tests and blood gases in acute neuromuscular respiratory failure. Eur. J. Neurol. 2012, 19, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Shebl, E.; Burns, B. Respiratory Failure; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Epstein, S.K.; Singh, N. Respiratory acidosis. Respir. Care 2001, 46, 366–383. [Google Scholar] [PubMed]
- Roussos, C.; Koutsoukou, A. Respiratory failure. Eur. Respir. J. Suppl. 2003, 47, 3s–14s. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Sweberg, T. Acute respiratory failure. Crit. Care Clin. 2013, 29, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, J.; Mandrekar, J.; Wijdicks, E.F.; Rabinstein, A.A. Predictors of extubation failure in myasthenic crisis. Arch. Neurol. 2008, 65, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.I.; Fernández-Pérez, E.R.; Pendem, S.; Brown, D.R.; Wijdicks, E.F.; Gajic, O. Mechanical ventilation in patients with Guillain-Barré syndrome. Respir. Care 2006, 51, 1403–1407. [Google Scholar] [PubMed]
- Kalita, J.; Kumar, M.; Misra, U.K. Serial single breath count is a reliable tool for monitoring respiratory functions in Guillain-Barré Syndrome. J. Clin. Neurosci. 2020, 72, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Rabinstein, A.A.; Wijdicks, E.F. Warning signs of imminent respiratory failure in neurological patients. Semin. Neurol. 2003, 23, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Vitacca, M.; Clini, E.; Facchetti, D.; Pagani, M.; Poloni, M.; Porta, R.; Ambrosino, N. Breathing pattern and respiratory mechanics in patients with amyotrophic lateral sclerosis. Eur. Respir. J. 1997, 10, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.K.C.; Lee, P.C.S.; Ansary, S.; Asha, S.; Wong, K.K.H.; Yee, B.J.; Ng, A.T. Role of venous blood gases in hypercapnic respiratory failure chronic obstructive pulmonary disease patients presenting to the emergency department. Intern. Med. J. 2019, 49, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.M.; Leicht, R.V.; Carlsson, C.J.; Elvekjaer, M.; Porsbjerg, C.; Aasvang, E.K.; Meyhoff, C.S. Agreement Between Transcutaneous Monitoring and Arterial Blood Gases During COPD Exacerbation. Respir. Care 2021, 66, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Soler-Cataluña, J.J.; Martínez-García, M.A.; Román Sánchez, P.; Salcedo, E.; Navarro, M.; Ochando, R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005, 60, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Hadjikoutis, S.; Wiles, C.M. Venous serum chloride and bicarbonate measurements in the evaluation of respiratory function in motor neuron disease. QJM 2001, 94, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Mayer, S.A.; Gungor, Y.; Swarup, R.; Webster, E.A.; Chang, I.; Brannagan, T.H.; Fink, M.E.; Rowland, L.P. Myasthenic crisis: Clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology 1997, 48, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Kuo, P.H.; Fan, P.C.; Wu, H.D.; Shih, F.Y.; Yang, P.C. The role of non-invasive ventilation and factors predicting extubation outcome in myasthenic crisis. Neurocrit. Care 2009, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).