A Device Strategy-Matched Comparison Analysis among Different Intermacs Profiles: A Single Center Experience
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Study Outcomes
2.3. Definitions
2.4. LVAD Management
2.5. Statistical Analysis
2.6. Study Oversight
3. Results
3.1. Baseline Characteristics and LVAD Implantation
3.2. Overall Results
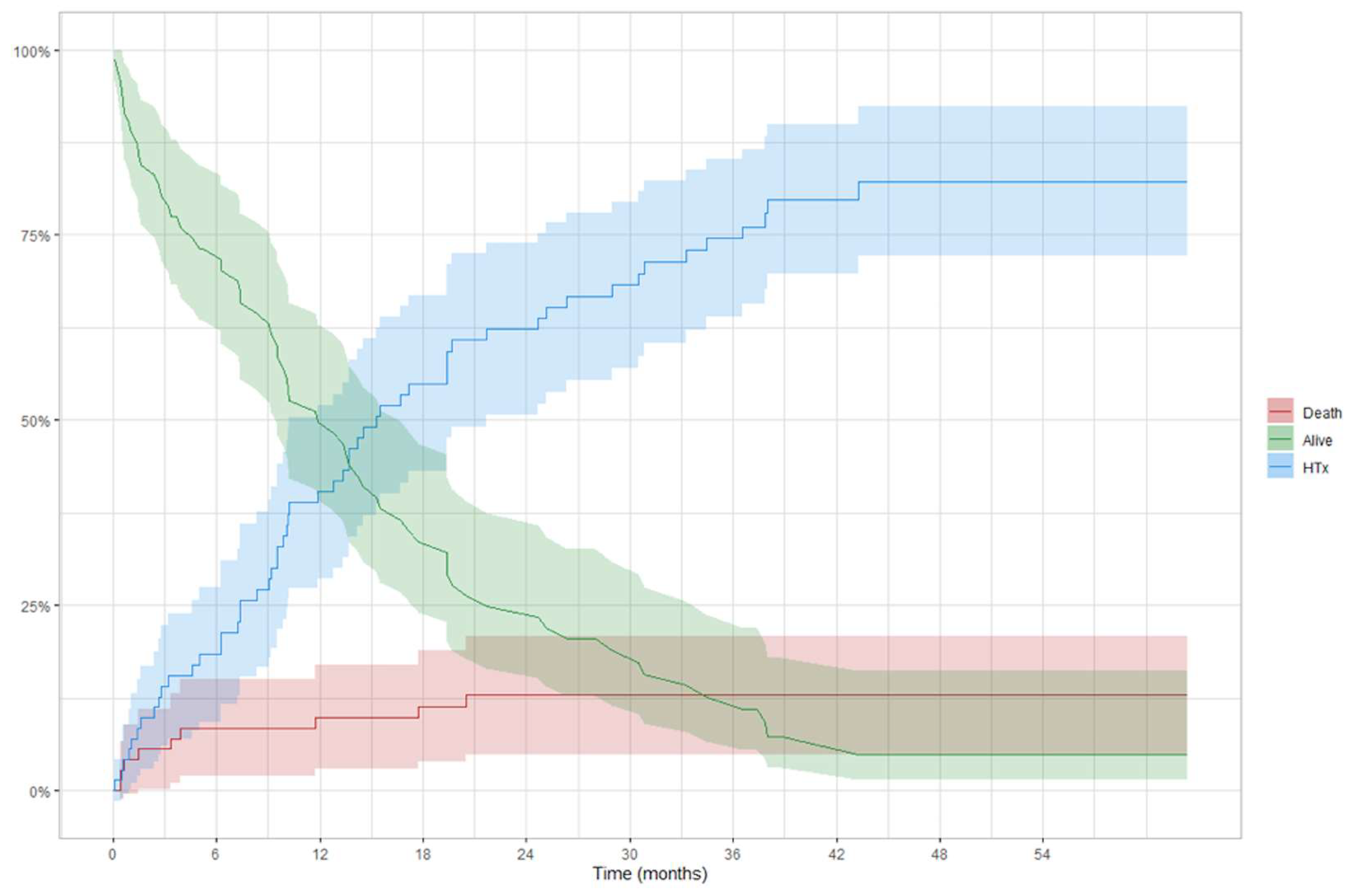
3.3. Intention to Treat Analysis
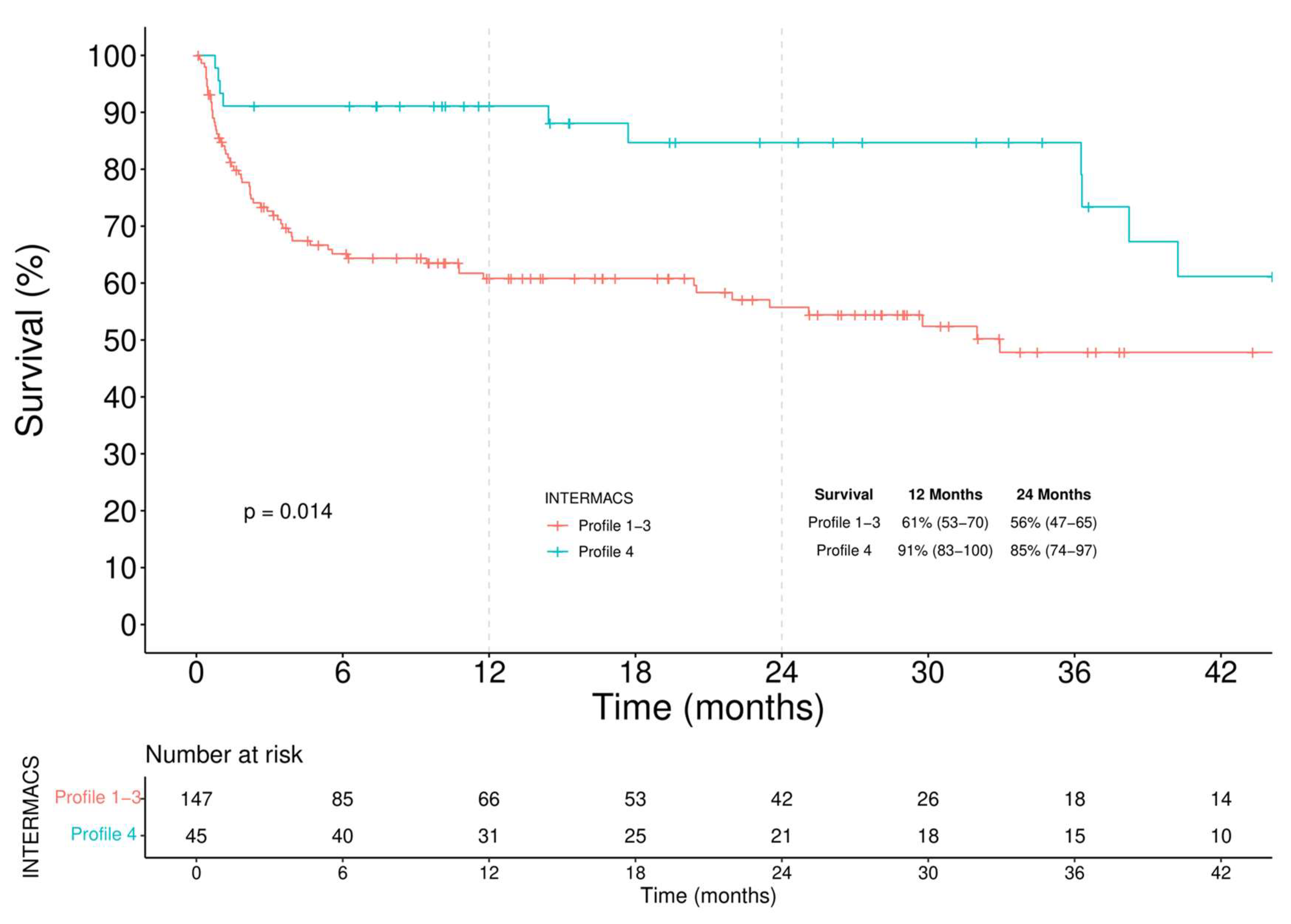
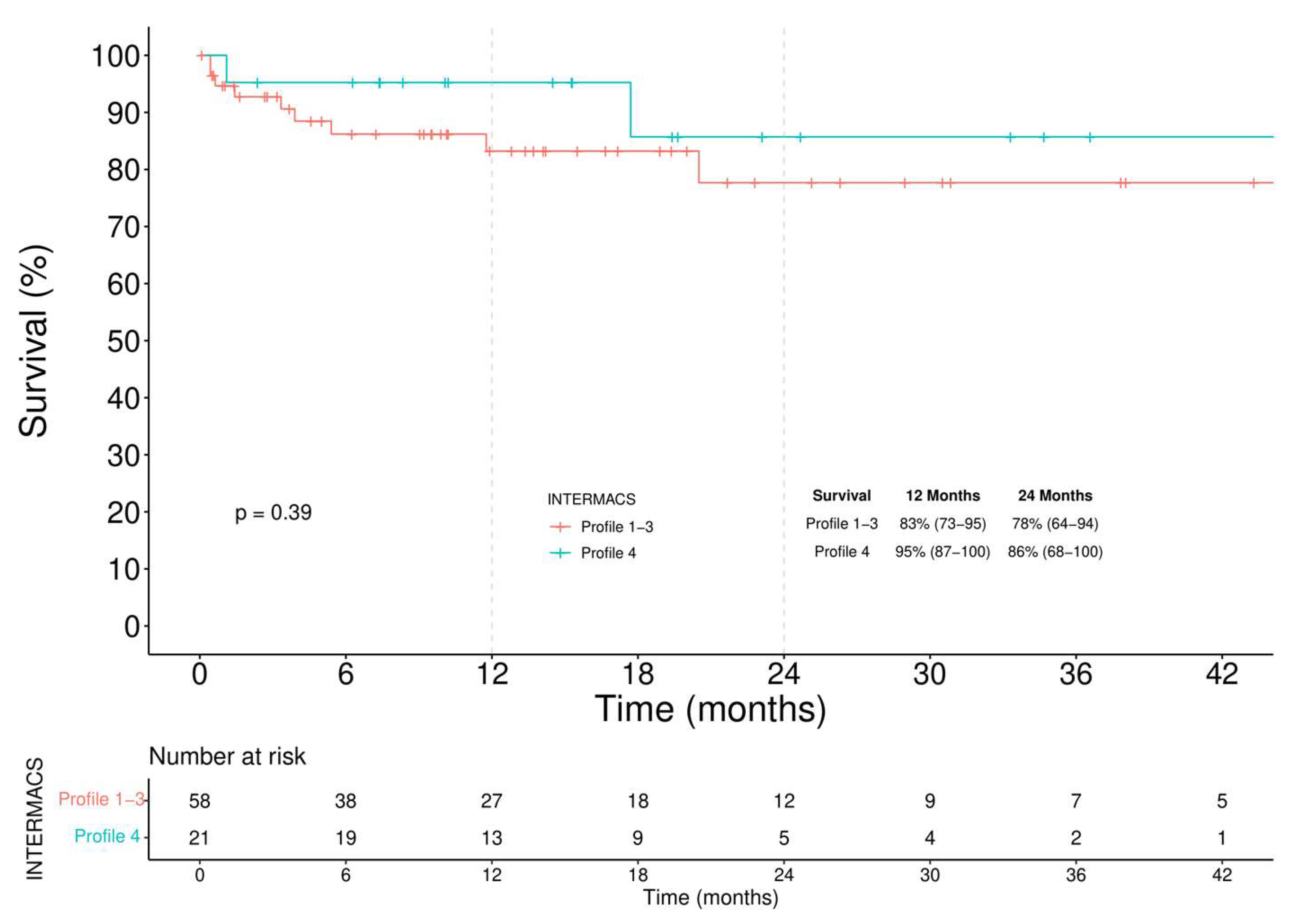
3.3.1. Survival
3.3.2. Major Adverse Events
4. Discussion
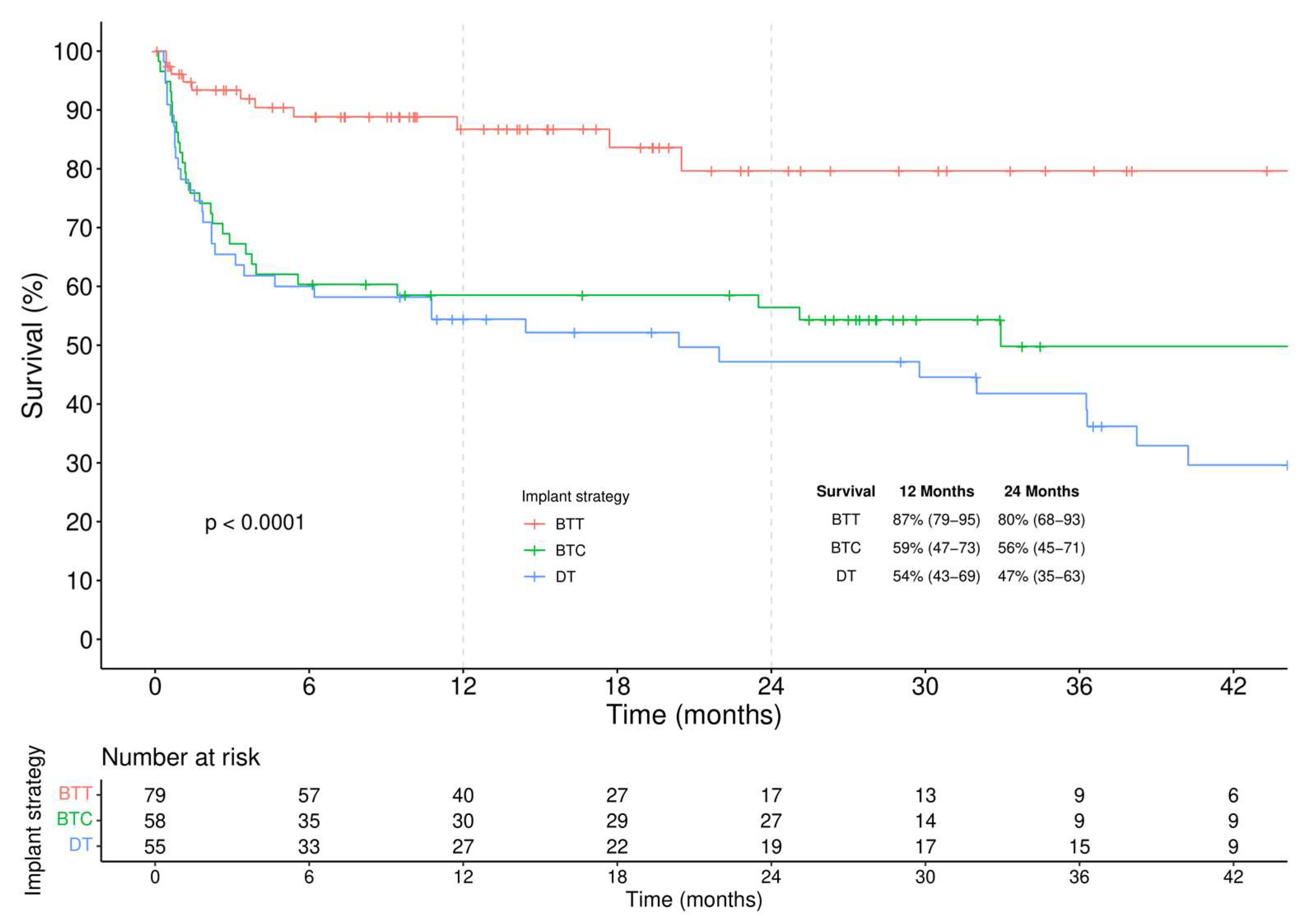
- (a)
- among BTT patients a similar survival regardless of the INTERMACS profile was observed, though a higher incidence of RVAD implantation was seen in the critically ill group.
- (b)
- among BTC patients, slightly improved survival is seen in elective patients.
- (c)
- among DT patients, higher survival is evident for elective patients.
5. Study Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Ve, H.; Jankpwska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; Frigerio, M.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology: Advanced heart failure: HFA position statement. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef] [PubMed]
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef]
- Rose, E.A.; Gelijns, A.C.; Moskowitz, A.J.; Heitjan, D.F.; Stevenson, L.W.; Dembitsky, W.; Long, J.W.; Ascheim, D.D.; Tierney, A.R.; Levitan, R.G.; et al. Long-Term Use of a Left Ventricular Assist Device for End-Stage Heart Failure. N. Engl. J. Med. 2001, 345, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Kormos, R.L.; Cowger, J.; Pagani, F.D.; Teuteberg, J.J.; Goldstein, D.J.; Jacobs, J.P.; Higgins, R.S.; Stevenson, L.W.; Stehlik, J.; Atluri, P.; et al. The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J. Heart Lung Transplant. 2019, 38, 114–126. [Google Scholar] [CrossRef]
- Teuteberg, J.J.; Cleveland, J.C.; Cowger, J.; Higgins, R.S.; Goldstein, D.J.; Keebler, M.; Kirklin, J.K.; Myers, S.L.; Salerno, C.T.; Stehlik, J.; et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann. Thorac. Surg. 2020, 109, 649–660. [Google Scholar] [CrossRef] [Green Version]
- de By, T.M.M.H.; Mohacsi, P.; Gahl, B.; Zittermann, A.; Krabatsch, T.; Gustafsson, F.; Meyns, B.; Netuka, I.; Caliskan, K.; Castedo, E.; et al. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS) of the European Association for Cardio-Thoracic Surgery (EACTS): Second report. Eur. J. Cardio Thorac. Surg. 2018, 53, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Potapov, E.V.; Antonides, C.; Crespo-Leiro, M.G.; Combes, A.; Färber, G.; Hannan, M.M.; Kukucka, M.; De Jonge, N.; Loforte, A.; Lund, L.H.; et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur. J. Cardio Thorac. Surg. 2019, 56, 230–270. [Google Scholar] [CrossRef]
- Casarotto, D.; Bottio, T.; Gambino, A.; Testolin, L.; Gerosa, G. The last to die is hope: Prolonged mechanical circulatory support with a Novacor left ventricular assist device as a bridge to transplantation. J. Thorac. Cardiovasc. Surg. 2003, 125, 417–418. [Google Scholar] [CrossRef]
- Apostolo, A.; Paolillo, S.; Contini, M.; Vignati, C.; Tarzia, V.; Campodonico, J.; Mapelli, M.; Massetti, M.; Bejko, J.; Righini, F.; et al. Comprehensive effects of left ventricular assist device speed changes on alveolar gas exchange, sleep ventilatory pattern, and exercise performance. J. Heart Lung Transplant. 2018, 37, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Estep, J.D.; Starling, R.C.; Horstmanshof, D.A.; Milano, C.A.; Selzman, C.H.; Shah, K.B.; Loebe, M.; Moazami, N.; Long, J.W.; Stehlik, J.; et al. ROADMAP Study Investigators. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: The ROADMAP Study 2-Year Results. JACC Heart Fail. 2017, 5, 518–527. [Google Scholar]
- Kormos, R.L.; Antonides, C.F.; Goldstein, D.J.; Cowger, J.A.; Starling, R.C.; Kirklin, J.K.; Rame, J.E.; Rosenthal, D.; Mooney, M.L.; Caliskan, K.; et al. Updated definitions of adverse events for trials and registries of mechanical circulatory support: A consensus statement of the mechanical circulatory support aca-demic research consortium. J. Heart Lung Transplant. 2020, 39, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Carrozzini, M.; Bejko, J.; Guariento, A.; Rubino, M.; Bianco, R.; Tarzia, V.; Gregori, D.; Bottio, T.; Gerosa, G. Minimally Invasive Implantation of Continuous Flow Left Ventricular Assist Devices: The Evolution of Surgical Techniques in a Single-Center Experience. Artif. Organs 2018, 43, E41–E52. [Google Scholar] [CrossRef]
- Carrozzini, M.; Bejko, J.; Gerosa, G.; Bottio, T. Bilateral mini-thoracotomy approach for minimally invasive implantation of HeartMate 3. Artif. Organs 2018, 43, 593–595. [Google Scholar] [CrossRef]
- Bottio, T.; Bejko, J.; Falasco, G.; Bortolussi, G.; Gallo, M.; Tarzia, V.; Gerosa, G. Less-invasive off-pump ventricular assist device im-plantation in regional paravertebral analgesia. J. Artif. Organs 2014, 17, 275–277. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 11 August 2021).
- Kittleson, M.M.; Shah, P.; Lala, A.; McLean, R.C.; Pamboukian, S.; Horstmanshof, D.A.; Thibodeau, J.; Shah, K.; Teuteberg, J.; Gilotra, N.A.; et al. INTERMACS profiles and outcomes of ambulatory advanced heart failure patients: A report from the REVIVAL Registry. J. Heart Lung Transplant. 2019, 39, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Ambardekar, A.V.; Kittleson, M.M.; Palardy, M.; Mountis, M.M.; Forde-McLean, R.C.; DeVore, A.D.; Pamboukian, S.V.; Thibodeau, J.T.; Teuteberg, J.J.; Cadaret, L.; et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. J. Heart Lung Transplant. 2018, 38, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Dell’Aquila, A.M.; Schneider, S.R.; Stypmann, J.; Ellger, B.; Redwan, B.; Schlarb, D.; Martens, S.; Sindermann, J.R. Survival results after implantation of intrapericardial third-generation centrifugal assist device: An INTERMACS-matched comparison analysis. Artif. Organs 2014, 38, 383–390. [Google Scholar] [CrossRef]
- Stepanenko, A.; Potapov, E.V.; Jurmann, B.; Lehmkuhl, H.B.; Dandel, M.; Siniawski, H.; Drews, T.; Hennig, E.; Kaufmann, F.; Jurmann, M.J.; et al. Outcomes of elective versus emergent permanent mechanical circulatory support in the elderly: A single-center experience. J. Heart Lung Transplant. 2010, 29, 61–65. [Google Scholar] [CrossRef]
- Caruso, R.; Verde, A.; Cabiati, M.; Milazzo, F.; Boroni, C.; Del Ry, S.; Parolini, M.; Vittori, C.; Paino, R.; Martinelli, L.; et al. Association of pre-operative interleukin-6 levels with Interagency Registry for Mechanically Assisted Circulatory Support profiles and intensive care unit stay in left ventricular assist device patients. J. Heart Lung Transplant. 2012, 31, 625–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, J.R.; Cheng, A.; Singh, R.; Williams, M.L.; Slaughter, M.S. Survival on the heart transplant waiting list: Impact of con-tinuous flow left ventricular assist device as bridge to transplant. Ann. Thorac. Surg. 2014, 98, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Carrozzini, M.; Bejko, J.; Gambino, A.; Tarzia, V.; Lanera, C.; Gregori, D.; Gerosa, G.; Bottio, T. Results of new-generation intrapericardial continuous flow left ventricular assist devices as a bridge-to-transplant. J. Cardiovasc. Med. 2018, 19, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Carrozzini, M.; Bottio, T.; Caraffa, R.; Bejko, J.; Bifulco, O.; Guariento, A.; Lombardi, C.M.; Metra, M.; Azzolina, D.; Gregori, D.; et al. Impact of Continuous Flow Left Ventricular Assist Device on Heart Transplant Candidates: A Multi-State Survival Analysis. J. Clin. Med. 2022, 11, 3425. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, O.; Bottio, T.; Caraffa, R.; Carrozzini, M.; Guariento, A.; Bejko, J.; Fedrigo, M.; Castellani, C.; Toscano, G.; Lorenzoni, G.; et al. Marginal versus Standard Donors in Heart Transplantation: Proper Selection Means Heart Transplant Benefit. J. Clin. Med. 2022, 11, 2665. [Google Scholar] [CrossRef]
- Bejko, J.; Toto, F.; Gregori, D.; Gerosa, G.; Bottio, T. Left ventricle assist devices and driveline’s infection incidence: A single-centre experience. J. Artif. Organs 2017, 21, 52–60. [Google Scholar] [CrossRef]


| Baseline Characteristic | Overall (n = 192) | Profile 1–3 (n = 147) | Profile 4 (n = 45) | p |
|---|---|---|---|---|
| Age at Implant | 62.4 (54.5–66.9) | 60.6 (52.6–66.2) | 64.9 (61.3–68.7) | 0.001 |
| Gender (male) | 167 (87%) | 124 (84.4%) | 43 (95.6%) | 0.073 |
| Cardiac Diagnosis | 0.389 | |||
| DCM | 86 (44.8%) | 69 (49.6%) | 17 (37.8%) | |
| IHD | 94 (49.0%) | 68 (46.3%) | 26 (57.8%) | |
| Other | 12 (6.2%) | 10 (6.8%) | 2 (4.4%) | |
| INTERMACS class | ||||
| 1 | 60 (31.2%) | |||
| 2 | 27 (14.1%) | |||
| 3 | 60 (31.2%) | |||
| 4 | 45 (23.4%) | |||
| Device Strategy | 0.898 | |||
| Bridge to transplantation (BTT) | 79 (41.1%) | 58 (39.5%) | 21 (46.7%) | |
| Bridge to candidacy (BTC) | 58 (30.2%) | 50 (34.0%) | 8 (17.7%) | |
| Destination therapy (DT) | 55 (28.7%) | 39 (26.5%) | 16 (35.6%) | |
| Type of device | 0.528 | |||
| Jarvik | 62 (32.3%) | 45 (30.6%) | 17 (37.8%) | |
| HVAD | 54 (28.1%) | 44 (29.9%) | 10 (22.2%) | |
| Heart Mate 3 | 76 (39.6%) | 58 (39.5%) | 18 (40.0%) | |
| Smoker | 80 (41.7%) | 61 (41.5%) | 19 (42.2%) | 0.931 |
| Dyslipidaemia | 95 (49.5%) | 66 (44.9%) | 29 (64.4%) | 0.027 |
| Hypertension | 109 (56.8%) | 78 (53.1%) | 31 (68.9%) | 0.085 |
| AF preop | 73 (38.0%) | 56 (31.3%) | 17 (37.8%) | 0.969 |
| Cancer | 17 (8.9%) | 14 (9.5%) | 3 (6.7%) | 0.766 |
| Diabetes | 58 (30.2%) | 43 (29.3%) | 15 (33.3%) | 0.584 |
| Peripheral vascular disease | 50 (26.0%) | 32 (21.8%) | 18 (40.0%) | 0.020 |
| COPD | 17 (8.9%) | 13 (8.8%) | 4 (8.9%) | 0.993 |
| BSA (m2) | 1.88 (1.77–2.00) | 1.88 (1.74–2.00) | 1.90 (1.80–1.99) | 0.573 |
| ICD | 129 (67.2%) | 88 (59.9%) | 41 (91.1%) | <0.001 |
| Redo | 30 (15.6%) | 20 (13.6%) | 10 (22.2%) | 0.167 |
| Cardiac Index (L/min/m2) | 2.00 (1.95–2.05) | 1.98 (1.95–2.00) | 2.00 (1.92–2.05) | 0.040 |
| Preop vO2 Peak (mL/min/m2) | 11.0 (9.0–12.0) | 11.0 (9.0–12.0) | 11.0 (10.5–12.5) | 0.136 |
| Preop CVVH | 18 (9.4%) | 18 (12.2%) | 0 (0.0%) | 0.008 |
| Preop CVVH days | 5.5 (3.8–8.5) | 5.5 (3.8–8.5) | ||
| Preop ECMO | 48 (25.0%) | |||
| Days | 5.0 (2.0–10.0) | |||
| Preop temporary-LVAD | 27 (14.1%) | |||
| Days | 7.0 (4.0–11.0) | |||
| Preop Bi-VAD | 2 (1.0%) | |||
| Days | 12.0 (10.0–14.0) | |||
| Preop Intubation | 35 (18.2%) | 35 (23.8%) | 0 (0.0%) | <0.001 |
| Days | 3.5 (1.0–6.0) | |||
| Preoperative Labs | Overall (n = 192) | Profile 1–3 (n = 147) | Profile 4 (n = 45) | p |
| Haemoglobin g/L | 11.0 (9.6–12.6) | 10.6 (9.4–12.3) | 12.1 (10.6–13.5) | 0.040 |
| Platelets count 109/L | 209 (155–269) | 211 (155–278) | 201 (159–239) | 0.759 |
| D-dimer | 664 (277–1914) | 824 (310–2322) | 301 (202–552) | 0.010 |
| Bilirubin (mg/dL) | 0.95 (0.58–1.60) | 1.05 (0.64–1.89) | 0.74 (0.45–1.13) | <0.001 |
| AST (U/L) | 28 (20–42) | 30 (21–46) | 22 (16–30) | 0.002 |
| ALT (U/L) | 25 (17–41) | 26 (18–46) | 22 (15–35) | 0.007 |
| Amilasi (U/L) | 28 (19–60) | 30 (20–67) | 25 (16–36) | 0.001 |
| LAD (U/L) | 267 (192–421) | 287 (211–450) | 208 (160–273) | 0.057 |
| CRP (mg/L) | 19 (4–84) | 32 (13–97) | 3 (3–11) | <0.001 |
| Na (mmol/L) | 137 (134–140) | 137 (134–139) | 140 (136–142) | 0.048 |
| BNP | 6267 (3555–12,460) | 8233 (3794–13,874) | 3818 (1199–7560) | 0.050 |
| GFR (ml/min/m2) | 64.0 (45.0–90.0) | 68.0 (49.0–90.0) | 56.0 (43.0–79.0) | 0.182 |
| Creatinine (mg/dL) | 1.26 (0.94–1.59) | 1.24 (0.90–1.56) | 1.39 (1.03–1.61) | 0.567 |
| Preoperative Echocardiogram | Overall (n = 192) | Profile 1–3 (n = 147) | Profile 4 (n = 45) | p |
| EF (%) | 20 (17–26) | 20 (17–24) | 25 (20–29) | 0.001 |
| iLVEDV (mL/m2) | 130 (110–157) | 129 (106–157) | 135 (114–162) | 0.373 |
| TAPSE (mm) | 15.0 (13.0–19.0) | 15.0 (12.0–18.1) | 18.2 (14.3–20.8) | 0.771 |
| sPAP (mmHg) | 43 (35–54) | 43 (36–53) | 44 (32–44) | 0.758 |
| RV FAC (%) | 31 (24–39) | 29 (22–38) | 36 (29–40) | 0.002 |
| TR ≥ moderate | 82 (42.7%) | 64 (43.5%) | 18 (40.0%) | 0.732 |
| MR ≥ moderate | 112 (58.3%) | 87 (59.2%) | 25 (55.6%) | 0.731 |
| AR ≥ moderate | 4 (2.1%) | 3 (2.0%) | 1 (2.2%) | 0.941 |
| LVAD Implantation | Overall (n = 192) | Profile 1–3 (n = 147) | Profile 4 (n = 45) | p |
|---|---|---|---|---|
| Surgical access | ||||
| Full sternotomy | 110 (57.3%) | 87 (59.2%) | 23 (51.1%) | 0.338 |
| Minimally invasive | 82 (42.7%) | 60 (40.8%) | 22 (48.9%) | 0.390 |
| Outflow graft anastomosis | ||||
| Ascending aorta | 154 (80.2%) | 122 (83.0%) | 32 (71.1%) | 0.090 |
| Descending aorta | 27 (14.1%) | 19 (12.9%) | 8 (17.8%) | 0.463 |
| Left subclavian artery | 11 (5.7%) | 6 (4.1%) | 5 (11.1%) | 0.134 |
| Associated procedures | 25 (13.0%) | 16 (10.9%) | 9 (20.0%) | 0.130 |
| Device Implantation | ||||
| CPB | 121 (63.0%) | 90 (61.2%) | 31 (68.9%) | 0.383 |
| ECMO | 31 (16.2%) | 29 (19.7%) | 2 (4.4%) | 0.019 |
| Off-pump | 40 (20.8%) | 28 (19.0%) | 12 (26.7%) | 0.297 |
| General anaesthesia | 143 (74.5%) | 104 (70.7%) | 39 (86.7%) | 0.033 |
| PVB analgesia | 48 (25.0%) | 42 (28.6%) | 6 (13.3%) | 0.048 |
| Duration of intervention (min) | 285 (240–370) | 288 (240–366) | 270 (235–400) | 0.415 |
| Duration of CPB (min) | 110 (82–150) | 110 (87–152) | 100 (67–136) | 0.135 |
| Postoperative Outcomes | Overall (n = 192) | Profile 1–3 (n = 147) | Profile 4 (n = 45) | p |
|---|---|---|---|---|
| Intubation days | 1 (1–3) | 1 (1–3) | 1 (1–2) | 0.117 |
| Tracheostomy | 15 (7.8%) | 13 (8.8%) | 2 (4.4%) | 0.527 |
| Postop CVVH | 65 (33.9%) | 57 (38.8%) | 8 (17.8%) | 0.006 |
| Days | 9.0 (4.0–17.0) | 10.0 (4.0–22.5) | 6.5 (3.5–10.0) | 0.039 |
| RVF total | 89 (46.3%) | 75 (51.0%) | 14 (31.1%) | 0.026 |
| Acute RVF | 63 (34.4%) | 54 (36.7%) | 9 (20.0%) | 0.046 |
| Early-acute RVF | 28 (14.6%) | 27 (18.4%) | 1 (2.2%) | 0.007 |
| Early-post-implant RVF | 38 (19.8%) | 32 (21.8%) | 6 (13.3%) | 0.563 |
| Chronic RVF | 30 (15.6%) | 24 (16.3%) | 6 (13.3%) | 0.815 |
| RVAD implantation | 42 (21.9%) | 39 (26.5%) | 3 (6.7%) | 0.015 |
| Days | 10.0 (6.0–13.0) | 9.5 (6.0–13.0) | 12.0 (3.0–12.0) | 0.823 |
| ECMO postop | 13 (6.8%) | 12 (8.2%) | 1 (2.2%) | 0.306 |
| Days | 3.0 (2.0–6.0) | 3.0 (1.3–6.8) | 4 | 0.954 |
| NO inhalation | 105 (54.7%) | 82 (55.8%) | 23 (51.1%) | 0.609 |
| MajorAdverseEvents | Overall (n = 192) | Profile I–III (n = 147) | Profile IV (n = 45) | p |
| Major bleeding | 71 (37.0) | 57 (38.8%) | 14 (31.1%) | 0.383 |
| Revision for bleeding | 60 (31.2%) | 50 (34.0%) | 10 (22.2%) | 0.147 |
| Cerebral event | 47 (24.5%) | 35 (23.8%) | 12 (26.7%) | 0.583 |
| Non-fatal cerebral complications | 28 (14.6%) | 20 (13.6%) | 8 (17.8%) | 0.367 |
| Ischemic | 22 (11.5%) | 17 (11.6) | 5 (11.1%) | 0.233 |
| Haemorragic | 6 (3.1%) | 3 (2.0%) | 3 (6.7%) | 0.187 |
| Fatal cerebral complications | 19 (9.9%) | 17 (11.6%) | 2 (4.4%) | 0.121 |
| Bowel complications | 26 (13.5%) | 19 (12.9%) | 7 (15.6%) | 0.629 |
| Fatal bowel complications | 5 (2.6%) | 5 (3.4%) | 0 (0.0%) | 0.593 |
| Intra hospital documented infection | 95 (49.5%) | 80 (54.4%) | 15 (33.3%) | 0.017 |
| VAD infection | 2 (1.0%) | 2 (1.4%) | 0 (0.0%) | 0.430 |
| VAD thrombosis | 15 (7.8%) | 12 (8.2%) | 3 (6.7%) | 0.743 |
| Thrombolysis | 7 (3.6%) | 7 (4.8%) | 0 (0.0%) | 0.203 |
| Driveline infection total | 37 (19.3%) | 28 (19.0%) | 9 (20.0%) | 0.504 |
| Within 6-months | 14 (7.3%) | 12 (8.2%) | 2 (4.4%) | 0.525 |
| Over 6-months | 23 (12.0%) | 18 (12.2%) | 5 (11.1%) | 0.925 |
| Postop-ICU (days) | 8.0 (4.0–18.0) | 10.0 (5.0–20.0) | 5.0 (3.0–15.3) | 0.010 |
| In-hospital stay (days) | 30.0 (20.0–45.0) | 30.0 (20.5–52.5) | 26.5 (20.0–41.5) | 0.037 |
| 30-days death | 25 (13.0%) | 22 (15.0%) | 3 (6.7%) | 0.206 |
| 90-days death | 43 (22.4%) | 39 (26.5%) | 4 (8.9%) | 0.014 |
| In-hospital mortality | 48 (25.0%) | 45 (30.6%) | 3 (6.7%) | 0.001 |
| Cause of intra-hospital mortality | <0.001 | |||
| Multiorgan failure | 26 | 26 (17.7%) | 0 (0.0%) | |
| Neurologic | 12 | 10 (6.8%) | 2 (4.4%) | |
| Bowel complications | 3 | 2 (1.4%) | 1 (2.2% | |
| Sepsis | 7 | 7 (4.8%) | 0 (0.0%) | |
| Rehospitalization | 102 (53.1%) | 76 (51.7%) | 26 (57.8%) | 0.648 |
| Number of rehospitalization | 2 (1–4) | 2 (1–4) | 2 (1–3) | 0.996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caraffa, R.; Bejko, J.; Carrozzini, M.; Bifulco, O.; Tarzia, V.; Lorenzoni, G.; Bottigliengo, D.; Gregori, D.; Castellani, C.; Bottio, T.; et al. A Device Strategy-Matched Comparison Analysis among Different Intermacs Profiles: A Single Center Experience. J. Clin. Med. 2022, 11, 4901. https://doi.org/10.3390/jcm11164901
Caraffa R, Bejko J, Carrozzini M, Bifulco O, Tarzia V, Lorenzoni G, Bottigliengo D, Gregori D, Castellani C, Bottio T, et al. A Device Strategy-Matched Comparison Analysis among Different Intermacs Profiles: A Single Center Experience. Journal of Clinical Medicine. 2022; 11(16):4901. https://doi.org/10.3390/jcm11164901
Chicago/Turabian StyleCaraffa, Raphael, Jonida Bejko, Massimiliano Carrozzini, Olimpia Bifulco, Vincenzo Tarzia, Giulia Lorenzoni, Daniele Bottigliengo, Dario Gregori, Chiara Castellani, Tomaso Bottio, and et al. 2022. "A Device Strategy-Matched Comparison Analysis among Different Intermacs Profiles: A Single Center Experience" Journal of Clinical Medicine 11, no. 16: 4901. https://doi.org/10.3390/jcm11164901
APA StyleCaraffa, R., Bejko, J., Carrozzini, M., Bifulco, O., Tarzia, V., Lorenzoni, G., Bottigliengo, D., Gregori, D., Castellani, C., Bottio, T., Angelini, A., & Gerosa, G. (2022). A Device Strategy-Matched Comparison Analysis among Different Intermacs Profiles: A Single Center Experience. Journal of Clinical Medicine, 11(16), 4901. https://doi.org/10.3390/jcm11164901








