Primary Catheter-Directed Thrombolysis for Porto-Mesenteric Venous Thrombosis (PMVT) in Non-Cirrhotic Patients
Abstract
:1. Introduction
2. Patients and Methods
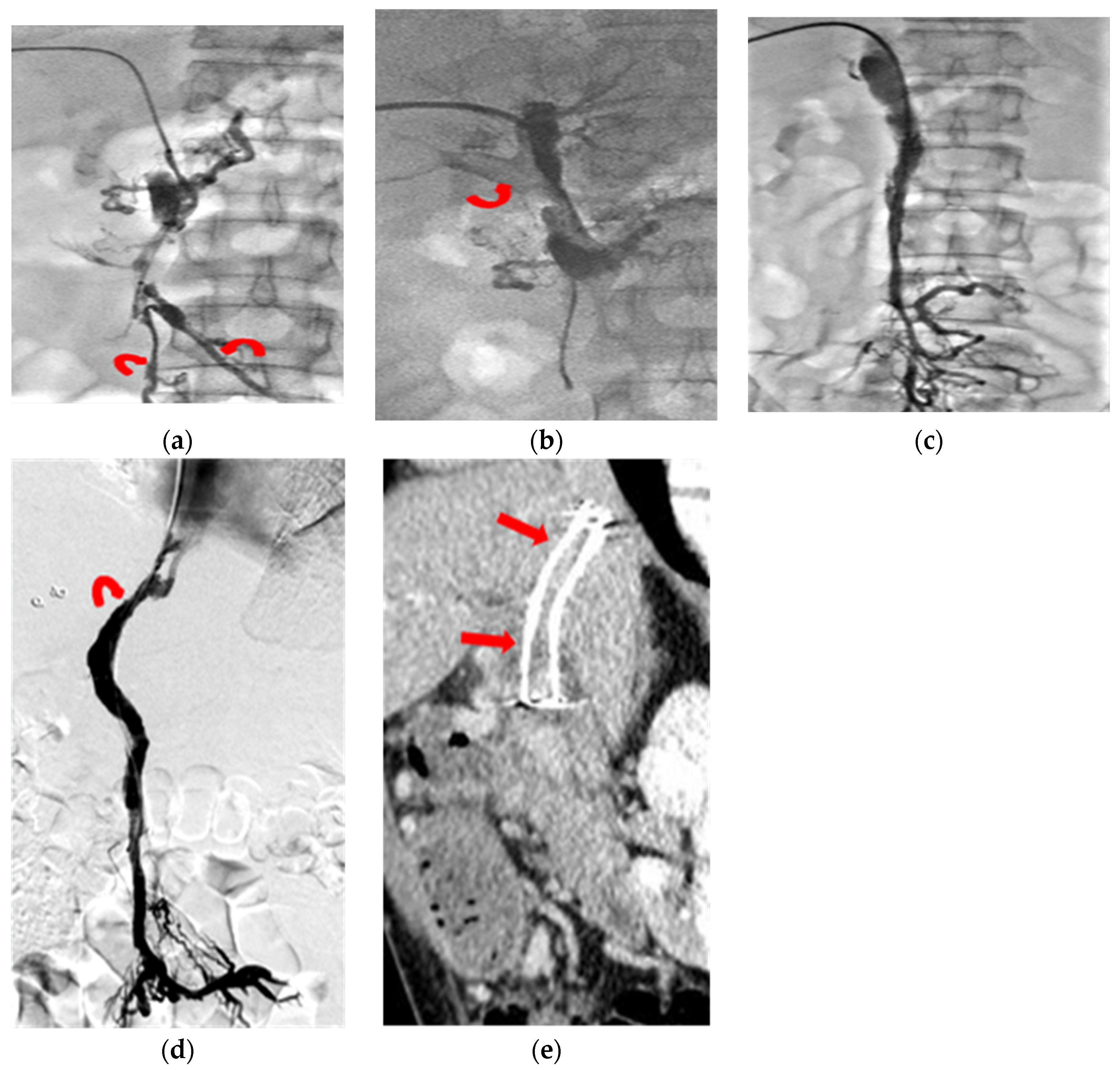
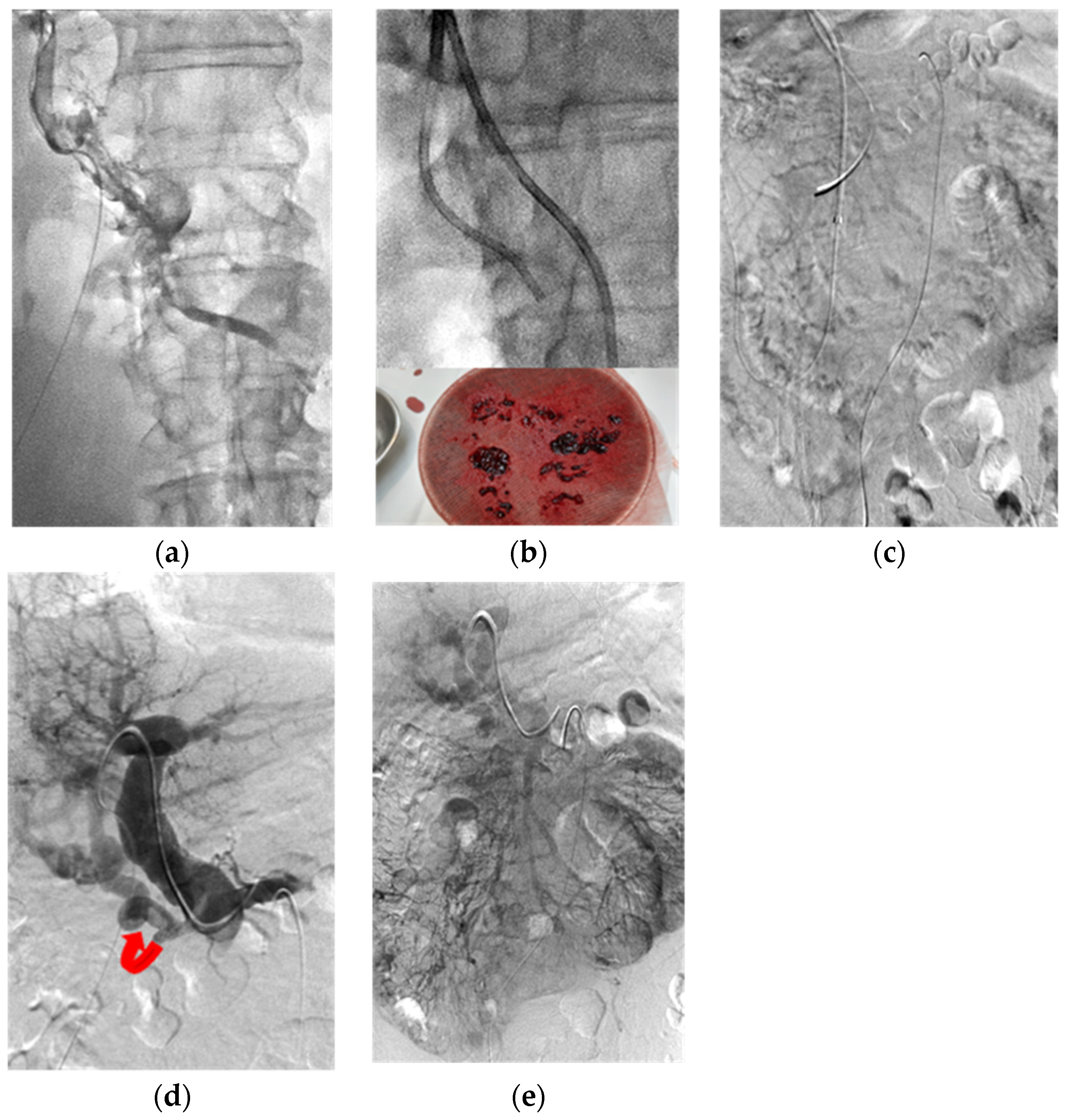
3. Thrombolysis Technique
4. Statistical Analysis
5. Results
6. Discussion
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Consent for Publication:
References
- Grendell, J.H.; Ockner, R.K. Mesenteric venous thrombosis. Gastroenterology 1982, 82, 358–372. [Google Scholar] [CrossRef]
- Søgaard, K.K.; Darvalics, B.; Horváth-Puhó, E.; Sørensen, H.T. Survival after splanchnic vein thrombosis: A 20-year nationwide cohort study. Thromb. Res. 2019, 141, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chawla, Y.K.; Bodh, V. Portal vein thrombosis. J. Clin. Exp. Hepatol. 2015, 5, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Levigard, R.B.; Salas, H.; Serrão, H.; Diniz, F.; Nogueira CA, V.; de Oliveira, A.A.; Pereira, G. Liver Growth and Portal Hypertension Improvement AfterPercutaneous Recanalization of Chronic Portal Vein Thrombosisin Non-Cirrhotic Participants. Cardiovasc. Interv. Radiol. 2022, 45, 582–589. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address eee. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J. Hepatol. 2016, 64, 179–202. [Google Scholar] [CrossRef]
- DeLeve, L.D.; Valla, D.C.; Garcia, T.G. American Association for the Study Liver D: Vascular disorders of the liver. Hepatology 2009, 49, 1729–1764. [Google Scholar] [CrossRef]
- Benmassaoud, A.; AlRubaiy, L.; Yu, D.; Chowdary, P.; Sekhar, M.; Parikh, P.; Patch, D. A stepwise thrombolysis regimen in the management of acute portal vein thrombosis in patients with evidence of intestinal ischaemia. Aliment Pharm. 2019, 50, 1049–1058. [Google Scholar] [CrossRef]
- Bilbao, J.I.; Elorz, M.; Vivas, I.; Martínez-Cuesta, A.; Bastarrika, G.; Benito, A. Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Treatment of Venous Symptomatic Chronic Portal Thrombosis in Non-Cirrhotic Patients. Cardiovasc. Interv. Radiol. 2004, 27, 474–480. [Google Scholar] [CrossRef]
- Hollingshead, M.; Burke, C.T.; Mauro, M.A.; Weeks, S.M.; Dixon, R.G.; Jaques, P.F. Transcatheter thrombolytic therapy for acute mesenteric and portal vein thrombosis. J. Vasc. Interv. Radiol. 2005, 16, 651–661. [Google Scholar] [CrossRef]
- Liu, F.Y.; Wang, M.Q.; Fan, Q.S.; Duan, F.; Wang, Z.J.; Song, P. Interventional treatment for symptomatic acute-subacute portal and superior mesenteric vein thrombosis. World J. Gastroenterol. 2009, 15, 5028–5034. [Google Scholar] [CrossRef]
- Luo, X.; Nie, L.; Zhou, B.; Yao, D.; Ma, H.; Jiang, M.; Li, X. Transjugular intrahepatic portosystemic shunt for the treatment of portal hypertension in noncirrhotic patients with portal cavernoma. Gastroenterol. Res. Pract. 2014, 2014, 659726. [Google Scholar] [CrossRef]
- Rosenqvist, K.; Ebeling Barbier, C.; Rorsman, F.; Sangfelt, P.; Nyman, R. Treatment of acute portomesenteric venous thrombosis with thrombectomy through a transjugular intrahepatic portosystemic shunt: A single-center experience. Acta Radiol. 2018, 59, 953–958. [Google Scholar] [CrossRef]
- Klinger, C.; Riecken, B.; Schmidt, A.; De Gottardi, A.; Meier, B.; Bosch, J.; Caca, K. Transjugular local thrombolysis with/without TIPS in patients with acute non-cirrhotic, non-malignant portal vein thrombosis. Dig. Liver Dis. 2017, 49, 1345–1352. [Google Scholar] [CrossRef]
- Rabuffi, P.; Vagnarelli, S.; Bruni, A.; Antonuccio, G.; Ambrogi, C. Percutaneous Pharmaco-Mechanical Thrombectomy of Acute Symptomatic Superior Mesenteric Vein Thrombosis. Cardiovasc. Interv. Radiol. 2020, 43, 46–54. [Google Scholar] [CrossRef]
- Smalberg, J.H.; Spaander, M.V.; Jie KS, G.; Pattynama, P.M.; van Buuren, H.R.; van den Berg, B.; Leebeek, F.W. Risks and benefits of transcatheter thrombolytic therapy in patients with splanchnic venous thrombosis. Thromb. Haemost. 2008, 100, 1084–1088. [Google Scholar] [CrossRef]
- Song, J.H.; He, X.; Lou, W.S.; Chen, L.; Chen, G.P.; Su, H.B.; Gu, J.P. Application of percutaneousAngioJet thrombectomy in patients with acute symptomaticportal and superior mesenteric venous thrombosis. Zhonghua Yi Xue Za Zhi 2017, 97, 991–995. [Google Scholar]
- Acosta, S.; Alhadad, A.; Svensson, P.; Ekberg, O. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. Br. J. Surg. 2008, 95, 1245–1251. [Google Scholar] [CrossRef]
- Harnik, I.G.; Brandt, L.J. Mesenteric venous thrombosis. Vasc. Med. 2010, 15, 407–418. [Google Scholar] [CrossRef]
- Kumar, S.; Sarr, M.G.; Kamath, P.S. Mesenteric venous thrombosis. N. Engl. J. Med. 2001, 345, 1683–1688. [Google Scholar] [CrossRef]
- Hall, T.C.; Garcea, G.; Metcalfe, M.; Bilku, D.; Dennison, A.R. Management of acute non-cirrhotic and non-malignant portal vein thrombosis: A systematic review. World J. Surg. 2011, 35, 2510–2520. [Google Scholar] [CrossRef]
- Plessier, A.; Darwish-Murad, S.; Hernandez-Guerra, M.; Consigny, Y.; Fabris, F.; Trebicka, J. Acute portal vein thrombosis unrelated to cirrhosis: A prospective multicenter follow-up study. Hepatology 2010, 51, 210–218. [Google Scholar] [CrossRef]
- Amitrano, L.; Guardascione, M.A.; Scaglione, M.; Pezzullo, L.; Sangiuliano, N.; Armellino, M.F.; Balzano, A. Prognostic factors in noncirrhotic patients with splanchnic vein thromboses. Am. J. Gastroenterol. 2007, 102, 2464–2470. [Google Scholar] [CrossRef]
- Turnes, J.; García–Pagán, J.C.; González, M.; Aracil, C.; Calleja, J.L.; Ripoll, C.; Bosch, J. Portal hypertension-related complications after acute portal vein thrombosis: Impact of early anticoagulation. Clin. Gastroenterol. Hepatol. 2008, 6, 1412–1417. [Google Scholar] [CrossRef]
- Fanelli, F.; Angeloni, S.; Salvatori, F.M.; Marzano, C.; Boatta, E.; Merli, M.; Riggio, O. Transjugular intrahepatic portosystemic shunt with expanded-polytetrafuoroethylene-covered stents in non-cirrhotic patients with portal cavernoma. Dig. Liver Dis. 2011, 43, 78–84. [Google Scholar] [CrossRef]
- Qi, X.; Han, G.; Yin, Z.; He, C.; Wang, J.; Guo, W.; Fan, D. Transjugular intrahepatic portosystemic shunt for portal cavernoma with symptomatic portal hypertension in non-cirrhotic patients. Dig. Dis. Sci. 2012, 57, 1072–1082. [Google Scholar] [CrossRef]
- Fonseca, A.L.; Cleary, M.A.; Cholewczynski, W.; Sumpio, B.E.; Atweh, N.A. Omental vein catheter thrombolysis for acute porto-mesenteric vein thrombosis. Ann. Vasc. Surg. 2013, 27, 497.e1–497.e4. [Google Scholar] [CrossRef]
- Liu, K.; Li, W.D.; Du, X.L.; Li, C.L.; Li, X.Q. Comparison of Systemic Thrombolysis Versus Indirect Thrombolysis via the Superior Mesenteric Artery in Patients with Acute Portal Vein Thrombosis. Ann. Vasc. Surg. 2017, 39, 264–269. [Google Scholar] [CrossRef]
- Chamarthy, M.R.; Anderson, M.E.; Pillai, A.K.; Kalva, S.P. Thrombolysis and Transjugular Intrahepatic Portosystemic Shunt Creation for Acute and Subacute Portal Vein Thrombosis. Tech. Vasc. Interv. Radiol. 2016, 19, 42–51. [Google Scholar] [CrossRef]
- Semiz-Oysu, A.; Keussen, I.; Cwikiel, W. Interventional radiological management of prehepatic obstruction of [corrected] the splanchnic venous system. Cardiovasc. Interv. Radiol. 2007, 30, 688–695. [Google Scholar] [CrossRef]
- Senzolo, M.; Tibbals, J.; Cholongitas, E.; Triantos, C.K.; Burroughs, A.K.; Patch, D. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with and without cavernous transformation. Aliment Pharm. 2006, 23, 767–775. [Google Scholar] [CrossRef]
- Perarnau, J.M.; Baju, A.; D’alteroche, L.; Viguier, J.; Ayoub, J. Feasibility and long-term evolution of TIPS in cirrhotic patients with portal thrombosis. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, P.; Li, Y.; Ma, S.; Zhao, J.; Zeng, Q. Percutaneous transhepatic balloon-assisted transjugular intrahepatic portosystemic shunt for chronic, totally occluded, portal vein thrombosis with symptomatic portal hypertension: Procedure technique, safety, and clinical applications. Eur. Radiol. 2015, 25, 3431–3437. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Han, G. Transjugular intrahepatic portosystemic shunt in the treatment of portal vein thrombosis: A critical review of literature. Hepatol. Int. 2012, 6, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.L.; Li, M.F.; Hsiao, C.C.; Wu, C.J.; Wu, T.H. Endovascular management of aorto-iliac occlusive disease (Leriche syndrome). J. Formos. Med. Assoc. 2020, 120, 1485–1492. [Google Scholar] [CrossRef]
- Lopera, J.E.; Correa, G.; Brazzini, A.; Ustunsoz, B.; Patel, S.; Janchai, A.; Castaneda-Zuniga, W. Percutaneous transhepatic treatment of symptomatic mesenteric venous thrombosis. J. Vasc. Surg. 2002, 36, 1058–1061. [Google Scholar] [CrossRef]
- Aytekin, C.; Boyvat, F.; Kurt, A.; Yologlu, Z.; Coskun, M. Catheter-directed thrombolysis with transjugular access in portal vein thrombosis secondary to pancreatitis. Eur. J. Radiol. 2001, 39, 80–82. [Google Scholar] [CrossRef]
- Kercher, K.W.; Sing, R.F.; Watson, K.W.; Matthews, B.D.; LeQuire, M.H.; Heniford, B.T. Transhepatic thrombolysis in acute portal vein thrombosis after laparoscopic splenectomy. Surg. Laparosc. Endosc. Percutaneous Tech. 2002, 12, 131–136. [Google Scholar] [CrossRef]
- Schoots, I.G.; Koffeman, G.I.; Legemate, D.A.; Levi, M.; Van Gulik, T.M. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br. J. Surg. 2004, 91, 17–27. [Google Scholar] [CrossRef]



| No | Sex | Age | Symptoms | Onset | Etiology | A/C | Cavernoma | Wall Edema | Ascites | Peripheral Venules |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 69 | abdominal pain | 3 days | unknown | acute | nil | y | nil | nil |
| 2 | F | 9 | abdominal pain | 6 months | nephrotic syndrome | chronic | y | y | y | nil |
| 3 | M | 37 | abdominal pain | 7 days | antiphospholipid antibody syndrome | acute | nil | y | nil | PV + MV |
| 4 | M | 47 | abdominal pain | 14 days | unknown | acute | nil | y | nil | MV |
| 5 | F | 11 | variceal bleeding | 3 years | protein C deficiency | chronic | y | y | y | nil |
| 6 | M | 26 | variceal bleeding | 4 months | myeloproliferative disorder | chronic | y | y | nil | nil |
| 7 | F | 73 | abdominal pain | 2 months | unknown | chronic | y | y | nil | PV + MV |
| 8 | M | 96 | tarry stool | 12 days | unknown | chronic | nil | y | nil | nil |
| 9 | M | 35 | abdominal pain | 26 days | myeloproliferative disorder | subacute | y | y | y | PV + MV |
| 10 | M | 40 | abdominal pain | 3 days | antithrombin III deficiency | chronic | y | y | y | PV + MV |
| 11 | M | 76 | fever of unknown origin | 14 days | antithrombin III deficiency | acute | nil | thinning | nil | PV |
| 12 | M | 49 | abdominal pain | 2 days | myeloproliferative disorder | acute | nil | y | y | PV + MV |
| 13 | M | 59 | abdominal pain | 4 days | unknown | chronic | y | y | y | PV + MV |
| 14 | F | 51 | abdominal pain | 14 days | unknown | subacute | nil | y | y | PV + MV |
| 15 | M | 56 | tarry stool | 23 days | pancreatitis | chronic | nil | y | y | nil |
| 16 | M | 44 | abdominal pain | 22 days | unknown | chronic | y | y | nil | PV |
| No | Route | L/R | Lysis Days | UK Dose (Million) | BMS Size (mm) | Recanalization | Follow-Up Image | Status | Survival |
|---|---|---|---|---|---|---|---|---|---|
| 1 | TH | R | 0.4 | 0.4 | 7 × 60 | CR | CT-1m | patent | 4 months-lfu |
| 2 | TH | R | 12 | 6.8 | 8 × 100(2) | CR | US-128m | patent | 150 months |
| 3 | TH + SMA | L | 9 | 8.6 | 6 × 120 | CR | CT-105m | patent | 119 months |
| 4 | TH + SMA | L | 3 | 4.5 | 6 × 60 | CR | CT-79m | patent | 110 months |
| 5 | TH | R | 6 | 2.4 | 8 × 60 | CR | CT-25m | occlusion | 106 months |
| 6 | TH | R | 13 | 9.6 | 8 × 100 | CR | CT-26m | patent | 105 months |
| 7 | TH + SMA | R | 2 | 2.8 | nil | refused further CDT | nil | excluded | Expired––9 days |
| 8 | TH | R | 3 | 0 | 7 × 60 | CR | nil | uncertain | Expired––1.5m |
| 9 | TH + SMA + TIPS | R | 19 | 16.4 | 8 × 80 | PR | CT-1m | re-occluded | 2 months-lfu |
| 10 | TH + SMA | L | 12 | 11.6 | nil | CR | CT-65m | patent | 68 months |
| 11 | TH | L | 3 | 3.6 | 8 × 37 | CR | US-36m | patent | 41 months |
| 12 | TH + IMA + TIPS | R | 12 | 10.8 | 10 × 80 | PR | nil | uncertain | Expired––30 days |
| 13 | TH + SMA | L | 6 | 6.4 | nil | CR | MR-15m | patent | 25 months |
| 14 | TH + SMA | R | 21 | 20.4 | 8 × 40 | PR: refused TIPS | CT-1m | re-occluded | 18 months |
| 15 | TH | R | 1 | 1.2 | 10 × 37 | CR | CT-5m | patent | 14 months |
| 16 | TH + TIPS | L | 15 | 12.9 | 8 × 60 | CR | CT-2m | patent | 5 months |
| Author | Year | Cirrhosis/Non-C | Route TJ/TH/SMA | Acute/Chronic | Thrombolytic Agent | Thrombectomy Device | Technical Success | CR/PR |
|---|---|---|---|---|---|---|---|---|
| Bilbao [8] | 2004 | 0/6 | TJ/TH | chronic | nil/bare metal stent | nil | 100% | nr |
| Hollingshead [9] | 2005 | 0/20 | TH/SMA | acute | urokinase/rt-PA | C-aspiration | 100% | 15%/60% |
| Smalberg [15] | 2008 | 2/10 | TH/TJ | acute | rt-PA | nil | 100% | 25%/33.3% |
| Liu [10] | 2009 | 14/32 | TJ/TH/SMA | acute | urokinase | C-aspiration | 100% | 56.5%/17.4% |
| Fanelli [24] | 2011 | 0/12 | TJ | chronic | nil/stentgraft | nil | 83.3% | 90%/10% |
| Qi [25] | 2012 | 0/20 | TJ | chronic | nil/bare metal stent | nil | 35% | nr |
| Luo [11] | 2014 | 0/15 | TJ | chronic | nil/stentgraft | nil | 73.3% | nr |
| Song [16] | 2017 | 0/8 | TH | acute | urokinase | yes | 100% | 37.5%/62.5% |
| Klinger [13] | 2017 | 0/17 | TJ | acute | Urokinase/rt-PA | C-aspiration | 94.1% | 56.3%/43.8% |
| Rosenqvist [12] | 2018 | 2/4 | TJ | acute | alteplase | yes | 100% | 83.3%/16.7% |
| Rabuffi [14] | 2020 | 1/7 | TJ/TH | acute | urokinase | yes | 100% | nr |
| Benmassaoud [7] | 2019 | 0/14 | TJ | acute | rt-PA | yes | 78.6% | 36.4%/45.4% |
| current study | 2022 | 0/16 | TH/SMA/TJ | acute/chronic | urokinase | C-aspiration | 100% | 80%/20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.-L.; Liang, H.-L.; Chen, W.-C.; Li, M.-F. Primary Catheter-Directed Thrombolysis for Porto-Mesenteric Venous Thrombosis (PMVT) in Non-Cirrhotic Patients. J. Clin. Med. 2022, 11, 4721. https://doi.org/10.3390/jcm11164721
Chiang C-L, Liang H-L, Chen W-C, Li M-F. Primary Catheter-Directed Thrombolysis for Porto-Mesenteric Venous Thrombosis (PMVT) in Non-Cirrhotic Patients. Journal of Clinical Medicine. 2022; 11(16):4721. https://doi.org/10.3390/jcm11164721
Chicago/Turabian StyleChiang, Chia-Ling, Huei-Lung Liang, Wen-Chi Chen, and Ming-Feng Li. 2022. "Primary Catheter-Directed Thrombolysis for Porto-Mesenteric Venous Thrombosis (PMVT) in Non-Cirrhotic Patients" Journal of Clinical Medicine 11, no. 16: 4721. https://doi.org/10.3390/jcm11164721
APA StyleChiang, C.-L., Liang, H.-L., Chen, W.-C., & Li, M.-F. (2022). Primary Catheter-Directed Thrombolysis for Porto-Mesenteric Venous Thrombosis (PMVT) in Non-Cirrhotic Patients. Journal of Clinical Medicine, 11(16), 4721. https://doi.org/10.3390/jcm11164721






