Anti-Diabetic Therapy, Heart Failure and Oxidative Stress: An Update
Abstract
1. Introduction
2. Materials and Methods
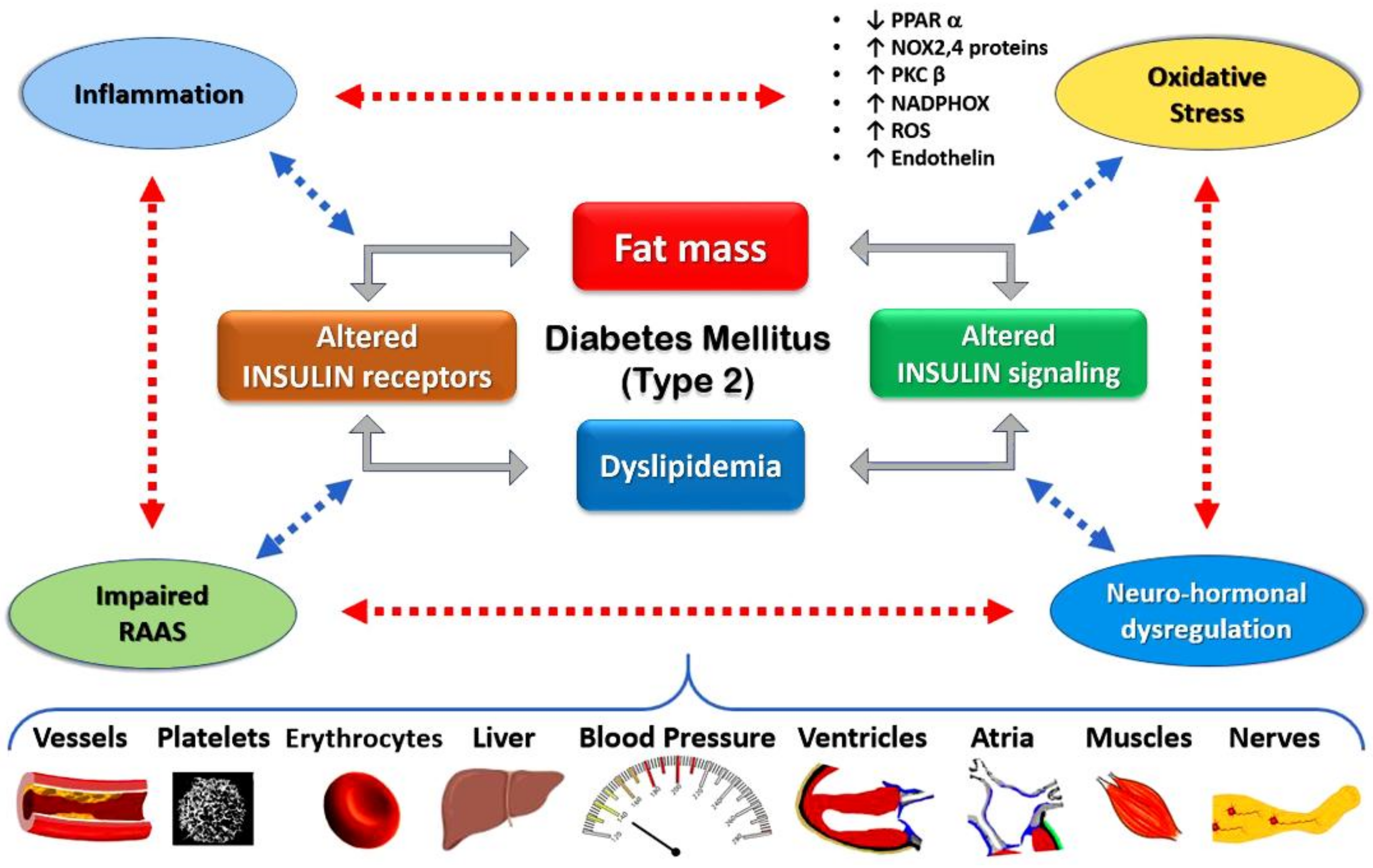
3. Epidemiology and Pathophysiological Pathway
4. Oxidative Stress in Diabetic Cardiomyopathy and β-Cell Dysfunction
- Subnormal expression of antioxidants (SOD, CAT, GPx) in β-cells due to hyperglycemia, high FFA-induced ROS and reactive nitrogen species (RNS) accumulation [19].
- Chronic exposure of β-cells to oxidative stress inhibits insulin secretion by opening ATP-sensitive K+ channels and suppressing calcium influx, which results from the ROS-induced overproduction of cyclin-dependent kinase inhibitor p21 [20].
- Chronic exposure of β-cells to elevated FFA, which decreases mitochondrial membrane potential and leads to uncoupled protein-2 accumulation, can also activate β-cell ATP-sensitive K+ channels to inhibit insulin production [21].
- Reduced transcriptional activity of insulin genes by nuclear accumulation of pancreas duodenal homeobox factor 1, which is a key transcription factor responsible for maintaining β-cell function) by oxidative stress [22].
- Excessive long-chain acyl CoA will be generated in the process of increased β-cell fatty acid metabolism, which can keep β-cell ATP-sensitive K+ channels open to suppress ATP generation and insulin secretion [25].
- ROS overproduction reduces insulin secretion by suppressing the expression of MaFA, a member of the fundamental leucine zipper family of transcription factors involved in the transcription of insulin genes [22].
5. Oxidative-Stress-Mediated Cardiac Hypertrophy/Heart Failure and Diabetes
- NOX proteins: ROS-generating enzymes
- b.
- Metabolic disorders: obesity and diabetes
- c.
- Mitochondrial dysfunction
- d.
- Inflammation
- e.
- Dysregulated autophagy and protein homeostasis
6. Anti-Diabetic Drug Categories and Their Action on Oxidative Stress
6.1. Metformin
6.2. Thiazolidinediones
6.3. Sulfonylureas
6.4. Meglitinides
6.5. Alpha Glucosidase Inhibitors
6.6. Insulin
6.7. Dipeptidyl Peptidase 4 Inhibitors
6.8. Sodium–Glucose Cotransporter-2 (SGLT-2) Inhibitors
6.9. Studies on the Effect of SGLT2 Inhibitors on Cardiovascular Outcomes and Heart Failure
6.10. Glucagon-Likepeptide-1 (GLP-1) Receptor Agonists
7. Conclusions
8. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| (in alphabetical order) | |
| ACE | Acarbose cardiovascular evaluation |
| AGE | Advanced glucose end-products |
| AMPK | Adenosine monophosphate-activated protein kinase |
| ARNI | Angiotensin receptor neprilysin inhibitor |
| CAD | Coronary artery disease |
| CKD | Chronic kidney disease |
| CANVAS | Canagliflozin Cardiovascular Assessment Study |
| CANSAS-R | Canaglifozin Cardiovascular Assessment Study-Renal |
| CARMELIN | Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes |
| CAROLINA | Cardiovascular Outcome Study of Linagliptin versus Glimepiride in Patients with Type 2 Diabetes |
| CREDENCE | Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation |
| CV | Cardiovascular |
| CVD-REAL | Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors |
| CVOTs | Cardiovascular outcome trials |
| DAPA-HF | Dapagliflozin in patients with HF and reduced ejection fraction |
| DECLARE-TIMI 58 | Program, Dapagliflozin Effect on Cardiovascular Events-Thrombolysis In Myocardial Infarction |
| DEFENCE | effectiveness on vascular endothelial function and glycemic control |
| DEFINE-HF | Dapagliflozin Effects on Biomarkers, Symptoms and Functional Status in Patients with HF with Reduced Ejection Fraction |
| DEVOTE | A Trial Comparing Cardiovascular Safety of Insulin Degludec Versus Insulin Glargine |
| DM | Diabetes mellitus |
| DNA | Deoxyribonucleic acid |
| DPP4 | Dipeptidyl peptidase 4 |
| DREAM | Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication |
| ELIXA | Evaluation of Lixisenatide in Acute Coronary Syndrome |
| EMMY | Impact of Empagliflozin on cardiac function and biomarkers of heart failure in patients with acute myocardial infarction |
| TOSCA.IT | The Thiazolidinediones Or Sulfonylureas and Cardiovascular Accidents Intervention Trial |
| UGPD | University Group Diabetes Program |
| Akt | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| ASK1 | Apoptosis-signal-regulating kinase 1 |
| BCL10 | B-cell lymphoma/leukemia 10 |
| CARD9 | Caspase recruitment domain family member 9 |
| CDC20 | Cell division cycle protein 20 homolog |
| CHCHD3 | Coiled-coil helix coiled-coil helix domain-containing protein 3 |
| CTRP3 | C1q-tumour necrosis factor-related protein 3 |
| CTRP9 | C1q-tumour necrosis factor-related protein 9 |
| ENDOG | Endonuclease G |
| Erk1/2 | Extracellular signal-regulated kinase ½ |
| FGF21 | Fibroblast growth factor 21 |
| FNDC5 | Fibronectin type III domain containing 5 |
| FOXO1 | Forkhead box O1 |
| FOXO3a | Forkhead box O3 |
| GSK3β | Glycogen synthase kinase 3 beta |
| HDAC4 | Histone deacetylase 4 |
| Hsp22 | Heat shock protein 22 |
| JAK2 | Janus kinase 2 |
| Jnk1/2 | c-Jun N-terminal kinases1/2 |
| LC3 | Microtubule-associated proteins 1A/1B light chain 3B |
| LKB1 | Loss of tumor suppressor liver kinase B1 |
| LPS | Lipopolysaccharide |
| LOX | Lysyl oxidase |
| MAPK | Mitogen-activated protein kinase |
| MICOS | Mitochondrial contact site and cristae organizing system |
| mitoKATP | Mitochondrial ATP-sensitive potassium channel |
| MnSOD | Manganese superoxide dismutase |
| MnTBAP | Superoxide scavenger Mn (III) tetrakis (4-benzoic acid) porphyrin chloride |
| MTG1 | Mitochondrial ribosome associated GTPase 1 |
| mTOR | Mammalian target of rapamycin |
| NCOA4 | Nuclear receptor coactivator 4 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOX | Nicotinamide adenine dinucleotide phosphate oxidase |
| NOX2 | NADPH oxidase 2 |
| NOX4 | NADPH oxidase 4 |
| NOX5 | NADPH oxidase 5 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PI3K | Phosphoinositide 3-kinase |
| PIKfyve | FYVE finger-containing phosphoinositide kinase |
| PKA | Protein kinase A |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PPARδ | Peroxisome proliferator-activated receptor delta |
| ROS | Reactive oxygen species |
| RPS6 | Ribosomal Protein S6 |
| SAM50 | Sorting and assembly machinery 50 |
| SFXN1 | Sideroflexin1 |
| SIRT1 | Sirtuin 1 |
| SIRT3 | Sirtuin 3 |
| STAT3 | Signal transducer and activator of transcription 3 |
| STVNa | Isosteviol sodium |
| TAK1 | Transforming growth factor beta-activated kinase 1 |
| TIM50 | Translocase of inner mitochondrial membrane 50 |
| TLR4 | Toll-like receptor 4 |
| TRPC3 | Transient receptor potential channel, canonical 3 |
| 4E-BP1 | Eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 |
References
- Lehrke, M.; Marx, N. Diabetes mellitus and heart failure. Am. J. Med. 2017, 130, S40–S50. [Google Scholar] [CrossRef] [PubMed]
- Bowes, C.D.; Lien, L.F.; Butler, J. Clinical aspects of heart failure in individuals with diabetes. Diabetologia 2019, 62, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A.; Rutter, G.A. The role of oxidative stress and hypoxia in pancreatic Beta-Cell dysfunction in diabetes mellitus. Antioxid. Redox. Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–46. [Google Scholar] [PubMed]
- Franchini, A.M.; Hunt, D.; Melendez, J.A.; Drake, J.R. FcγR-driven release of IL-6 by macrophages requires NOX2-dependent production of reactive oxygen species. J. Biol. Chem. 2013, 288, 25098–25108. [Google Scholar] [CrossRef]
- Huang, X.; Sun, M.; Li, D.; Liu, J.; Guo, H.; Dong, Y.; Jiang, L.; Pan, Q.; Man, Y.; Wang, S.; et al. Augmented NADPH oxidase activity and p22phox expression in monocytes underlie oxidative stress of patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2011, 91, 371–380. [Google Scholar] [CrossRef]
- Elumalai, S.; Karunakaran, U.; Moon, J.S.; Won, K.C. NADPH Oxidase (NOX) targeting in diabetes: A special emphasis on pancreatic β-cell dysfunction. Cells 2021, 10, 1573. [Google Scholar] [CrossRef]
- Cattadori, G.; Pantanetti, P.; Ambrosio, G. Glucose-lowering drugs and heart failure: Implications of recent cardiovascular outcome trials in type 2 diabetes. Diabetes Res. Clin. Pract. 2019, 157, 107835. [Google Scholar] [CrossRef]
- Kenny, H.C.; Abel, E.D. Heart failure in type 2 diabetes mellitus: Impact of glucose-lowering agents, heart failure therapies, and novel therapeutic strategies. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef]
- Bell, D.S.H.; Goncalves, E. Heart failure in the patient with diabetes: Epidemiology, aetiology, prognosis, therapy and the effect of glucose lowering medications. Diabetes Obes. Metab. 2019, 21, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Vasko, P.; Jonsson, Å.; Edner, M.; Dahlström, U.; Lund, L.H. The Swedish Heart Failure Registry: A living, ongoing quality assurance and research in heart failure. Ups. J. Med. Sci. 2019, 124, 65–69. [Google Scholar] [CrossRef]
- Nassif, M.E.; Kosiborod, M.A. Review of cardiovascular outcomes trials of glucose-lowering therapies and their effects on heart failure outcomes. Am. J. Cardiol. 2019, 124, S12–S19. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.P.; Robinson, E.; Edgar, K.S.; McMullan, R.; O’Neill, K.M.; Alderdice, M.; Amirkhah, R.; Dunne, P.D.; McDermott, B.J.; Grieve, D.J. Downregulation of PPARα during experimental left ventricular hypertrophy is critically dependent on Nox2 NADPH oxidase signalling. Int. J. Mol. Sci. 2020, 21, 4406. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Cong, S.; Chan, X.; Yap, E.P.; Yu, F.; Hausenloy, D.J. Oxidative stress in cardiac hypertrophy: From molecular mechanisms to novel therapeutic targets. Free Radic. Biol. Med. 2021, 166, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Dihoum, A.; Mordi, I.R.; Choy, A.M.; Rena, G.; Lang, C.C. Left ventricular hypertrophy in diabetic cardiomyopathy: A target for intervention. Front. Cardiovasc. Med. 2021, 8, 746382. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, S.; Gao, X.; Mao, X.; Wang, T.; Wong, G.T.; Vanhoutte, P.M.; Irwin, M.G.; Xia, Z. PKCbeta inhibition with ruboxistaurin reduces oxidative stress and attenuates left ventricular hypertrophy and dysfunction in rats with streptozotocin-induced diabetes. Clin. Sci. 2012, 122, 161–173. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Oxidative stress and beta-cell dysfunction. Pflügers Arch. -Eur. J. Physiol. 2010, 460, 703–718. [Google Scholar] [CrossRef]
- Maechler, P.; Jornot, L.; Wollheim, C.B. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic β cells. J. Biol. Chem. 1999, 274, 27905–27913. [Google Scholar] [CrossRef]
- Lameloise, N.; Muzzin, P.; Prentki, M.; Assimacopoulos-Jeannet, F. Uncoupling protein 2: A possible link between fatty acid excess and impaired glucose-induced insulin secretion? Diabetes 2001, 50, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.A.; Artner, I.; Henderson, E.; Means, A.; Sander, M.; Stein, R. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc. Natl. Acad. Sci. USA 2004, 101, 2930–2933. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, D.; Kajimoto, Y.; Kaneto, H. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH2-terminal kinase. Diabetes 2003, 52, 2896–2904. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, D.; Kaneto, H.; Nakatani, Y. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J. Biol. Chem. 2006, 281, 1091–1098. [Google Scholar] [CrossRef]
- Robertson, R.P.; Harmon, J.; Tran, P.O. β-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004, 53 (Suppl. 1), S119–S124. [Google Scholar] [CrossRef]
- Gurzov, E.N.; Eizirik, D.L. Bcl-2 proteins in diabetes: Mitochondrial pathways of β-cell death and dysfunction. Trends Cell Biol. 2011, 21, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Heimberg, H.; Heremans, Y.; Jobin, C.; Leemans, R.; Cardozo, A.K.; Darville, M.; Eiziriket, D.L. Inhibition of cytokine-induced NF-kappaB activation by adenovirus-mediated expression of a NF-kappaB super-repressor prevents beta-cell apoptosis. Diabetes 2001, 50, 2219–2224. [Google Scholar] [CrossRef]
- Ago, T.; Kuroda, J.; Pain, J.; Fu, C.; Li, H.; Sadoshima, J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010, 106, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Ago, T.; Matsushima, S.; Zhai, P.; Schneider, M.D.; Sadoshima, J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA 2010, 107, 15565–15570. [Google Scholar] [CrossRef]
- Okabe, K.; Matsushima, S.; Ikeda, S.; Ikeda, M.; Ishikita, A.; Tadokoro, T.; Enzan, N.; Yamamoto, T.; Sada, M.; Deguchi, H.; et al. DPP (dipeptidyl peptidase)-4 inhibitor attenuates ang II (angiotensin II)-Induced cardiac hypertrophy via GLP (Glucagon-Like peptide)-1-dependent suppression of nox (nicotinamide adenine dinucleotide phosphate oxidase) 4-HDAC (histone deacetylase) 4 pathway. Hypertension 2020, 75, 991–1001. [Google Scholar] [PubMed]
- Schnelle, M.; Sawyer, I.; Anilkumar, N.; Mohamed, B.A.; Richards, D.A.; Toischer, K.; Zhang, M.; Catibog, N.; Sawyer, G.; Mongue-Din, H.; et al. NADPH oxidase-4 promotes eccentric cardiac hypertrophy in response to volume overload. Cardiovasc. Res. 2019, 117, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Brewer, A.C.; Schroder, K.; Santos, C.X.; Grieve, D.J.; Wang, M.; Anilkumar, N.; Yu, B.; Dong, X.; Walker, S.J.; et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18121–18126. [Google Scholar] [CrossRef] [PubMed]
- Nabeebaccus, A.; Hafstad, A.; Eykyn, T.; Yin, X.; Brewer, A.; Zhang, M.; Mayr, M.; Shah, A. Cardiac-targeted NADPH oxidase 4 in the adaptive cardiac remodelling of the murine heart. Lancet 2015, 385, S73. [Google Scholar] [CrossRef]
- Zhao, G.J.; Zhao, C.L.; Ouyang, S.; Deng, K.Q.; Zhu, L.; Montezano, A.C.; Zhang, C.; Hu, F.; Zhu, X.Y.; Tian, S.; et al. Ca2+-Dependent NOX5 (NADPH oxidase 5) exaggerates cardiac hypertrophy through reactive oxygen species production. Hypertension 2020, 76, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Galan, M.; Varona, S.; Guadall, A.; Orriols, M.; Navas, M.; Aguilo, S.; de Diego, A.; Navarro, M.A.; Garcia-Dorado, D.; Rodriguez-Sinovas, A.; et al. Lysyl oxidase overexpression accelerates cardiac remodeling and aggravates angiotensin II-induced hypertrophy. FASEB J. 2017, 31, 3787–3799. [Google Scholar] [CrossRef]
- Bhatti, S.N.; Li, J.M. Nox2 dependent redox-regulation of Akt and ERK1/2 to promote left ventricular hypertrophy in dietary obesity of mice. Biochem. Biophys. Res. Commun. 2020, 528, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gu, J.; Xu, Z.; Zhang, Z.; Bai, T.; Xu, J.; Cai, J.; Barnes, G.; Liu, Q.J.; Freedman, J.H.; et al. Zinc rescues obesity-induced cardiac hypertrophy via stimulating metallothionein to suppress oxidative stress-activated BCL10/CARD9/p38 MAPK pathway. J. Cell Mol. Med. 2017, 21, 1182–1192. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Zuo, A.; Zhao, X.; Li, T.; Li, J.; Lei, S.; Chen, J.; Xu, D.; Song, C.; Liu, T.; Li, C.; et al. CTRP9 knockout exaggerates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy through inhibiting the LKB1/AMPK pathway. J. Cell Mol. Med. 2020, 24, 2635–2647. [Google Scholar] [CrossRef]
- Terai, K.; Hiramoto, Y.; Masaki, M.; Sugiyama, S.; Kuroda, T.; Hori, M.; Kawase, I.; Hirota, H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol. Cell Biol. 2005, 25, 9554–9575. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.Q.; Zhao, M.; Wu, Q.; Yang, S.; Cui, Y.L.; Yu, X.J.; Liu, J.; Zang, W.J. Regulation of mitochondrial cristae remodelling by acetylcholine alleviates palmitate-induced cardiomyocyte hypertrophy. Free Radic. Biol. Med. 2019, 145, 103–117. [Google Scholar] [CrossRef]
- Tronchere, H.; Cinato, M.; Timotin, A.; Guitou, L.; Villedieu, C.; Thibault, H.; Baetz, D.; Payrastre, B.; Valet, P.; Parini, A.; et al. Inhibition of PIKfyve prevents myocardial apoptosis and hypertrophy through activation of SIRT3 in obese mice. EMBO Mol. Med. 2017, 9, 770–785. [Google Scholar] [CrossRef]
- Cheng, K.C.; Chang, W.T.; Li, Y.; Cheng, Y.Z.; Cheng, J.T.; Chen, Z.C. GW0742 activates peroxisome proliferator-activated receptor delta to reduce free radicals and alleviate cardiac hypertrophy induced by hyperglycemia in cultured H9c2 cells. J. Cell. Biochem. 2018, 119, 9532–9542. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Li, Q.; Gao, X.; Sugano, E.; Tomita, H.; Yang, L.; Shi, S. Thioredoxin 2 offers protection against mitochondrial oxidative stress in H9c2 cells and against myocardial hypertrophy induced by hyperglycemia. Int. J. Mol. Sci. 2017, 18, 1958. [Google Scholar] [CrossRef] [PubMed]
- Galatou, E.; Kelly, T.; Lazou, A. The PPARbeta/delta agonist GW0742 modulates signaling pathways associated with cardiac myocyte growth via a non-genomic redox mechanism. Mol. Cell. Biochem. 2014, 395, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Novoa, U.; Arauna, D.; Moran, M.; Nunez, M.; Zagmutt, S.; Saldivia, S.; Valdes, C.; Villasenor, J.; Zambrano, C.G.; Gonzalez, D.R. High-intensity exercise reduces cardiac fibrosis and hypertrophy but does not restore the nitroso-redox imbalance in diabetic cardiomyopathy. Oxid. Med. Cell Longev. 2017, 2017, 7921363. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Hohn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Tang, S.G.; Liu, X.Y.; Wang, S.P.; Wang, H.H.; Jovanovic, A.; Tan, W. Trimetazidine prevents diabetic cardiomyopathy by inhibiting Nox2/TRPC3-induced oxidative stress. J. Pharmacol. Sci. 2019, 139, 311–318. [Google Scholar] [CrossRef]
- Tang, S.G.; Liu, X.Y.; Ye, J.M.; Hu, T.T.; Yang, Y.Y.; Han, T.; Tan, W. Isosteviol ameliorates diabetic cardiomyopathy in rats by inhibiting ERK and NF-kappaB signaling pathways. J. Endocrinol. 2018, 238, 47–60. [Google Scholar] [CrossRef]
- Li, J.; Minczuk, K.; Massey, J.C.; Howell, N.L.; Roy, R.J.; Paul, S.; Patrie, J.T.; Kramer, C.M.; Epstein, F.H.; Carey, R.M.; et al. Metformin improves cardiac metabolism and function, and prevents left ventricular hypertrophy in spontaneously hypertensive rats. J. Am. Heart Assoc. 2020, 9, e015154. [Google Scholar] [CrossRef] [PubMed]
- An, H.S.; Lee, J.Y.; Choi, E.B.; Jeong, E.A.; Shin, H.J.; Kim, K.E.; Park, K.A.; Jin, Z.; Lee, J.E.; Koh, J.S.; et al. Caloric restriction reverses left ventricular hypertrophy through the regulation of cardiac iron homeostasis in impaired leptin signaling mice. Sci. Rep. 2020, 10, 7176. [Google Scholar] [CrossRef] [PubMed]
- David, C.E.B.; Lucas, A.M.B.; Araujo, M.T.S.; Coelho, B.N.; Neto, J.B.S.; Portela, B.R.C.; Varela, A.L.N.; Kowaltowski, A.J.; Facundo, H.T. Calorie restriction attenuates hypertrophy-induced redox imbalance and mitochondrial ATP-sensitive K+ channel repression. J. Nutr. Biochem. 2018, 62, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Brubaker, P.; Morgan, T.; Haykowsky, M.; Hundley, G.; Kraus, W.E.; Eggebeen, J.; Nicklas, B.J. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. J. Am. Med. Assoc. 2016, 315, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, M.; Lazzeroni, D.; Pisano, A.; Girolami, F.; Alfieri, O.; La Canna, G.; d’Amati, G.; Olivotto, I.; Rimoldi, O.E.; Foglieni, C.; et al. Mitochondrial energetics and Ca2+-activated ATPase in obstructive hypertrophic cardiomyopathy. J. Clin. Med. 2020, 9, 1799. [Google Scholar] [CrossRef]
- Ma, T.; Lin, S.; Wang, B.; Wang, Q.; Xia, W.; Zhang, H.; Cui, Y.; He, C.; Wu, H.; Sun, F.; et al. TRPC3 deficiency attenuates high salt-induced cardiac hypertrophy by alleviating cardiac mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2019, 519, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Huang, Z.; Luo, X.; Liu, M.; Wang, L.; Qi, Z.; Huang, S.; Zhong, J.; Chen, J.X.; Li, L.; et al. Ferritinophagy activation and sideroflexin1-dependent mitochondria iron overload is involved in apelin-13-induced cardiomyocytes hypertrophy. Free Radic. Biol. Med. 2019, 134, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Chen, T.; Wanagat, J.; Laflamme, M.; Marcinek, D.J.; Emond, M.J.; Ngo, C.P.; Prolla, T.A.; Rabinovitch, P.S. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 2010, 9, 536–544. [Google Scholar] [CrossRef]
- Blasco, N.; Camara, Y.; Nunez, E.; Bea, A.; Bares, G.; Forne, C.; Ruiz-Meana, M.; Giron, C.; Barba, I.; Garcia-Arumi, E.; et al. Cardiomyocyte hypertrophy induced by Endonuclease G deficiency requires reactive oxygen radicals accumulation and is inhibitable by the micropeptide humanin. Redox Biol. 2018, 16, 146–156. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, Y.; Weng, X.; Lu, Y.; Li, W.; Tang, K.; Chen, W.; Liu, Z.; Qi, X.; Zheng, J.; et al. Novel role of mitochondrial GTPases 1 in pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2019, 128, 105–116. [Google Scholar] [CrossRef]
- Tang, K.; Zhao, Y.; Li, H.; Zhu, M.; Li, W.; Liu, W.; Zhu, G.; Xu, D.; Peng, W.; Xu, Y.W. Translocase of inner membrane 50 functions as a novel protective regulator of pathological cardiac hypertrophy. J. Am. Heart Assoc. 2017, 6, e004346. [Google Scholar] [CrossRef]
- Fang, L.; Ellims, A.H.; Beale, A.L.; Taylor, A.J.; Murphy, A.; Dart, A.M. Systemic inflammation is associated with myocardial fibrosis, diastolic dysfunction, and cardiac hypertrophy in patients with hypertrophic cardiomyopathy. Am. J. Transl. Res. 2017, 9, 5063–5073. [Google Scholar] [PubMed]
- Yang, Y.; Lv, J.; Jiang, S.; Ma, Z.; Wang, D.; Hu, W.; Deng, C.; Fan, C.; Di, S.; Sun, Y.; et al. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis. 2016, 7, e2234. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.O.R.; He, Y.; Bertagnolli, M.; Mai-Vo, T.A.; Fernandes, R.O.; Boudreau, F.; Cloutier, A.; Luu, T.M.; Nuyt, A.M. TLR (Toll-Like receptor) 4 antagonism prevents left ventricular hypertrophy and dysfunction caused by neonatal hyperoxia exposure in rats. Hypertension 2019, 74, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Katare, P.B.; Bagul, P.K.; Dinda, A.K.; Banerjee, S.K. Toll-like receptor 4 inhibition improves oxidative stress and mitochondrial health in isoproterenol-induced cardiac hypertrophy in rats. Front. Immunol. 2017, 8, 719. [Google Scholar] [CrossRef]
- Chen, D.; Li, Z.; Bao, P.; Chen, M.; Zhang, M.; Yan, F.; Xu, Y.; Ji, C.; Hu, X.; Sanchis, D.; et al. Nrf2 deficiency aggravates Angiotensin II-induced cardiac injury by increasing hypertrophy and enhancing IL-6/STAT3-dependent inflammation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Yin, G.; Huang, C.; Wang, H.; Gao, J.; Luo, J.; Zhang, Z.; Wang, J.; Hong, J.; Chai, X. Peroxiredoxin-1 ameliorates pressure overload-induced cardiac hypertrophy and fibrosis. Biomed. Pharmacother. 2020, 129, 110357. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.G.; Yuan, Y.P.; Zhang, X.; Xu, S.C.; Kong, C.Y.; Song, P.; Li, N.; Tang, Q.Z. C1q-tumour necrosis factor-related protein-3 exacerbates cardiac hypertrophy in mice. Cardiovasc. Res. 2019, 115, 1067–1077. [Google Scholar] [CrossRef]
- Geng, Z.; Fan, W.Y.; Zhou, B.; Ye, C.; Tong, Y.; Zhou, Y.B.; Xiong, X.Q. FNDC5 attenuates obesity-induced cardiac hypertrophy by inactivating JAK2/STAT3-associated inflammation and oxidative stress. J. Transl. Med. 2019, 17, 107. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Z.; Xue, M.; Yi, X.; Liang, J.; Niu, C.; Chen, G.; Shen, Y.; Zhang, H.; Zheng, J.; et al. Fibroblast growth factor 21 protects the heart from angiotensin II-induced cardiac hypertrophy and dysfunction via SIRT1. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Ni, X.; Hu, K.Q.; Meng, F.L.; Li, M.; Ma, X.L.; Meng, T.T.; Wu, H.H.; Ge, D.; Zhao, J.; et al. Cilostazol alleviate nicotine induced cardiomyocytes hypertrophy through modulation of autophagy by CTSB/ROS/p38MAPK/JNK feedback loop. Int. J. Biol. Sci. 2020, 16, 2001–2013. [Google Scholar] [CrossRef]
- Xie, Y.P.; Lai, S.; Lin, Q.Y.; Xie, X.; Liao, J.W.; Wang, H.X.; Tian, C.; Li, H.H. CDC20 regulates cardiac hypertrophy via targeting LC3-dependent autophagy. Theranostics 2018, 8, 5995–6007. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gao, Y.; Gao, R.; Yang, W.; Dong, Z.; Moses, R.E.; Sun, A.; Li, X.; Ge, J. The proteasome activator REGgamma accelerates cardiac hypertrophy by declining PP2Acalpha-SOD2 pathway. Cell Death Differ. 2020, 27, 2952–2972. [Google Scholar] [CrossRef]
- Morin, D.; Long, R.; Panel, M.; Laure, L.; Taranu, A.; Gueguen, C.; Pons, S.; Leoni, V.; Caccia, C.; Vatner, S.F.; et al. Hsp22 overexpression induces myocardial hypertrophy, senescence and reduced life span through enhanced oxidative stress. Free Radic. Biol. Med. 2019, 137, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, B.A.; Bhat, D.L.; McGuire, D.K.; Cahn, A.; Mosenzon, O.; Steg, G.; Im, K.; Kanevsky, E.; Gurmu, Y.; Raz, I.; et al. Metformin Use and Clinical Outcomes among Patients with Diabetes with or without Heart Failure or Kidney Dysfunction: Observations from the SAVOR-TIMI 53 Trial. Circulation 2019, 140, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, X.; Zheng, Y.; Li, J.; Wang, Y.; Lin, Y.; He, Z.; Zhao, W.; Chen, C.; Qiu, K.; et al. Association of glucose-lowering medications with cardiovascular outcomes: An umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020, 8, 192–205. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Khan, M.S.; Solomon, N.; DeVore, A.D.; Sharma, A.; Felker, G.M.; Hernandez, A.F.; Heidenreich, P.A.; Matsouaka, R.A.; Green, J.B.; Butler, J.; et al. Clinical outcomes with metformin and sulfonylurea therapies among patients with heart failure and diabetes. Heart Fail. 2022, 10, 198–210. [Google Scholar] [CrossRef]
- Halabi, A.; Sen, J.; Huynh, Q.; Marwick, T.H. Metformin treatment in heart failure with preserved ejection fraction: A systematic review and meta-regression analysis. Cardiovasc. Diabetol. 2020, 19, 124. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yang, Q.; Xu, C.; Zheng, Y.; Wang, L.; Wu, L.; Zeng, M.; Luo, M. Metformin prevents methylglyoxal-induced apoptosis by suppressing oxidative stress in vitro and in vivo. Cell Death Dis. 2022, 13, 29. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Min, X.; Yuan, T.; Wei, J.; Cai, Z. The association between metformin treatment and outcomes in type 2 diabetes mellitus patients with heart failure with preserved ejection fraction: A retrospective study. Front. Cardiovasc. Med. 2021, 8, 648212. [Google Scholar] [CrossRef]
- Benes, J.; Kotrc, M.; Kroupova, K.; Wohlfahrt, P.; Kovar, J.; Franekova, J.; Hegarova, M.; Hoskova, L.; Hoskova, E.; Pelikanova, T.; et al. Abstract 13045: Metformin in management of diabetic patients with advanced heart failure (HFrEFf): A propensity score-matched analysis. Circulation 2021, 144, A13045. [Google Scholar]
- Gerstein, H.C.; Yusuf, S.; Bosch, J.; Pogue, J.; Sheridan, P.; Dinccag, N.; Hanefeld, M.; Hoogwerf, B.; Laakso, M.; Mohan, V.; et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 2006, 368, 1096–1105. [Google Scholar] [PubMed]
- Erdmann, E.; Dormandy, J.; Wilcox, R.; Massi-Benedetti, M.; Charbonnel, B. PROactive 07: Pioglitazone in the treatment of type 2 diabetes: Results of the PROactive study. Vasc. Health Risk. Manag. 2007, 3, 355–370. [Google Scholar] [PubMed]
- Vaccaro, O.; Masulli, M.; Bonora, E.; Prato, S.D.; Nicolucci, A.; Rivellese, A.A.; Riccardi, G.; TOSCA. IT Study Group. The TOSCA. IT trial: A study designed to evaluate the effect of pioglitazone versus sulfonylureas on cardiovascular disease in type 2 diabetes. Diabetes Care 2012, 35, e82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inzucchi, S.E.; Furie, K.L. The IRIS (Insulin Resistance Intervention after Stroke) trial: A new perspective on pioglitazone. J. Diabetes 2016, 8, 607–609. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Thiazolidinediones: The forgotten diabetes medications. Curr. Diabetes Rep. 2019, 19, 151. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Meng, L.; Gong, M.; Li, J.; Shi, W.; Qiu, J.; Yang, Y.; Zhao, J.; Suo, J.; et al. Pioglitazone inhibits diabetes-induced atrial mitochondrial oxidative stress and improves mitochondrial biogenesis, dynamics, and function through the PPAR-γ/PGC-1α signaling pathway. Front. Pharmacol. 2021, 12, 658362. [Google Scholar] [CrossRef]
- Li, J.; Shen, X. Effect of rosiglitazone on inflammatory cytokines and oxidative stress after intensive insulin therapy in patients with newly diagnosed type 2 diabetes. Diabetol. Metab. Syndr. 2019, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Esubi, J.U.; Olojede, S.O.; Lawal, S.K.; Medubi, L.J.; Adekoya, A.J.; Dauda, F.F.; Olusegun, A.P.; Osinubi, A.A. Comparative studies on safety of glimepiride and glipizide on renal microarchitecture and oxidative stress markers of pregnant streptozotocin-induced diabetic wistar rats. J. Pharm. Pharmacol. Res. 2019, 3, 3–19. [Google Scholar]
- Holman, R.R.; Haffner, S.M.; McMurray, J.J.; Bethel, M.A.; Holzhauer, B.; Hua, T.A.; Belenkov, Y.; Boolell, M.; Buse, J.B.; Buckley, B.M.; et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N. Engl. J. Med. 2010, 362, 1463–1476. [Google Scholar]
- Wang, L.; Guo, L.; Zhang, L.; Zhou, Y.; He, Q.; Zhang, Z.; Wang, M. Effects of glucose load and nateglinide intervention on endothelial function and oxidative stress. J. Diabetes Res. 2013, 2013, 849295. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Coleman, R.L.; Chan, J.C.N.; Chiasson, J.; Feng, H.; Ge, J.; Gerstein, H.C.; Gray, R.; Huo, Y.; Lang, Z.; et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 877–886. [Google Scholar] [CrossRef]
- Chiasson, J.L.; Gomis, R.; Hanefeld, M.; Josse, G.R.; Karasik, A.; Laakso, M. The STOP-NIDDM Trial: An international study on the efficacy of an a-glucosidase inhibitor to prevent type 2 diabetes in a population with impaired glucose tolerance: Rationale, design, and preliminary screening data. Diabetes Care 1998, 21, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Jalalvand, F.; Amoli, M.M.; Yaghmaei, P.; Kimiagar, M.; Ebrahim-Habibi, A. Acarbose versus trans-chalcone: Comparing the effect of two glycosidase inhibitors on obese mice. Arch. Endocrinol. Metab. 2015, 59, 202–209. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Bosch, J.; Dagenais, G.R.; Díaz, R.; Jung, H.; Maggioni, A.P.; Pogue, J.; Probstfield, J.; Ramachandran, A.; Riddle, M.C.; et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 2012, 367, 319–328. [Google Scholar] [PubMed]
- Pratley, R.E.; Husain, M.; Lingvay, I.; Pieber, T.R.; Mark, T.; Saevereid, H.A.; Møller, D.V.; Zinman, B.; DEVOTE Study Group. Heart failure with insulin degludec versus glargine U100 in patients with type 2 diabetes at high risk of cardiovascular disease: DEVOTE 14. Cardiovasc. Diabetol. 2019, 18, 156. [Google Scholar] [CrossRef]
- Jang, S.Y.; Jang, J.; Yang, D.H.; Cho, H.; Lim, S.; Jeon, E.; Lee, S.E.; Kim, J.; Kang, S.; Baek, S.H.; et al. Impact of insulin therapy on the mortality of acute heart failure patients with diabetes mellitus. Cardiovasc. Diabetol. 2021, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Valente, T.; Valente, F.; Lucchesi, M.B.B.; Punaro, G.R.; Mouro, M.G.; Gabbay, M.A.L.; Higa, E.M.S.; Dib, S.A. Relationship between short and long-term glycemic variability and oxidative stress in type 1 diabetes mellitus under daily life insulin treatment. Arch. Endocrinol. Metab. 2021, 65, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Zhou, M.; Xie, Y.; Dong, X.; Bai, F.; Zhang, J. DPHC from Alpinia officinarum ameliorates oxidative stress and insulin resistance via activation of Nrf2/ARE pathway in db/db mice and high glucose-treated HepG2 cells. Front. Pharmacol. 2022, 12, 792977. [Google Scholar] [CrossRef]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. SAVOR-TIMI 53 steering committee and investigators. saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–13126. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Cannon, C.P.; Heller, S.R.; Nissen, S.E.; Bergenstal, R.M.; Bakris, G.L.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 2013, 369, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; BuseJ, B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of linagliptinvs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk the CARMELINA randomized clinical trial. JAMA 2019, 321, 69–79. [Google Scholar] [CrossRef]
- Rosenstock, J.; Kahn, S.E.; Johansen, O.E.; Zinman, B.; Espeland, M.A.; Woerle, H.J.; Pfarr, E.; Keller, A.; Mattheus, M.; Baanstra, D.; et al. Effect of linagliptinvs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes the CAROLINA randomized clinical trial. JAMA 2019, 322, 1155–1166. [Google Scholar] [CrossRef]
- Sano, M. Mechanism by which dipeptidyl peptidase-4 inhibitors increase the risk of heart failure and possible differences in heart failure risk. J. Cardiol. 2019, 73, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Luceri, C.; Dicembrini, I.; Tatti, L.; Scavone, F.; Giovannelli, L.; Mannucci, E.; Lodovici, M. Effect of dipeptidyl-peptidase 4 inhibitors on circulating oxidative stress biomarkers in patients with type 2 diabetes mellitus. Antioxidants 2020, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Williams, D.M.; Evans, M. Are SGLT-2 inhibitors the future of heart failure treatment? The EMPEROR-Preserved and EMPEROR-Reduced Trials. Diabetes Ther. 2020, 11, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, C.; Warden, B.A. Mechanisms and evidence for heart failure benefits from SGLT2 inhibitors. Curr. Cardiol. Rep. 2019, 21, 130. [Google Scholar] [CrossRef]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Shigiyama, F.; Kumashiro, N.; Miyagi, M.; Ikehara, K.; Kanda, E.; Uchino, H.; Hirose, T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc. Diabetol. 2017, 16, 84. [Google Scholar] [CrossRef]
- Palmiero, G.; Cesaro, A.; Vetrano, E.; Pafundi, P.C.; Galiero, R.; Caturano, A.; Moscarella, E.; Gragnano, F.; Salvatore, T.; Rinaldi, L.; et al. Impact of SGLT2 inhibitors on heart failure: From pathophysiology to clinical effects. Int. J. Mol. Sci. 2021, 22, 5863. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; Antonio, R.S.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Januzzi, J.L. Preventing and treating heart failure with sodium glucose co-transporter 2 inhibitors. Am. J. Cardiol. 2019, 124, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; McMurray, J.J.V. The serendipitous story of SGLT2 inhibitors in heart failure: New insights from DECLARE-TIMI 58. Circulation 2019, 139, 2537–2541. [Google Scholar] [CrossRef]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Oh, T.J.; Lee, G.; Maeng, H.J.; Lee, D.H.; Kim, K.M.; Choi, S.H.; Jang, H.C.; Lee, H.S.; Park, K.S.; et al. The beneficialeffects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE-mice fed a western diet. Diabetologia 2017, 60, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.C.; Kratzsch, J.; Linke, A.; Schoene, N.; Adams, V.; Gielen, S.; Erbs, S.; Moebius-Winkler, S.; Schuler, G. Elevated serumlevels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. Eur. J. Heart Fail. 2003, 5, 33–40. [Google Scholar] [CrossRef]
- Paulus, W.J. Unfolding discoveries in heart failure. N. Engl. J. Med. 2020, 382, 679–682. [Google Scholar] [CrossRef]
- Lim, C.T.; Kola, B.; Korbonits, M. AMPK as a mediator of hormonal signalling. J. Mol. Endocrinol. 2009, 44, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Autophagy-dependent and -independent modulation of oxidative and organellar stress in the diabetic heart by glucose-lowering drugs. Cardiovasc. Diabetol. 2020, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lódi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovács, Á.; Fülöp, G.Á.; Falcão-Pires, I.; et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc. Res. 2021, 117, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Imprialos, K.; Stavropoulos, K.; Papademetriou, V. Sodium-glucose cotransporter-2 inhibitors, reverse j-curve pattern, and mortality in heart failure. Heart Fail. Clin. 2019, 15, 519–530. [Google Scholar] [CrossRef]
- Giorgino, F.; Caruso, I.; Moellmann, J.; Lehrke, M. Differential indication for SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with established atherosclerotic heart disease or at risk for congestive heart failure. Metabolism 2020, 104, 154045. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Collins, S.P. Dapagliflozin in heart failure: New frontiers. Eur. J. Heart Fail. 2019, 21, 1412–1414. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Sattar, N.; Januzzi, J.; Verma, S.; Lund, L.H.; Fitchett, D.; Zeller, C.; George, J.T.; Brueckmann, M.; Ofstad, A.P.; et al. Empagliflozin is associated with a lower risk of post-acute heart failure rehospitalization and mortality: Insights from the EMPA-REG OUTCOME trial. Circulation 2019, 139, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Fitchett, D.; Inzucchi, S.E.; Cannon, C.P.; McGuire, D.K.; Scirica, B.M.; Johansen, O.E.; Sambevski, S.; Kaspers, S.; Pfarr, E.; George, J.T.; et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation 2019, 139, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Patorno, E.; Najafzadeh, M.; Pawar, A.; Franklin, J.M.; Déruaz-Luyet, A.; Brodovicz, K.G.; Santiago Ortiz, A.J.; Bessette, L.G.; Kulldorff, M.; Schneeweiss, S. The EMPagliflozin comparative effectIveness and SafEty (EMPRISE) study programme: Design and exposure accrual for an evaluation of empagliflozin in routine clinical care. Endocrinol. Diabetes Metab. 2019, 3, e00103. [Google Scholar] [CrossRef] [PubMed]
- Tripolt, N.J.; Kolesnik, E.; Pferschy, P.N.; Verheyen, N.; Ablasser, K.; Sailer, S.; Alber, H.; Berger, R.; Kaulfersch, C.; Leitner, K.; et al. Impact of EMpagliflozin on cardiac function and biomarkers of heart failure in patients with acute MYocardial infarction—The EMMY trial. Am. Heart J. 2020, 221, 39–47. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.; Choi, D.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Khan, M.S.; Marx, N.; Lam, C.S.P.; Schnaidt, S.; Ofstad, A.P.; Brueckmann, M.; Jamal, W.; et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: Results from the EMPEROR-reduced trial. Circulation 2021, 143, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Omar, M.; Kistorp, C.; Poulsen, M.K.; Tuxen, C.; Gustafsson, I.; Køber, L.; Gustafsson, F.; Fosbøl, E.; Bruun, N.E.; et al. Empagliflozin in heart failure patients with reduced ejection fraction: A randomized clinical trial (Empire HF). Trials 2019, 20, 374. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Claggett, B.L.; Solomon, S.D. How do SGLT-2 inhibitors work to prevent heart failure? JACC Heart Fail. 2018, 20, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Figtree, G.A.; Radholm, K.; Barrett, T.D.; Perkovic, V.; Mahaffey, K.W.; Zeeuw, D.; Fulcher, G.; Matthews, D.R.; Shaw, W.; Neal, B. Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes: Results from the CANVAS program. Circulation 2019, 139, 2591–2593. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Kato, E.T.; Silverman, M.G.; Mosenzon, O.; Zelniker, T.A.; Cahn, A.; Furtado, R.H.M.; Kuder, J.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019, 139, 2528–2536. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Singh, R. Heart failure hospitalization with SGLT-2 inhibitors: A systematic review and meta-analysis of randomized controlled and observational studies. Expert Rev. Clin. Pharmacol. 2019, 12, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Butler, J. SGLT-2 inhibitors in heart failure: A new therapeutic avenue. Nat. Med. 2019, 25, 1653–1654. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Jhund, P.S.; Docherty, K.F.; Diez, M.; Petrie, M.C.; Verma, S.; Nicolau, J.C.; Merkely, B.; Kitakaze, M.; DeMets, D.L.; et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: Results from the DAPA-HF trial. Circulation 2020, 141, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.A.; Serenelli, M.; Nicolau, J.C.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age insights from DAPA-HF. Circulation 2020, 141, 100–111. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Tang, F.; Khariton, Y.; Husain, M.; Inzucchi, S.E.; McGuire, D.K.; Pitt, B.; Scirica, B.M.; Austin, B.; et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: The DEFINE-HF trial. Circulation 2019, 140, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, B.; Ueda, P.; Eliasson, B.; Svensson, A.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Wintzell, V.; Melbye, M.; et al. Use of sodium glucose cotransporter 2 inhibitors and risk of major cardiovascular events and heart failure: Scandinavian register based cohort study. BMJ 2019, 366, I4772. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.F.; Chen, Y.L.; Chiou, T.T.; Chu, T.; Li, L.; Ng, H.; Lee, W.; Lee, C. Emergence of SGLT2 inhibitors as powerful antioxidants in human diseases. Antioxidants 2021, 10, 1166. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Mao, L.; Zhang, L.; Zhu, Y.; Xu, Y.; Cheng, Y.; Sun, R.; Zhang, Y.; Ke, J.; et al. SGLT2 inhibition restrains thyroid cancer growth via G1/S phase transition arrest and apoptosis mediated by DNA damage response signaling pathways. Cancer Cell Int. 2022, 22, 74. [Google Scholar] [CrossRef]
- Ala, M.; Khoshdel, M.R.F.; Dehpour, A.R. Empagliflozin enhances autophagy, mitochondrial biogenesis, and antioxidant defense and ameliorates renal ischemia/reperfusion in nondiabetic rats. Oxid. Med. Cell. Longev. 2022, 2022, 1197061. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Heo, Y.J.; Choi, S.E.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. Hepatoprotective effects of gemigliptin and empagliflozin in a murine model of diet-induced non-alcoholic fatty liver disease. Biochem. Biophys. Res. Commun. 2022, 588, 154–160. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. LEADER trial investigators. liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2017, 376, 890. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; White, J.; Pagidipati, N.J.; Lokhnygina, Y.; Wainstein, J.; Murin, J.; Iqbal, N.; Öhman, P.; Lopes, R.D.; Reicher, B.; et al. Effect of once-weekly exenatide in patients with type 2 diabetes with and without heart failure and heart failure-related outcomes: Insights from the EXSCEL trial. Circulation 2019, 140, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B., Sr.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Vardeni, O. The sweet spot: Heart failure prevention with SGLT2 inhibitors. Am. J. Med. 2020, 133, 182–185. [Google Scholar] [CrossRef]
- Lambadiari, V.; Thymis, J.; Kouretas, D.; Skaperda, Z.; Tekos, F.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of a 12-month treatment with glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on oxidant and antioxidant biomarkers in patients with type 2 diabetes. Antioxidants 2021, 10, 1379. [Google Scholar] [CrossRef] [PubMed]

| Sodium–glucose cotransporter-2 (SGLT-2) inhibitors: Block renal glucose reuptake and promote loss of glucose in the urine, thus improving blood pressure via glucose and sodium excretion. |
| Canagliflozin (Invokana) taken by mouth once daily Dapagliflozin (Farxiga, Forxiga) taken by mouth once daily Empagliflozin (Jardiance) taken by mouth once daily Ertugliflozin (Steglatro) taken by mouth once daily Ipragliflozin (Suglat) taken by mouth once daily Luseogliflozin (Lusefi) taken by mouth once daily Remogliflozin etabonate (Remo, Remozen) taken by mouth once daily Sotagliflozin (Zynquista) taken by mouth once daily Tofogliflozin (Apleway, Deberza) taken by mouth once daily |
| Glucagon-like peptide-1 (GLP-1) receptor agonists: Increase insulin secretion and inhibit glucagon release via stimulation of the GLP-1 receptors |
| Dulaglutide (Trulicity) taken by injection weekly Exenatide extended release (Bydureon) taken by injection weekly Exenatide (Byetta) taken by injection twice daily Liraglutide (Victoza) taken by injection daily Lixisenatide (Adlyxin) taken by injection daily Semaglutide (Ozempic) taken by injection weekly Semaglutide (Rybelsus) taken by mouth once daily |
| Trial | Medication Used | Results | Meta-Analyses |
|---|---|---|---|
| EMPA-REG OUTCOME [40,76,77,78,79,80,81] | Empagliflozin vs. placebo |
| Savarese et al.: reduction in HF-associated readmissions and all composite post-acute HF period Fitchett et al.: regardless of the CV risk, the positive effects concerned all groups of patients |
| EMPRISE [82] | Empagliflozin vs. sitagliptin |
| |
| EMMY [83] | Empagliflozin vs. placebo |
| |
| EMPEROR-PRESERVED [84] | Empagliflozin vs. placebo in patients with preserved ejection fraction |
| |
| EMPEROR-REDUCED [85] | Empagliflozin vs. placebo in patients with reduced ejection fraction |
| |
| EMPIRE-HF [86] | Empagliflozin vs. placebo in patients with reduced ejection fraction |
| |
| CANVAS Programm [39,76,87,88] | Canagliflozin vs. placebo |
| Figtree et al.: canagliflozin reduced the risk of HF events in general in patients with type 2 DM, regardless of the presence of reduced or preserved ejection fraction |
| CREDENCE [39,77] | Canagliflozin vs. placebo |
| |
| CVD REAL [76] | SGLT-2 inhibitors vs. other anti-diabetic factors |
| |
| DECLARE-TIMI 58 [39,76,89,90,91] | Dapagliflozin vs. placebo |
| Furtado et al.: reduced risk of MACE in DM patients compared to MI patients, similar risk reduction in cardiovascular mortality/heart failure hospitalization with a greater absolute risk reduction estimated at 1.9% for patients with prior MI vs. 0.6% in patients without prior MI Verma et al.: dapagliflozin had a positive effect in hospitalizations due to HF averse events and led to a greater reduction in HF complications or cardiovascular mortality in patients with HF with reduced ejection fraction (≤45%) in comparison with patients without reduced ejection fraction Kato et al.: dapagliflozin leads to a reduction of hospitalizations for HF regardless of the presence of reduced ejection fraction; the reduction of cardiovascular mortality and all-cause mortality was observed only in patients with reduced ejection fraction |
| DAPA-HF [94,95,96,97,98] | Dapagliflozin vs. placebo |
| Kosiborod et al.: dapagliflozin improved health status and quality of life and reduced cardiovascular mortality Martinez et al.: safe use of dapagliflozin in elderly |
| DEFINE-HF [99] | Dapagliflozin vs. placebo |
| |
| VERTIS-CV [71] | Ertugliflozin vs. placebo |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koniari, I.; Velissaris, D.; Kounis, N.G.; Koufou, E.; Artopoulou, E.; de Gregorio, C.; Mplani, V.; Paraskevas, T.; Tsigkas, G.; Hung, M.-Y.; et al. Anti-Diabetic Therapy, Heart Failure and Oxidative Stress: An Update. J. Clin. Med. 2022, 11, 4660. https://doi.org/10.3390/jcm11164660
Koniari I, Velissaris D, Kounis NG, Koufou E, Artopoulou E, de Gregorio C, Mplani V, Paraskevas T, Tsigkas G, Hung M-Y, et al. Anti-Diabetic Therapy, Heart Failure and Oxidative Stress: An Update. Journal of Clinical Medicine. 2022; 11(16):4660. https://doi.org/10.3390/jcm11164660
Chicago/Turabian StyleKoniari, Ioanna, Dimitrios Velissaris, Nicholas G. Kounis, Eleni Koufou, Eleni Artopoulou, Cesare de Gregorio, Virginia Mplani, Themistoklis Paraskevas, Grigorios Tsigkas, Ming-Yow Hung, and et al. 2022. "Anti-Diabetic Therapy, Heart Failure and Oxidative Stress: An Update" Journal of Clinical Medicine 11, no. 16: 4660. https://doi.org/10.3390/jcm11164660
APA StyleKoniari, I., Velissaris, D., Kounis, N. G., Koufou, E., Artopoulou, E., de Gregorio, C., Mplani, V., Paraskevas, T., Tsigkas, G., Hung, M.-Y., Plotas, P., Lambadiari, V., & Ikonomidis, I. (2022). Anti-Diabetic Therapy, Heart Failure and Oxidative Stress: An Update. Journal of Clinical Medicine, 11(16), 4660. https://doi.org/10.3390/jcm11164660











