Atopic Dermatitis: Striving for Reliable Biomarkers
Abstract
1. Introduction
2. Definition and Subtypes of Biomarkers
3. Biomarkers in AD
3.1. Biomarkers for Disease Clinical Severity and Monitoring Disease Activity
3.2. Prognostic and Screening Biomarkers
3.3. Predictive Biomarkers
3.4. Diagnostic Biomarkers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Eichenfield, L.F.; Tom, W.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Ziehfreund, S.; Tizek, L.; Hangel, N.; Fritzsche, M.; Weidinger, S.; Smith, C.; Bryce, P.; Greco, D.; Bogaard, E.; Flohr, C.; et al. Requirements and expectations of high-quality biomarkers for atopic dermatitis and psoriasis in 2021—A two-round Delphi survey among international experts. J. Eur. Acad. Dermatol. Venereol. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Moyle, M.; Cevikbas, F.; Harden, J.L.; Guttman-Yassky, E. Understanding the immune landscape in atopic dermatitis: The era of biologics and emerging therapeutic approaches. Exp. Dermatol. 2019, 28, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Allam, J.-P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.-U.; Wollenberg, A.; et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Bakker, D.S.; Nierkens, S.; Knol, E.F.; Giovannone, B.; Delemarre, E.M.; van der Schaft, J.; van Wijk, F.; de Bruin-Weller, M.S.; Drylewicz, J.; Thijs, J.L. Confirmation of multiple endotypes in atopic dermatitis based on serum biomarkers. J. Allergy Clin. Immunol. 2021, 147, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef]
- Mansouri, Y.; Guttman-Yassky, E. Immune Pathways in Atopic Dermatitis, and Definition of Biomarkers through Broad and Targeted Therapeutics. J. Clin. Med. 2015, 4, 858–873. [Google Scholar] [CrossRef]
- Bieber, T.; D’Erme, A.M.; Akdis, C.A.; Traidl-Hoffmann, C.; Lauener, R.; Schäppi, G.; Schmid-Grendelmeier, P. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J. Allergy Clin. Immunol. 2017, 139, S58–S64. [Google Scholar] [CrossRef]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J. Allergy Clin. Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Y.; Liu, X.; Zhang, J.; Su, H.; Wen, H.; Li, W.; Yao, X. Efficacy and safety of dupilumab for the treatment of adult atopic dermatitis: A meta-analysis of randomized clinical trials. J. Allergy Clin. Immunol. 2017, 140, 888–891.e6. [Google Scholar] [CrossRef]
- Simpson, E.L.; Flohr, C.; Eichenfield, L.F.; Bieber, T.; Sofen, H.; Taïeb, A.; Owen, R.; Putnam, W.; Castro, M.; DeBusk, K.; et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J. Am. Acad. Dermatol. 2018, 78, 863–871.e11. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Silverberg, J.I.; Nemoto, O.; Forman, S.B.; Wilke, A.; Prescilla, R.; de la Peña, A.; Nunes, F.P.; Janes, J.; Gamalo, M.; et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: A phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J. Am. Acad. Dermatol. 2019, 80, 913–921.e9. [Google Scholar] [CrossRef] [PubMed]
- Thijs, J.; Krastev, T.; Weidinger, S.; Buckens, C.F.; De Bruin-Weller, M.; Bruijnzeel-Koomen, C.; Flohr, C.; Hijnen, D. Biomarkers for atopic dermatitis: A systematic review and meta-analysis. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- WHO International Programme on Chemical Safety Biomarkers in Risk Assessment: Validity and Validation. 2001. Available online: http://www.inchem.org/documents/ehc/ehc/ehc222.htm (accessed on 20 June 2022).
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource. 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326791/ (accessed on 20 June 2022).
- EMA. Glossary: Biomarker. 2020. Available online: https://www.ema.europa.eu/en/glossary/biomarker (accessed on 20 June 2022).
- Renert-Yuval, Y.; Thyssen, J.P.; Bissonnette, R.; Bieber, T.; Kabashima, K.; Hijnen, D.; Guttman-Yassky, E. Biomarkers in atopic dermatitis—a review on behalf of the International Eczema Council. J. Allergy Clin. Immunol. 2021, 147, 1174–1190.e1. [Google Scholar] [CrossRef] [PubMed]
- Gromova, M.; Vaggelas, A.; Dallmann, G.; Seimetz, D. Biomarkers: Opportunities and Challenges for Drug Development in the Current Regulatory Landscape. Biomark. Insights 2020, 15, 1177271920974652. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Diaz, A.; Pavel, A.B.; Fernandes, M.; Lefferdink, R.; Erickson, T.; Canter, T.; Rangel, S.; Peng, X.; Li, R.; et al. Use of Tape Strips to Detect Immune and Barrier Abnormalities in the Skin of Children With Early-Onset Atopic Dermatitis. JAMA Dermatol. 2019, 155, 1358–1370. [Google Scholar] [CrossRef]
- Castelo-Soccio, L. Stripping Away Barriers to Find Relevant Skin Biomarkers for Pediatric Atopic Dermatitis. JAMA Dermatol. 2019, 155, 1342. [Google Scholar] [CrossRef] [PubMed]
- Pavel, A.B.; Renert-Yuval, Y.; Wu, J.; Del Duca, E.; Diaz, A.; Lefferdink, R.; Fang, M.M.; Canter, T.; Rangel, S.M.; Zhang, N.; et al. Tape strips from early-onset pediatric atopic dermatitis highlight disease abnormalities in nonlesional skin. Allergy 2020, 76, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Goleva, E.; Kim, P.S.; Norquest, K.; Bronchick, C.; Taylor, P.; Leung, D.Y. Side-by-Side Comparison of Skin Biopsies and Skin Tape Stripping Highlights Abnormal Stratum Corneum in Atopic Dermatitis. J. Investig. Dermatol. 2019, 139, 2387–2389.e1. [Google Scholar] [CrossRef]
- Thijs, J.L.; Fiechter, R.; Giovannone, B.; de Bruin-Weller, M.S.; Knol, E.F.; Bruijnzeel-Koomen, C.A.F.M.; Drylewicz, J.; Nierkens, S.; Hijnen, D. Biomarkers detected in dried blood spots from atopic dermatitis patients strongly correlate with disease severity. Allergy 2019, 74, 2240–2243. [Google Scholar] [CrossRef]
- Ungar, B.; Garcet, S.; Gonzalez, J.; Dhingra, N.; da Rosa, J.C.; Shemer, A.; Krueger, J.G.; Suarez-Farinas, M.; Guttman-Yassky, E. An Integrated Model of Atopic Dermatitis Biomarkers Highlights the Systemic Nature of the Disease. J. Investig. Dermatol. 2017, 137, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Thijs, J.L.; Van Seggelen, W.; Bruijnzeel-Koomen, C.; De Bruin-Weller, M.; Hijnen, D. New Developments in Biomarkers for Atopic Dermatitis. J. Clin. Med. 2015, 4, 479–487. [Google Scholar] [CrossRef]
- He, H.; Del Duca, E.; Diaz, A.; Kim, H.J.; Gay-Mimbrera, J.; Zhang, N.; Wu, J.; Beaziz, J.; Estrada, Y.; Krueger, J.G.; et al. Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities. J. Allergy Clin. Immunol. 2021, 147, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Thijs, J.L.; Nierkens, S.; Herath, A.; Bruijnzeel-Koomen, C.A.; Knol, E.; Giovannone, B.; De Bruin-Weller, M.S.; Hijnen, D. A panel of biomarkers for disease severity in atopic dermatitis. Clin. Exp. Allergy 2015, 45, 698–701. [Google Scholar] [CrossRef]
- Thijs, J.L.; Drylewicz, J.; Fiechter, R.; Strickland, I.; Sleeman, M.A.; Herath, A.; May, R.D.; Bruijnzeel-Koomen, C.A.; Knol, E.F.; Giovannone, B.; et al. EASI p-EASI: Utilizing a combination of serum biomarkers offers an objective measurement tool for disease severity in atopic dermatitis patients. J. Allergy Clin. Immunol. 2017, 140, 1703–1705. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, L.; Overbeek, S.A.; Wyllie, A.L.; Chu, M.L.J.N.; Bogaert, D.; De Jager, W.; Knippels, L.M.J.; Sanders, E.A.M.; Van Aalderen, W.M.C.; Garssen, J.; et al. Exploring Immune Development in Infants With Moderate to Severe Atopic Dermatitis. Front Immunol. Front. Immunol. 2018, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Yoshida, T.; Baba, M.; Nishimura, M.; Kakizaki, M.; Yoshie, O. Molecular Cloning of a Novel T Cell-directed CC Chemokine Expressed in Thymus by Signal Sequence Trap Using Epstein-Barr Virus Vector. J. Biol. Chem. 1996, 271, 21514–21521. [Google Scholar] [CrossRef]
- Song, T.W.; Sohn, M.H.; Kim, E.S.; Kim, K.W.; Kim, K.-E. Increased serum thymus and activation-regulated chemokine and cutaneous T cell-attracting chemokine levels in children with atopic dermatitis. Clin. Exp. Allergy 2006, 36, 346–351. [Google Scholar] [CrossRef]
- Hijnen, D.; De Bruin-Weller, M.; Oosting, B.; Lebre, C.; De Jong, E.; Bruijnzeel-Koomen, C.; Knol, E. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell–attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J. Allergy Clin. Immunol. 2004, 113, 334–340. [Google Scholar] [CrossRef]
- Machura, E.; Rusek-Zychma, M.; Jachimowicz, M.; Wrzask, M.; Mazur, B.; Kasperska-Zajac, A. Serum TARC and CTACK concentrations in children with atopic dermatitis, allergic asthma, and urticaria. Pediatr. Allergy Immunol. 2012, 23, 278–284. [Google Scholar] [CrossRef]
- Ahrens, B.; Schulz, G.; Bellach, J.; Niggemann, B.; Beyer, K. Chemokine levels in serum of children with atopic dermatitis with regard to severity and sensitization status. Pediatr. Allergy Immunol. 2015, 26, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Nagao, M.; Hiraguchi, Y.; Katsumata, H.; Nishimori, H.; Iguchi, K.; Kato, Y.; Higashiura, M.; Ogawauchi, I.; Tamaki, K. Serum measurement of thymus and activation-regulated chemokine/CCL17 in children with atopic dermatitis: Elevated normal levels in infancy and age-specific analysis in atopic dermatitis. Pediatr. Allergy Immunol. 2009, 20, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Landheer, J.; de Bruin-Weller, M.; Boonacker, C.; Hijnen, D.; Bruijnzeel-Koomen, C.; Röckmann, H. Utility of serum thymus and activation-regulated chemokine as a biomarker for monitoring of atopic dermatitis severity. J. Am. Acad. Dermatol. 2014, 71, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Yasukochi, Y.; Nakahara, T.; Abe, T.; Kido-Nakahara, M.; Kohda, F.; Takeuchi, S.; Hagihara, A.; Furue, M. Reduction of serum TARC levels in atopic dermatitis by topical anti-inflammatory treatments. Asian Pac. J. Allergy Immunol. 2014, 32, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Vekaria, A.S.; Brunner, P.M.; Aleisa, A.I.; Bonomo, L.; Lebwohl, M.G.; Israel, A.; Guttman-Yassky, E. Moderate-to-severe atopic dermatitis patients show increases in serum C-reactive protein levels, correlating with skin disease activity. F1000Research 2017, 6, 1712. [Google Scholar] [CrossRef] [PubMed]
- Kou, K.; Aihara, M.; Matsunaga, T.; Chen, H.; Taguri, M.; Morita, S.; Fujita, H.; Yamaguchi, Y.; Kambara, T.; Ikezawa, Z. Association of serum interleukin-18 and other biomarkers with disease severity in adults with atopic dermatitis. Arch. Dermatol. Res. 2012, 304, 305–312. [Google Scholar] [CrossRef]
- Morishima, Y.; Kawashima, H.; Takekuma, K.; Hoshika, A. Changes in serum lactate dehydrogenase activity in children with atopic dermatitis. Pediatr. Int. 2010, 52, 171–174. [Google Scholar] [CrossRef]
- Czech, W.; Krutmann, J.; Schopf, E.; Kapp, A. Serum eosinophil cationic protein (ECP) is a sensitive measure for disease activity in atopic dermatitis. Br. J. Dermatol. 1992, 126, 351–355. [Google Scholar] [CrossRef]
- Kägi, M.K.; Joller-Jemelka, H.; Wüthrich, B. Correlation of Eosinophils, Eosinophil Cationic Protein and Soluble lnterleukin-2 Receptor with the Clinical Activity of Atopic Dermatitis. Dermatology 1992, 185, 88–92. [Google Scholar] [CrossRef]
- Kou, K.; Okawa, T.; Yamaguchi, Y.; Ono, J.; Inoue, Y.; Kohno, M.; Matsukura, S.; Kambara, T.; Ohta, S.; Izuhara, K.; et al. Periostin levels correlate with disease severity and chronicity in patients with atopic dermatitis. Br. J. Dermatol. 2014, 171, 283–291. [Google Scholar] [CrossRef]
- Thijs, J.L.; Knipping, K.; Bruijnzeel-Koomen, C.A.F.; Garssen, J.; De Bruin-Weller, M.S.; Hijnen, D.J. Immunoglobulin free light chains in adult atopic dermatitis patients do not correlate with disease severity. Clin. Transl. Allergy 2016, 6, 44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aral, M.; Arican, O.; Gül, M.; Sasmaz, S.; Kocturk, S.A.; Kastal, U.; Ekerbicer, H.C. The relationship between serum levels of total IgE, IL-18, IL-12, IFN-gamma and disease severity in children with atopic dermatitis. Mediat. Inflamm. 2006, 2006, 73098. [Google Scholar] [CrossRef] [PubMed]
- Thijs, J.L.; de Bruin-Weller, M.S.; Hijnen, D. Current and Future Biomarkers in Atopic Dermatitis. Immunol. Allergy Clin. N. Am. 2017, 37, 51–61. [Google Scholar] [CrossRef]
- Masuoka, M.; Shiraishi, H.; Ohta, S.; Suzuki, S.; Arima, K.; Aoki, S.; Toda, S.; Inagaki, N.; Kurihara, Y.; Hayashida, S.; et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J. Clin. Investig. 2012, 122, 2590–2600. [Google Scholar] [CrossRef]
- Yamaguchi, Y. Periostin in skin tissue and skin-related diseases. Allergol. Int. 2014, 63, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.; Lee, K.S.; Ha, E.G.; Lee, S.J.; Kim, M.A.; Lee, S.W.; Jee, H.M.; Sheen, Y.H.; Jung, Y.H.; Han, M.Y. An association of periostin levels with the severity and chronicity of atopic dermatitis in children. Pediatr. Allergy Immunol. 2017, 28, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Villarreal, M.; Jepson, B.; Rafaels, N.; David, G.; Hanifin, J.; Taylor, P.; Boguniewicz, M.; Yoshida, T.; De Benedetto, A.; et al. Patients with Atopic Dermatitis Colonized with Staphylococcus aureus Have a Distinct Phenotype and Endotype. J. Investig. Dermatol. 2018, 138, 2224–2233. [Google Scholar] [CrossRef]
- Furue, M.; Yamamura, K.; Kido-Nakahara, M.; Nakahara, T.; Fukui, Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy 2018, 73, 29–36. [Google Scholar] [CrossRef]
- Kyoya, M.; Kawakami, T.; Soma, Y. Serum thymus and activation-regulated chemokine (TARC) and interleukin-31 levels as biomarkers for monitoring in adult atopic dermatitis. J. Dermatol. Sci. 2014, 75, 204–207. [Google Scholar] [CrossRef]
- Raap, U.; Weißmantel, S.; Gehring, M.; Eisenberg, A.M.; Kapp, A.; Fölster-Holst, R. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr. Allergy Immunol. 2012, 23, 285–288. [Google Scholar] [CrossRef]
- Neis, M.M.; Peters, B.; Dreuw, A.; Wenzel, J.; Bieber, T.; Mauch, C.; Krieg, T.; Stanzel, S.; Heinrich, P.C.; Merk, H.F. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J. Allergy Clin. Immunol. 2006, 118, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Raap, U.; Wichmann, K.; Bruder, M.; Ständer, S.; Wedi, B.; Kapp, A.; Werfel, T. Correlation of IL-31 serum levels with severity of atopic dermatitis. J. Allergy Clin. Immunol. 2008, 122, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Hvid, M.; Johansen, C.; Buchner, M.; Fölster-Holst, R.; Deleuran, M.; Vestergaard, C. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Ozceker, D.; Bulut, M.; Ozbay, A.C.; Dilek, F.; Koser, M.; Tamay, Z.; Guler, N. Assessment of IL-31 levels and disease severity in children with atopic dermatitis. Allergol. Immunopathol. 2018, 46, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Kezic, S.; O’Regan, G.M.; Lutter, R.; Jakasa, I.; Koster, E.S.; Saunders, S.; Caspers, P.; Kemperman, P.M.; Puppels, G.J.; Sandilands, A.; et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J. Allergy Clin. Immunol. 2012, 129, 1031–1039.e1. [Google Scholar] [CrossRef]
- Simpson, E.L.; Chalmers, J.R.; Hanifin, J.M.; Thomas, K.S.; Cork, M.J.; McLean, W.I.; Brown, S.J.; Chen, Z.; Chen, Y.; Williams, H.C. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J. Allergy Clin. Immunol. 2014, 134, 818–823. [Google Scholar] [CrossRef]
- Glatz, M.; Jo, J.-H.; Kennedy, E.A.; Polley, E.C.; Segre, J.A.; Simpson, E.L.; Kong, H.H. Emollient use alters skin barrier and microbes in infants at risk for developing atopic dermatitis. PLoS ONE 2018, 13, e0192443. [Google Scholar] [CrossRef]
- Paternoster, L.; Standl, M.; Waage, J.; Baurecht, H.; Hotze, M.; Strachan, D.P.; Curtin, J.A.; Bønnelykke, K.; Tian, C.; Takahashi, A.; et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. 2015, 47, 1449–1456. [Google Scholar] [CrossRef]
- Kezic, S.; O’Regan, G.M.; Yau, N.; Sandilands, A.; Chen, H.; Campbell, L.E.; Kroboth, K.; Watson, R.; Rowland, M.; Irwin McLean, W.H.; et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy 2011, 66, 934–940. [Google Scholar] [CrossRef]
- Weidinger, S.; O’Sullivan, M.; Illig, T.; Baurecht, H.; Depner, M.; Rodriguez, E.; Ruether, A.; Klopp, N.; Vogelberg, C.; Weiland, S.K.; et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J. Allergy Clin. Immunol. 2008, 121, 1203–1209.e1. [Google Scholar] [CrossRef]
- Barker, J.N.; Palmer, C.N.; Zhao, Y.; Liao, H.; Hull, P.R.; Lee, S.P.; Allen, M.H.; Meggitt, S.J.; Reynolds, N.J.; Trembath, R.C.; et al. Null Mutations in the Filaggrin Gene (FLG) Determine Major Susceptibility to Early-Onset Atopic Dermatitis that Persists into Adulthood. J. Investig. Dermatol. 2007, 127, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Tham, E.H.; Leung, D.Y. Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March. Allergy Asthma Immunol. Res. 2019, 11, 4–15. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Calatroni, A.; Zaramela, L.S.; LeBeau, P.K.; Dyjack, N.; Brar, K.; David, G.; Johnson, K.; Leung, S.; Ramirez-Gama, M.; et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci. Transl. Med. 2019, 11, eaav2685. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.-J.; Wang, Y.-J.; Lin, Y.-C.; Chang, C.-C.; Shieh, C.-C.; Lung, F.-W.; Guo, Y.L. Prediction of atopic dermatitis in 2-yr-old children by cord blood IgE, genetic polymorphisms in cytokine genes, and maternal mentality during pregnancy. Pediatr. Allergy Immunol. 2011, 22, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, H.; Okazaki, N.; Nagakura, T.; Korematsu, S.; Izumi, T. Elevated umbilical cord serum TARC/CCL17 levels predict the development of atopic dermatitis in infancy. Clin. Exp. Allergy 2011, 41, 186–191. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Lee, J.; Han, Y.; Jun, H.-Y.; Kim, H.; Choi, J.; Leung, D.Y.; Ahn, K. Epidermal thymic stromal lymphopoietin predicts the development of atopic dermatitis during infancy. J. Allergy Clin. Immunol. 2016, 137, 1282–1285.e4. [Google Scholar] [CrossRef] [PubMed]
- Ní Chaoimh, C.; Nico, C.; Puppels, G.J.; Caspers, P.J.; Wong, X.F.C.C.; Common, J.E.; Irvine, A.D.; Hourihane, J.O. In vivo Raman spectroscopy discriminates between FLG loss-of-function carriers vs. wild-type in day 1-4 neonates. Ann. Allergy, Asthma Immunol. 2020, 124, 500–504. [Google Scholar] [CrossRef]
- Horimukai, K.; Morita, K.; Narita, M.; Kondo, M.; Kabashima, S.; Inoue, E.; Sasaki, T.; Niizeki, H.; Saito, H.; Matsumoto, K.; et al. Transepidermal water loss measurement during infancy can predict the subsequent development of atopic dermatitis regardless of filaggrin mutations. Allergol. Int. 2015, 65, 103–108. [Google Scholar] [CrossRef]
- Lauffer, F.; Baghin, V.; Standl, M.; Stark, S.P.; Jargosch, M.; Wehrle, J.; Thomas, J.; Schmidt-Weber, C.B.; Biedermann, T.; Eyerich, S.; et al. Predicting persistence of atopic dermatitis in children using clinical attributes and serum proteins. Allergy 2021, 76, 1158–1172. [Google Scholar] [CrossRef]
- Staudacher, A.; Hinz, T.; Novak, N.; Von Bubnoff, D.; Bieber, T. Exaggerated IDO1 expression and activity in Langerhans cells from patients with atopic dermatitis upon viral stimulation: A potential predictive biomarker for high risk of Eczema herpeticum. Allergy 2015, 70, 1432–1439. [Google Scholar] [CrossRef]
- Brunner, P.M.; Pavel, A.B.; Khattri, S.; Leonard, A.; Malik, K.; Rose, S.; On, S.J.; Vekaria, A.S.; Traidl-Hoffmann, C.; Singer, G.K.; et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J. Allergy Clin. Immunol. 2018, 143, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Glickman, J.W.; Han, J.; Garcet, S.; Krueger, J.G.; Pavel, A.B.; Guttman-Yassky, E. Improving evaluation of drugs in atopic dermatitis by combining clinical and molecular measures. J. Allergy Clin. Immunol. Pract. 2020, 8, 3622–3625.e19. [Google Scholar] [CrossRef] [PubMed]
- Esaki, H.; Brunner, P.M.; Renert-Yuval, Y.; Czarnowicki, T.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; Johnson, D.B.; et al. Early-onset pediatric atopic dermatitis is T H 2 but also T H 17 polarized in skin. J. Allergy Clin. Immunol. 2016, 138, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; Esaki, H.; Gonzalez, J.; Malajian, D.; Shemer, A.; Noda, S.; Talasila, S.; Berry, A.; Gray, J.; Becker, L.; et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)+ TH2/TH1 cell imbalance, whereas adults acquire CLA+ TH22/TC22 cell subsets. J. Allergy Clin. Immunol. 2015, 136, 941–951.e3. [Google Scholar] [CrossRef]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; de Guzman Strong, C.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015, 136, 1254–1264. [Google Scholar] [CrossRef]
- Nomura, T.; Wu, J.; Kabashima, K.; Guttman-Yassky, E. Endophenotypic Variations of Atopic Dermatitis by Age, Race, and Ethnicity. J. Allergy Clin. Immunol. Pract. 2020, 8, 1840–1852. [Google Scholar] [CrossRef]
- Kaufman, B.P.; Guttman-Yassky, E.; Alexis, A.F. Atopic dermatitis in diverse racial and ethnic groups-Variations in epidemiology, genetics, clinical presentation and treatment. Exp. Dermatol. 2018, 27, 340–357. [Google Scholar] [CrossRef]
- Quaranta, M.; Knapp, B.; Garzorz, N.; Mattii, M.; Pullabhatla, V.; Pennino, D.; Andres, C.; Traidl-Hoffmann, C.; Cavani, A.; Theis, F.J.; et al. Intraindividual genome expression analysis reveals a specific molecular signature of psoriasis and eczema. Sci. Transl. Med. 2014, 6, 244ra90. [Google Scholar] [CrossRef]
- Garzorz-Stark, N.; Krause, L.; Lauffer, F.; Atenhan, A.; Thomas, J.; Stark, S.P.; Franz, R.; Weidinger, S.; Balato, A.; Mueller, N.S.; et al. A novel molecular disease classifier for psoriasis and eczema. Exp. Dermatol. 2016, 25, 767–774. [Google Scholar] [CrossRef]
- He, H.; Bissonnette, R.; Wu, J.; Diaz, A.; Proulx, E.S.-C.; Maari, C.; Jack, C.; Louis, M.; Estrada, Y.; Krueger, J.G.; et al. Tape strips detect distinct immune and barrier profiles in atopic dermatitis and psoriasis. J. Allergy Clin. Immunol. 2021, 147, 199–212. [Google Scholar] [CrossRef]
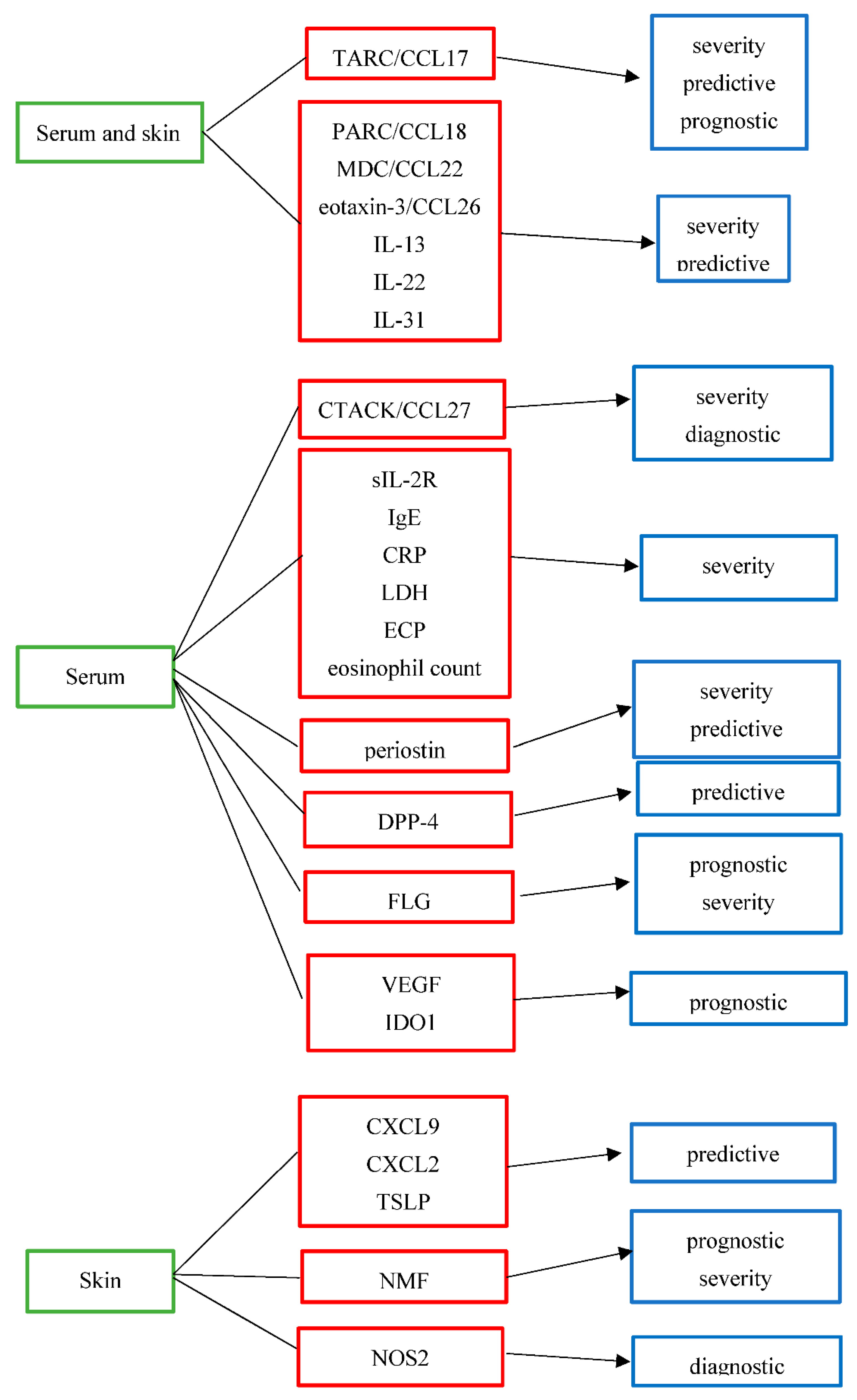
| Biomarker | Full Name | Purpose | Grade of Evidence | Biologic Origin |
|---|---|---|---|---|
| TARC/CCL17 [13,25,27,28,29,30,32,33,34,35,36,37,38,40,53,69] | thymus and activation-regulated chemokine/ C-C motif ligand 17 | severity predictive prognostic | high moderate low | serum and skin |
| PARC/CCL18 [25,27,28,29] | pulmonary and activation-regulated chemokine/C-C motif ligand 18 | severity predictive | moderate low | serum and skin |
| MDC/CCL22 [13,25,27,28,29,30,76] | macrophage-derived chemokine/C-C motif ligand 22 | severity predictive | moderate low | serum and skin |
| Eotaxin-3/CCL26 [25,27] | eosinophil-attracting chemokine/C-C motif ligand 26 | severity predictive | moderate low | serum and skin |
| IL-13 [9,25,27,77,78,79,80] | interleukin-13 | severity predictive | moderate low | serum and skin |
| IL-22 [25,27,28,29,75,77,78,79,80] | interleukin-22 | severity predictive | moderate low | serum and skin |
| IL-31 [13,52,53,54,55,56,57,58] | interleukin-31 | severity predictive | moderate low | serum and skin |
| CTACK/CCL27 [13,25,27,33,34,82,83,84] | cutaneous T-cell-attracting chemokine/ C-C motif ligand 27 | severity diagnostic/differential diagnosis from psoriasis | moderate low | serum |
| sIL-2R [13,28,29] | soluble IL-2 receptor | severity | moderate | serum |
| IgE [13,40,41,45,46] | immunoglobulin E | severity | moderate | serum |
| CRP [39] | C-reactive protein | severity | low | serum |
| LDH [13,39,40,41] | lactate dehydrogenase | severity | moderate | serum |
| ECP [13,42,43] | eosinophil cationic protein | severity | moderate | serum |
| Eosinophil count [40,41,43,44] | severity | moderate | serum | |
| Periostin [9,44,48,49,50] | severity predictive | moderate low | serum | |
| DPP-4 [9] | dipeptidyl peptidase-4 | predictive | low | serum |
| CXCL9 [76] | C-X-C motif ligand 9 | predictive | low | skin |
| CXCL2 [76] | C-X-C motif ligand 2 | predictive | low | skin |
| TSLP [70] | thymic stromal lymphopoietin protein | predictive | moderate | skin |
| NMF [59,71] | natural moisturizing factor | prognostic severity | moderate low | skin |
| FLG [47,59,62,65,66,67] | filaggrin | prognostic severity | moderate low | serum |
| VEGF [73] | vascular endothelial growth factor | prognostic | low | serum |
| IDO1 [74] | indoleamine 2,3-dioxygenase-1 | prognostic for eczema herpeticum | low | serum |
| NOS2 [82,83,84] | nitric oxide synthase 2 | diagnostic/differential diagnosis from psoriasis | low | skin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastraftsi, S.; Vrioni, G.; Bakakis, M.; Nicolaidou, E.; Rigopoulos, D.; Stratigos, A.J.; Gregoriou, S. Atopic Dermatitis: Striving for Reliable Biomarkers. J. Clin. Med. 2022, 11, 4639. https://doi.org/10.3390/jcm11164639
Mastraftsi S, Vrioni G, Bakakis M, Nicolaidou E, Rigopoulos D, Stratigos AJ, Gregoriou S. Atopic Dermatitis: Striving for Reliable Biomarkers. Journal of Clinical Medicine. 2022; 11(16):4639. https://doi.org/10.3390/jcm11164639
Chicago/Turabian StyleMastraftsi, Styliani, Georgia Vrioni, Michail Bakakis, Electra Nicolaidou, Dimitrios Rigopoulos, Alexander J. Stratigos, and Stamatios Gregoriou. 2022. "Atopic Dermatitis: Striving for Reliable Biomarkers" Journal of Clinical Medicine 11, no. 16: 4639. https://doi.org/10.3390/jcm11164639
APA StyleMastraftsi, S., Vrioni, G., Bakakis, M., Nicolaidou, E., Rigopoulos, D., Stratigos, A. J., & Gregoriou, S. (2022). Atopic Dermatitis: Striving for Reliable Biomarkers. Journal of Clinical Medicine, 11(16), 4639. https://doi.org/10.3390/jcm11164639








