Effect of Sinus Perforation with Flaplessly Placed Mini Dental Implants for Oral Rehabilitation: A 5-Year Clinical and Radiological Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Treatment Protocol
2.2. Radiological Examination
2.3. Clinical Rhinosinusitis Evaluation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vi, S.; Pham, D.; Du, Y.; Arora, H.; Tadakamadla, S. Mini-Implant-Retained Overdentures for the Rehabilitation of Completely Edentulous Maxillae: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 4377. [Google Scholar] [CrossRef] [PubMed]
- Van Doorne, L.; Fonteyne, E.; Matthys, C.; Bronkhorst, E.; Meijer, G.; De Bruyn, H. Longitudinal Oral Health-Related Quality of Life in maxillary mini dental implant overdentures after 3 years in function. Clin. Oral Implant. Res. 2020, 32, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.S.; Levine, A.; Bedos, C.; Mojon, P.; Rosberger, Z.; Feine, J.; Thomason, J.M. Refusal of implant supported mandibular overdentures by elderly patients. Gerodontology 2011, 28, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Turner, H.N.; Liman, E.R. The Cellular and Molecular Basis of Sour Taste. Annu. Rev. Physiol. 2022, 84, 41–58. [Google Scholar] [CrossRef]
- Damghani, S.; Masri, R.; Driscoll, C.F.; Romberg, E. The effect of number and distribution of unsplinted maxillary implants on the load transfer in implant-retained maxillary overdentures: An in vitro study. J. Prosthet. Dent. 2012, 107, 358–365. [Google Scholar] [CrossRef]
- Benzing, U.R.; Gall, H.; Weber, H. Biomechanical aspects of two different implant-prosthetic concepts for edentulous maxillae. Int. J. Oral Maxillofac. Implant. 1995, 10. [Google Scholar]
- Cowin, S.C. Wolff’s Law of Trabecular Architecture at Remodeling Equilibrium. J. Biomech. Eng. 1986, 108, 83–88. [Google Scholar] [CrossRef]
- Stanford, C.M.; Brand, R.A. Toward an understanding of implant occlusion and strain adaptive bone modeling and remodeling. J. Prosthet. Dent. 1999, 81, 553–561. [Google Scholar] [CrossRef]
- Sotto-Maior, B.S.; Mercuri, E.G.F.; Senna, P.M.; Assis, N.M.S.P.; Francischone, C.E.; Cury, A.A.D.B. Evaluation of bone remodeling around single dental implants of different lengths: A mechanobiological numerical simulation and validation using clinical data. Comput. Methods Biomech. Biomed. Eng. 2015, 19, 1–8. [Google Scholar] [CrossRef]
- Maló, P.; de Araújo Nobre, M.; Lopes, A.; Ferro, A.; Gravito, I. All-on-4® treatment concept for the rehabilitation of the completely edentulous mandible: A 7-year clinical and 5-year radiographic retrospective case series with risk assessment for implant failure and marginal bone level: Complete edentulous mandible rehabilitation. Clin. Implant Dent. Relat. Res. 2015, 17, e531–e541. [Google Scholar]
- Ausiello, P.; Tribst, J.P.M.; Ventre, M.; Salvati, E.; di Lauro, A.E.; Martorelli, M.; Lanzotti, A.; Watts, D.C. The role of cortical zone level and prosthetic platform angle in dental implant mechanical response: A 3D finite element analysis. Dent. Mater. 2021, 37, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Tribst, J.P.M.; de Morais, D.C.; de Matos, J.D.M.; Lopes, G.D.R.S.; Piva, A.M.D.O.D.; Borges, A.L.S.; Bottino, M.A.; Lanzotti, A.; Martorelli, M.; Ausiello, P. Influence of Framework Material and Posterior Implant Angulation in Full-Arch All-on-4 Implant-Supported Prosthesis Stress Concentration. Dent. J. 2022, 10, 12. [Google Scholar] [CrossRef]
- Gandhi, Y. Sinus Grafts: Science and Techniques—Then and Now. J. Maxillofac. Oral Surg. 2017, 16, 135–144. [Google Scholar] [CrossRef]
- Summers, R.B. A new concept in maxillary implant surgery: The osteotome technique. Compendium 1994, 15, 152–154. [Google Scholar]
- Park, W.; Herr, Y.; Chung, J.; Shin, S.; Han, J.; Lim, H. Long-term effects of sinus membrane perforation on dental implants placed with transcrestal sinus floor elevation: A case–control study. Clin. Implant Dent. Relat. Res. 2021, 23, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Choi, S.M.; Yoon, J.H.; Lee, E.J.; Yoon, J.; Kwon, S.H.; Yeo, C.D.; Ryu, J.S.; Lee, J.H.; You, Y.S.; et al. What Affects Postoperative Sinusitis and Implant Failure after Dental Implant: A Meta-analysis. Otolaryngol. Head Neck Surg. 2019, 160, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Sobiesk, J.L.; Munakomi, S. Anatomy, Head and Neck, Nasal Cavity. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Raghoebar, G.; van Weissenbruch, R.; Vissink, A. Rhino-sinusitis related to endosseous implants extending into the nasal cavity: A case report. Int. J. Oral Maxillofac. Surg. 2004, 33, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Park, H.I.; Ahn, K.; Kim, J.H.; Chung, Y.; Jang, Y.J.; Yu, M.S. Features of Odontogenic Sinusitis Associated With Dental Implants. Laryngoscope 2022. [Google Scholar] [CrossRef]
- Ragucci, G.M.; Elnayef, B.; Del Amo, F.S.-L.; Wang, H.-L.; Hernández-Alfaro, F.; Gargallo-Albiol, J. Influence of exposing dental implants into the sinus cavity on survival and complications rate: A systematic review. Int. J. Implant. Dent. 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Van Doorne, L.; De Kock, L.; De Moor, A.; Shtino, R.; Bronkhorst, E.; Meijer, G.; De Bruyn, H. Flaplessly placed 2.4-mm mini-implants for maxillary overdentures: A prospective multicentre clinical cohort study. Int. J. Oral Maxillofac. Surg. 2019, 49, 384–391. [Google Scholar] [CrossRef]
- Van Doorne, L.; Gholami, P.; D’Haese, J.; Hommez, G.; Meijer, G.; De Bruyn, H. Three-Dimensional Radiographic Outcome of Free-Handed Flaplessly Placed Mini Dental Implants in Edentulous Maxillae after 2-Years Function. J. Clin. Med. 2020, 9, 2120. [Google Scholar] [CrossRef] [PubMed]
- Jones, N. CT of the paranasal sinuses: A review of the correlation with clinical, surgical and histopathological findings. Clin. Otolaryngol. Allied Sci. 2002, 27, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, S.; Ustaoğlu, G.; Demiralp, K.; Çakmak, E.K. Evaluation of the Characteristics and Association Between Schneiderian Membrane Thickness and Nasal Septum Deviation. J. Craniofacial Surg. 2018, 29, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Rustogi, R.; Coucher, J.; Malan, J. New radiological typing system for rhinosinusitis and comparison with existing system: Initial finding. Australas. Radiol. 2007, 51, 339–345. [Google Scholar] [CrossRef] [PubMed]
- The 2007 Recommendations of the International Commission on Radiological Protection. Ann. ICRP 2007, 37, 1–332. [CrossRef]
- Janner, S.F.M.; Caversaccio, M.D.; Dubach, P.; Sendi, P.; Buser, D.; Bornstein, M.M. Characteristics and dimensions of the Schneiderian membrane: A radiographic analysis using cone beam computed tomography in patients referred for dental implant surgery in the posterior maxilla. Clin. Oral Implant. Res. 2011, 22, 1446–1453. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Lauber, R.; Sendi, P.; von Arx, T. Comparison of Periapical Radiography and Limited Cone-Beam Computed Tomography in Mandibular Molars for Analysis of Anatomical Landmarks before Apical Surgery. J. Endod. 2011, 37, 151–157. [Google Scholar] [CrossRef]
- Razi, B.; Perkovic, A.; Alvarado, R.; Stroud, A.; Ho, J.; Kalish, L.H.; Campbell, R.G.; Sacks, R.; Harvey, R.J. Sinus Radiological Findings in General Asymptomatic Populations: A Systematic Review of Incidental Mucosal Changes. Otolaryngol. Head Neck Surg. 2021, 167, 16–24. [Google Scholar] [CrossRef]
- Jung, J.-H.; Choi, B.-H.; Zhu, S.-J.; Lee, S.-H.; Huh, J.-Y.; You, T.-M.; Lee, H.-J.; Li, J. The effects of exposing dental implants to the maxillary sinus cavity on sinus complications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 102, 602–605. [Google Scholar] [CrossRef]
- Jung, J.-H.; Choi, B.-H.; Jeong, S.-M.; Li, J.; Lee, S.-H.; Lee, H.-J. A retrospective study of the effects on sinus complications of exposing dental implants to the maxillary sinus cavity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 103, 623–625. [Google Scholar] [CrossRef]
- Thor, A.; Sennerby, L.; Hirsch, J.M.; Rasmusson, L. Bone Formation at the Maxillary Sinus Floor Following Simultaneous Elevation of the Mucosal Lining and Implant Installation Without Graft Material: An Evaluation of 20 Patients Treated With 44 Astra Tech Implants. J. Oral Maxillofac. Surg. 2007, 65 (7 Suppl 1), 64–72. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, J.S.; Kim, H.T.; Lee, C.H.; Park, Y.H.; Bae, J.H. Clinical features and treatment outcomes of dental implant-related paranasal sinusitis: A 2-year prospective observational study. Clin. Oral Implant. Res. 2015, 27, e100–e104. [Google Scholar] [CrossRef] [PubMed]
- Troeltzsch, M.; Pache, C.; Troeltzsch, M.; Kaeppler, G.; Ehrenfeld, M.; Otto, S.; Probst, F. Etiology and clinical characteristics of symptomatic unilateral maxillary sinusitis: A review of 174 cases. J. Craniomaxillofac. Surg. 2015, 43, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Prasetyo, A.; Sadhana, U.; Budiman, J. Nasal Mucociliary Clearance in Smokers: A Systematic Review. Int. Arch. Otorhinolaryngol. 2020, 25, e160–e169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monje, A.; Diaz, K.T.; Aranda, L.; Insua, A.; Garcia-Nogales, A.; Wang, H.-L. Schneiderian Membrane Thickness and Clinical Implications for Sinus Augmentation: A Systematic Review and Meta-Regression Analyses. J. Periodontol. 2016, 87, 888–899. [Google Scholar] [CrossRef] [PubMed]
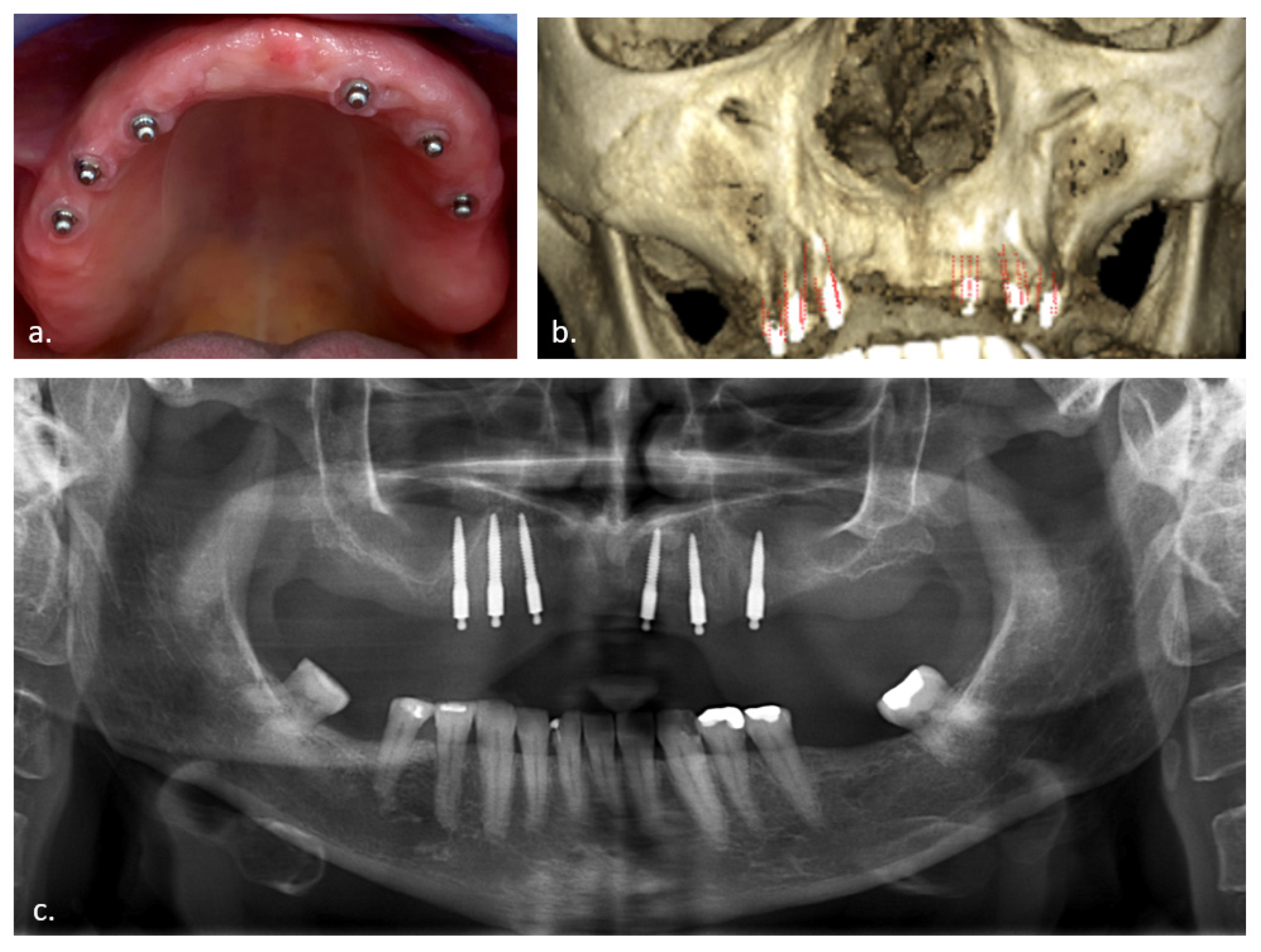
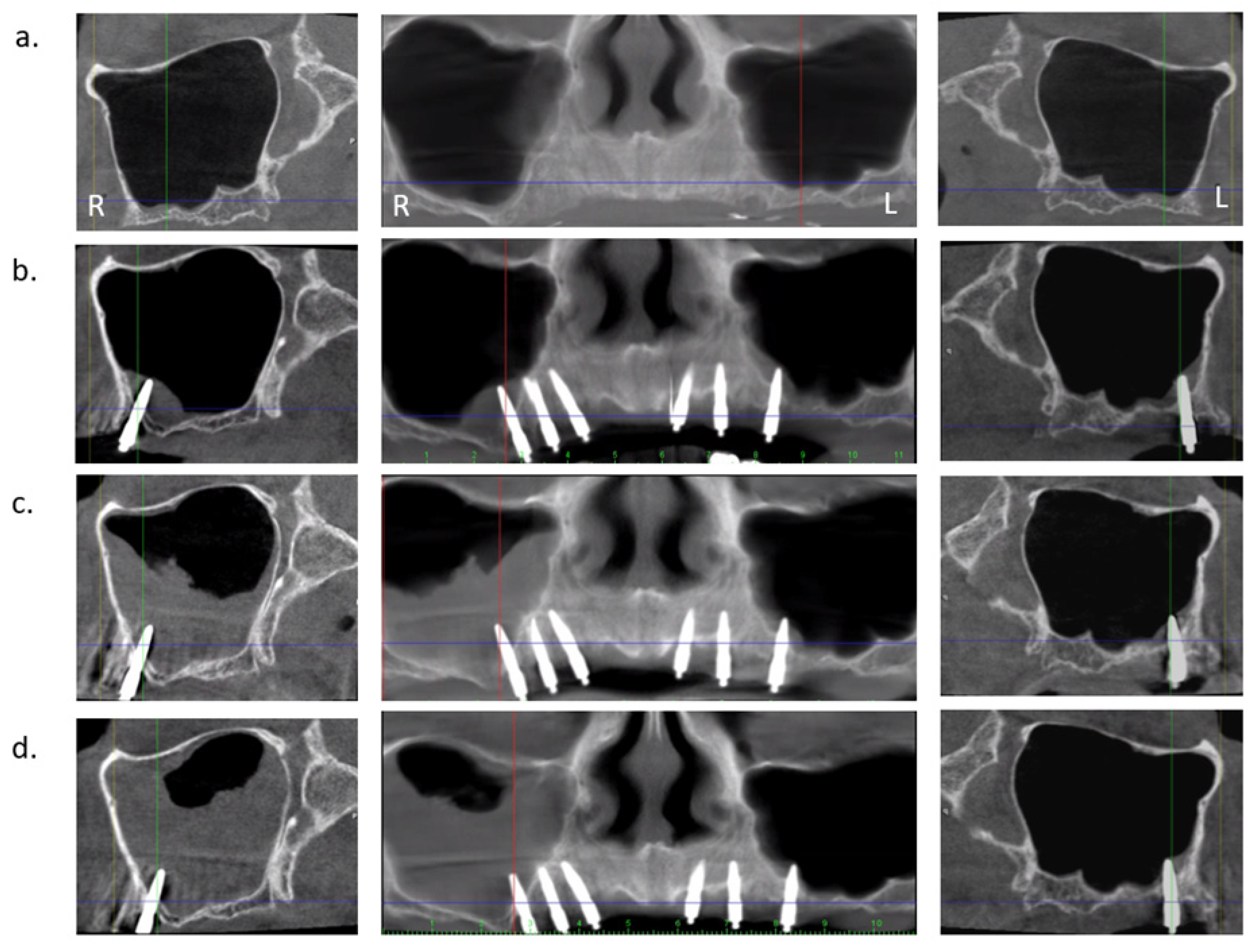
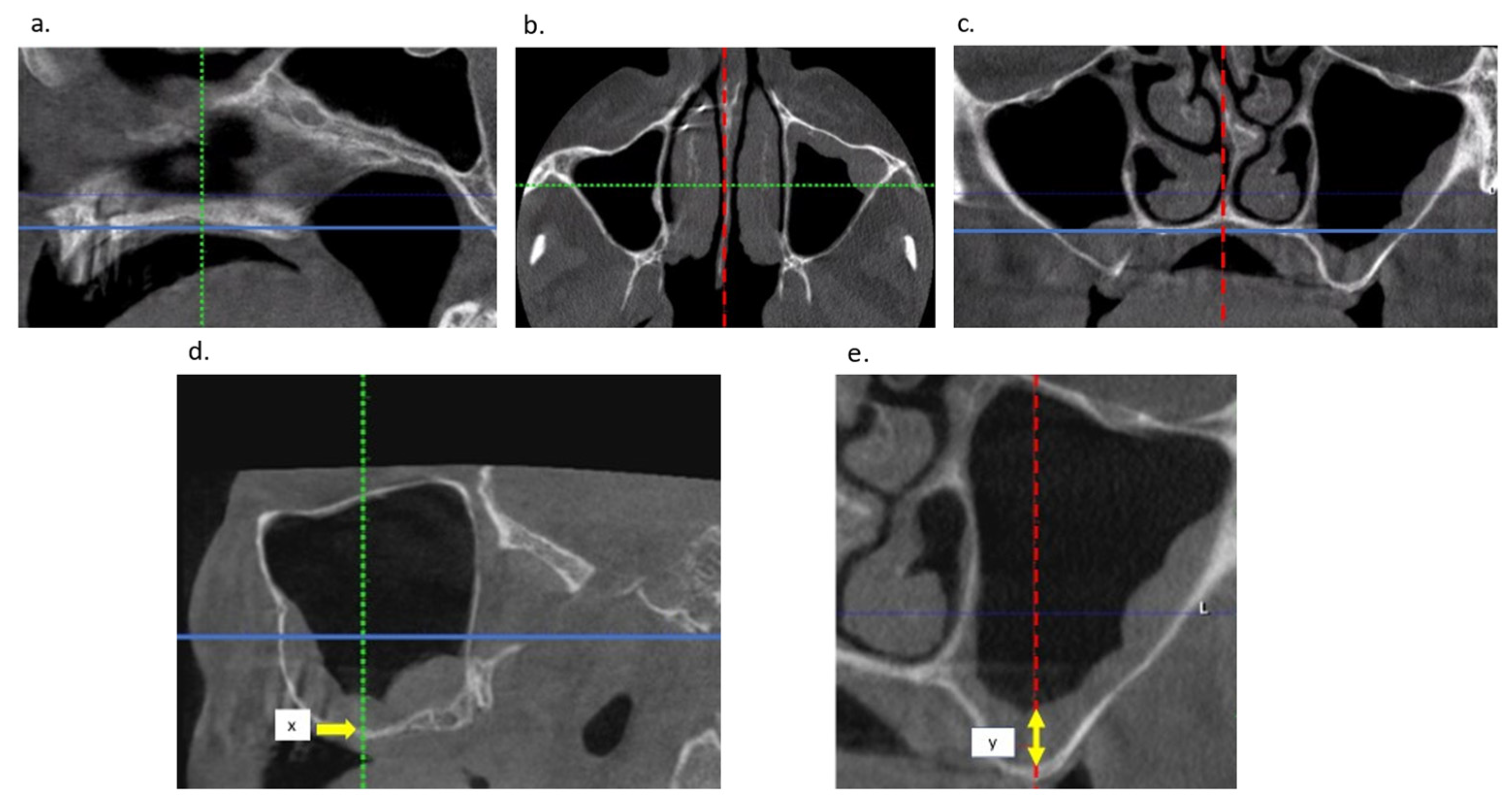
| Membrane Thickness (mm) | All Membranes (n = 54) | No Perforation (n = 21) | with Perforation (n = 33) |
|---|---|---|---|
| Baseline | 2.87 (SD: 4.20; range 0.0–20.6) | 2.49 (SD: 4.46; range 0.0–20.6) | 3.46 (SD: 3.78; range 0.0–11.2) |
| After 2 years | 3.15 (SD: 4.46; range 0.0–18.4) | 2.31 (SD: 4.29; range 0.0–18.4) | 4.49 (SD: 4.51; range 0.0–15.4) |
| After 5 years | 4.30 (SD: 7.13; range 0.0–35.6) | 1.87 (SD: 2.92; range 0.0–14.6) | 8.13 (SD: 9.81; range 0.0–35.6) |
| 2 Year | Estimate | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Intercept | 2.607 | [1.905…3.325] | <0.001 |
| Thickness baseline centered (mm) | 0.782 | [0.612…0.955] | <0.001 |
| Perforation length (mm) | 0.212 | [0.003…0.425] | 0.051 |
| 5 Years | |||
| Intercept | 2.163 | [1.284…3.038] | <0.001 |
| Thickness baseline centered (mm) | 0.597 | [0.381…0.812] | <0.001 |
| Perforation length (mm) | 0.638 | [0.378…0.897] | <0.001 |
| 2 Year | Estimate | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Intercept | 3.122 | [2.444…3.800] | <0.001 |
| Thickness baseline centered (mm) | 0.827 | [0.678…0.986] | <0.001 |
| Perforation length (mm) | 0.000 | [−0.201…0.203] | 0.999 |
| Smoking (yes vs. no) | 1.526 | [−2.673…-0.379] | 0.019 |
| Perforation length and smoking | 0.867 | [0.480…1.274] | <0.001 |
| 5 Year | |||
| Intercept | 2.556 | [1.511…3.601] | <0.001 |
| Thickness baseline centered (mm) | 0.605 | [0.394…0.818] | <0.001 |
| Perforation length (mm) | 0.534 | [0.233…0.830] | 0.001 |
| Smoking (yes vs. no) | −1.140 | [−2.917…0.636] | 0.236 |
| Perforation length and smoking | 0.351 | [0.378…0.897] | 0.251 |
| Complaints 2 Years | No Perforation (n: 10) | Perforation (n: 13) | Total (n: 23) |
|---|---|---|---|
| no | 7 (70.0%) | 8 (61.5%) | 15 (65%) |
| mild | 2 (20.0%) | 3 (23.1%) | 5 (21.7%) |
| moderate | 1 (10.0%) | 2 (15.4%) | 3 (13.0% |
| severe | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Complaints 5 Years | |||
| no | 6 (54.5%) | 6 (45.2%) | 12 (50.0%) |
| mild | 4 (36.4%) | 4 (30.8%) | 8 (33.3%) |
| moderate | 1 (9.1%) | 3 (23.1%) | 4 (16.7%) |
| severe | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Doorne, L.; Hommez, G.; Bronkhorst, E.; Meijer, G.; De Bruyn, H. Effect of Sinus Perforation with Flaplessly Placed Mini Dental Implants for Oral Rehabilitation: A 5-Year Clinical and Radiological Follow-Up. J. Clin. Med. 2022, 11, 4637. https://doi.org/10.3390/jcm11154637
Van Doorne L, Hommez G, Bronkhorst E, Meijer G, De Bruyn H. Effect of Sinus Perforation with Flaplessly Placed Mini Dental Implants for Oral Rehabilitation: A 5-Year Clinical and Radiological Follow-Up. Journal of Clinical Medicine. 2022; 11(15):4637. https://doi.org/10.3390/jcm11154637
Chicago/Turabian StyleVan Doorne, Luc, Geert Hommez, Ewald Bronkhorst, Gert Meijer, and Hugo De Bruyn. 2022. "Effect of Sinus Perforation with Flaplessly Placed Mini Dental Implants for Oral Rehabilitation: A 5-Year Clinical and Radiological Follow-Up" Journal of Clinical Medicine 11, no. 15: 4637. https://doi.org/10.3390/jcm11154637
APA StyleVan Doorne, L., Hommez, G., Bronkhorst, E., Meijer, G., & De Bruyn, H. (2022). Effect of Sinus Perforation with Flaplessly Placed Mini Dental Implants for Oral Rehabilitation: A 5-Year Clinical and Radiological Follow-Up. Journal of Clinical Medicine, 11(15), 4637. https://doi.org/10.3390/jcm11154637








