Increased Apoptotic Activity in Low-Risk Myelodysplastic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. IHC
2.3. Telomere Q-FISH
2.4. Prognostic Factors
2.5. Mutational Analysis
2.6. Statistics
3. Results
3.1. Patient Characteristics
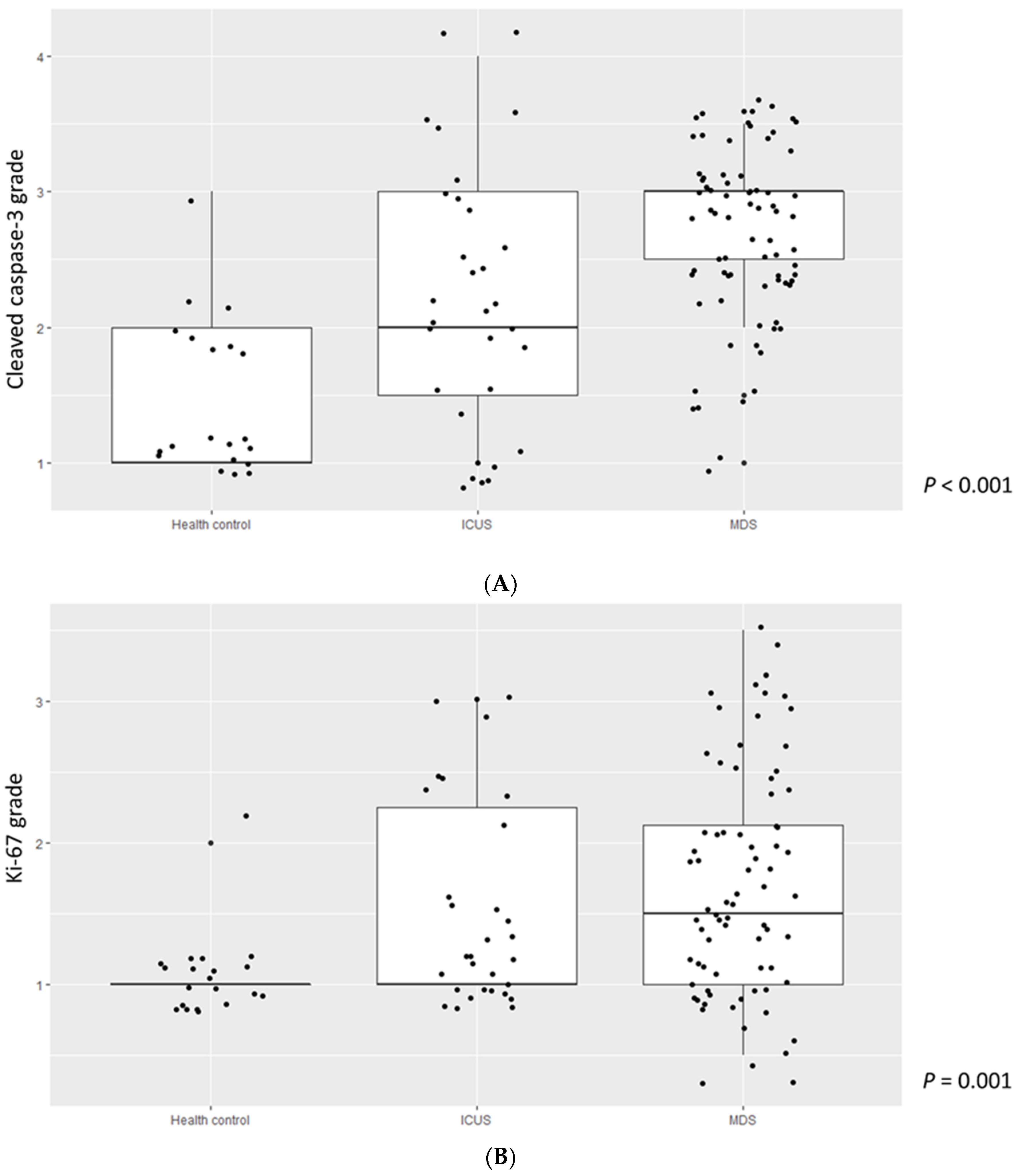
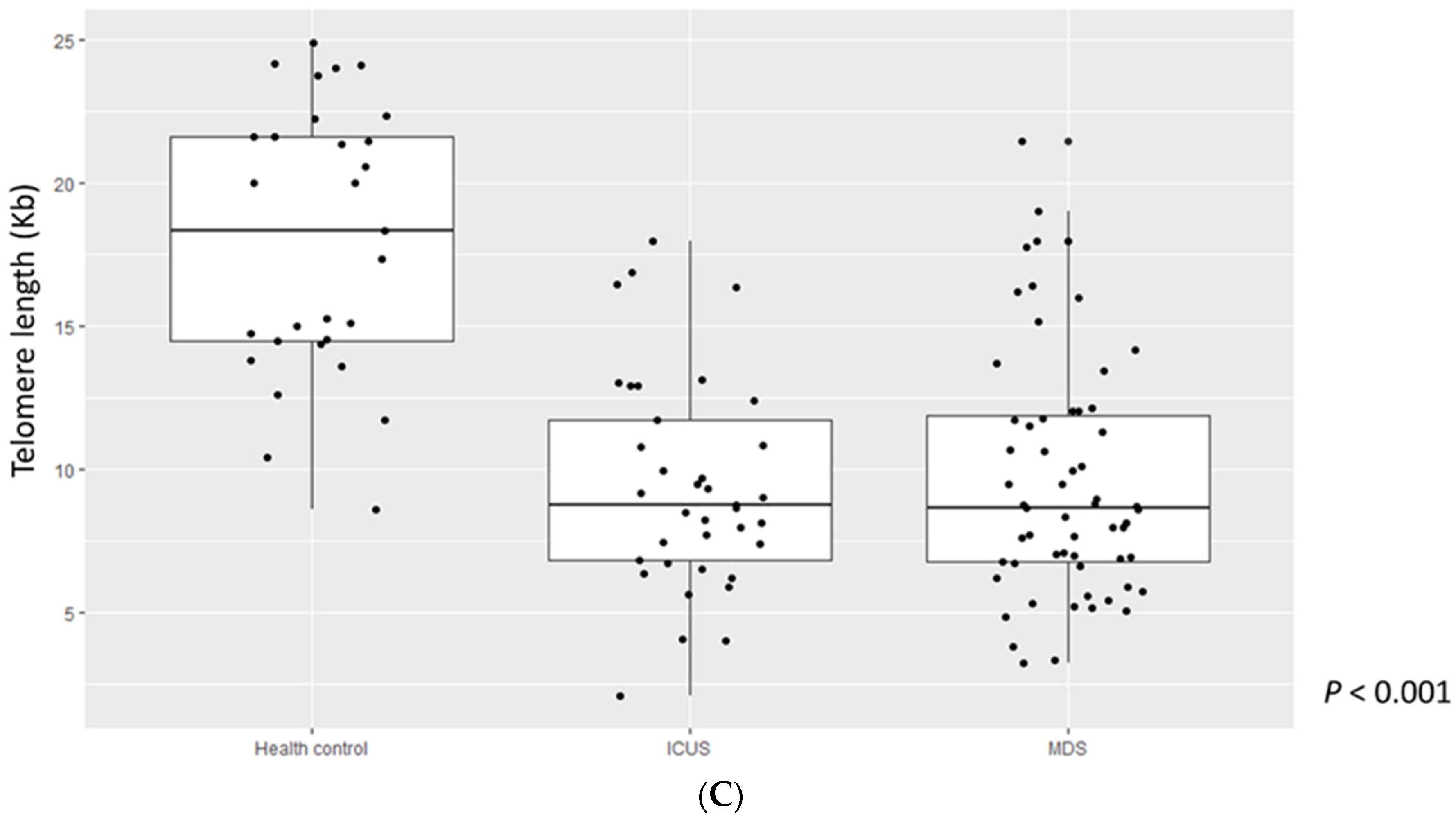
3.2. Comparison of Cleaved Caspase-3, Ki-67 Grade, and TL among MDS Patients, ICUS, and HCs
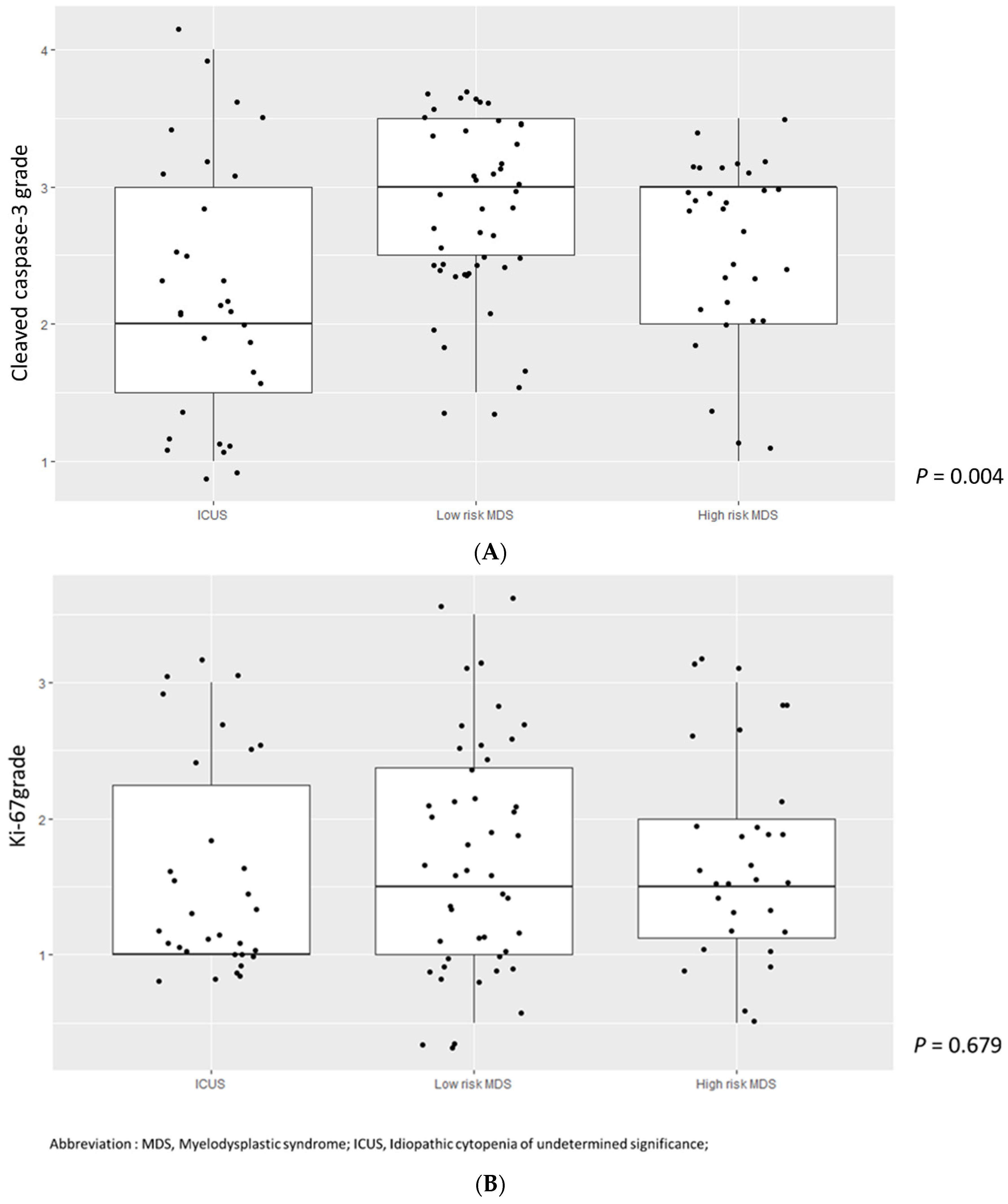
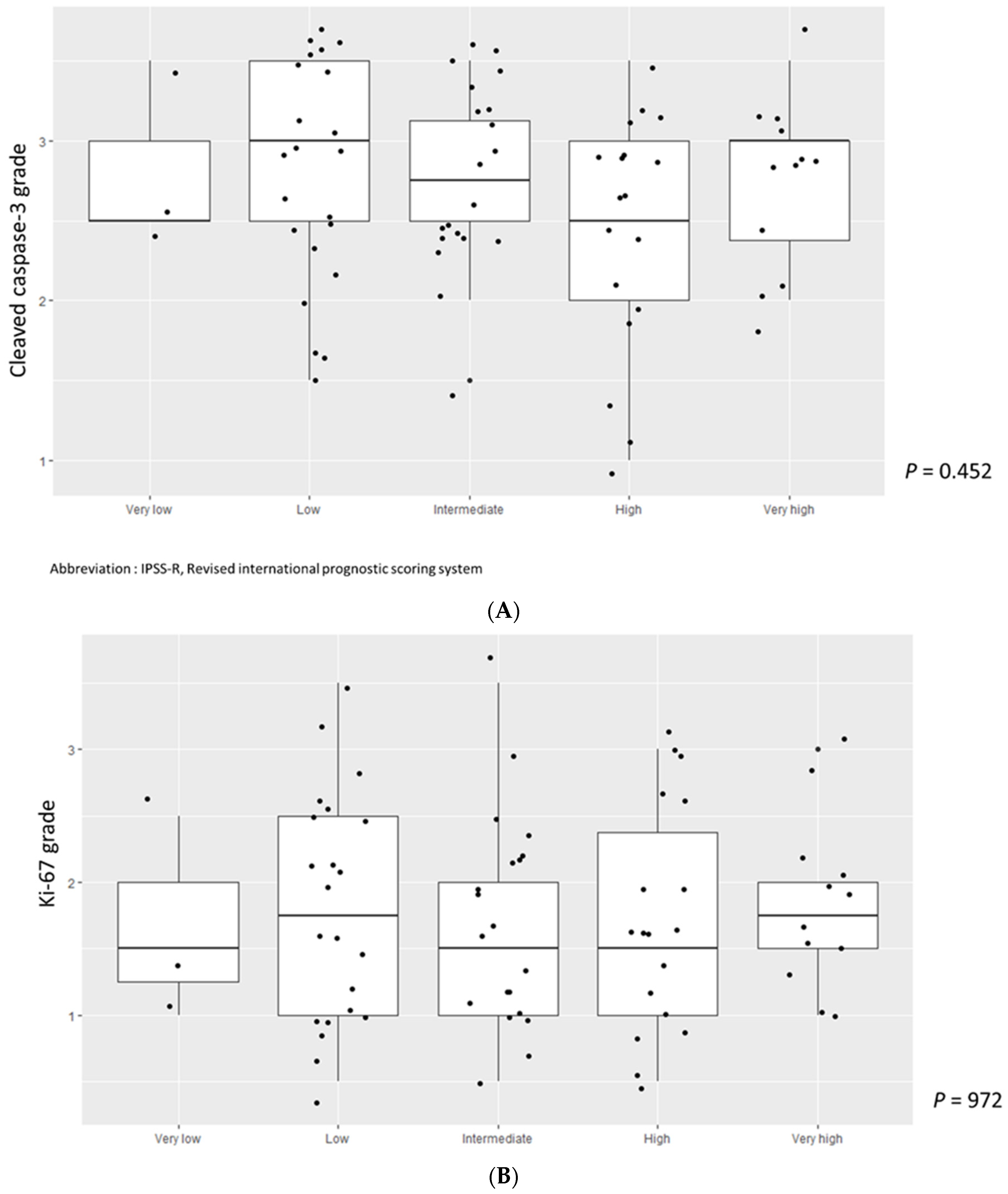
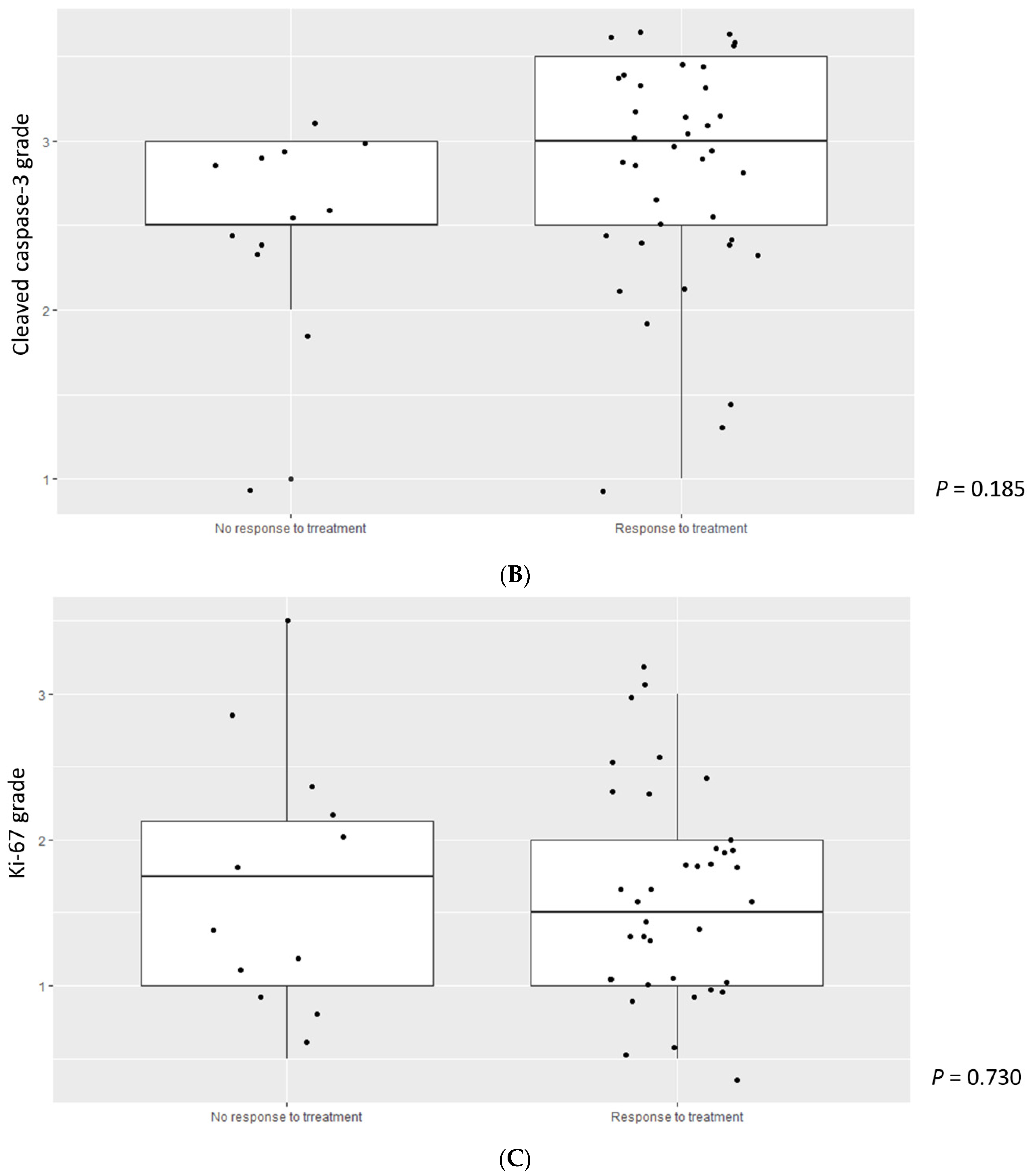
3.3. Higher Cleaved Caspase-3 Grade Was Enriched in Lower-Risk MDS
3.4. Ki-67, Caspase-3, and Genetics in MDS
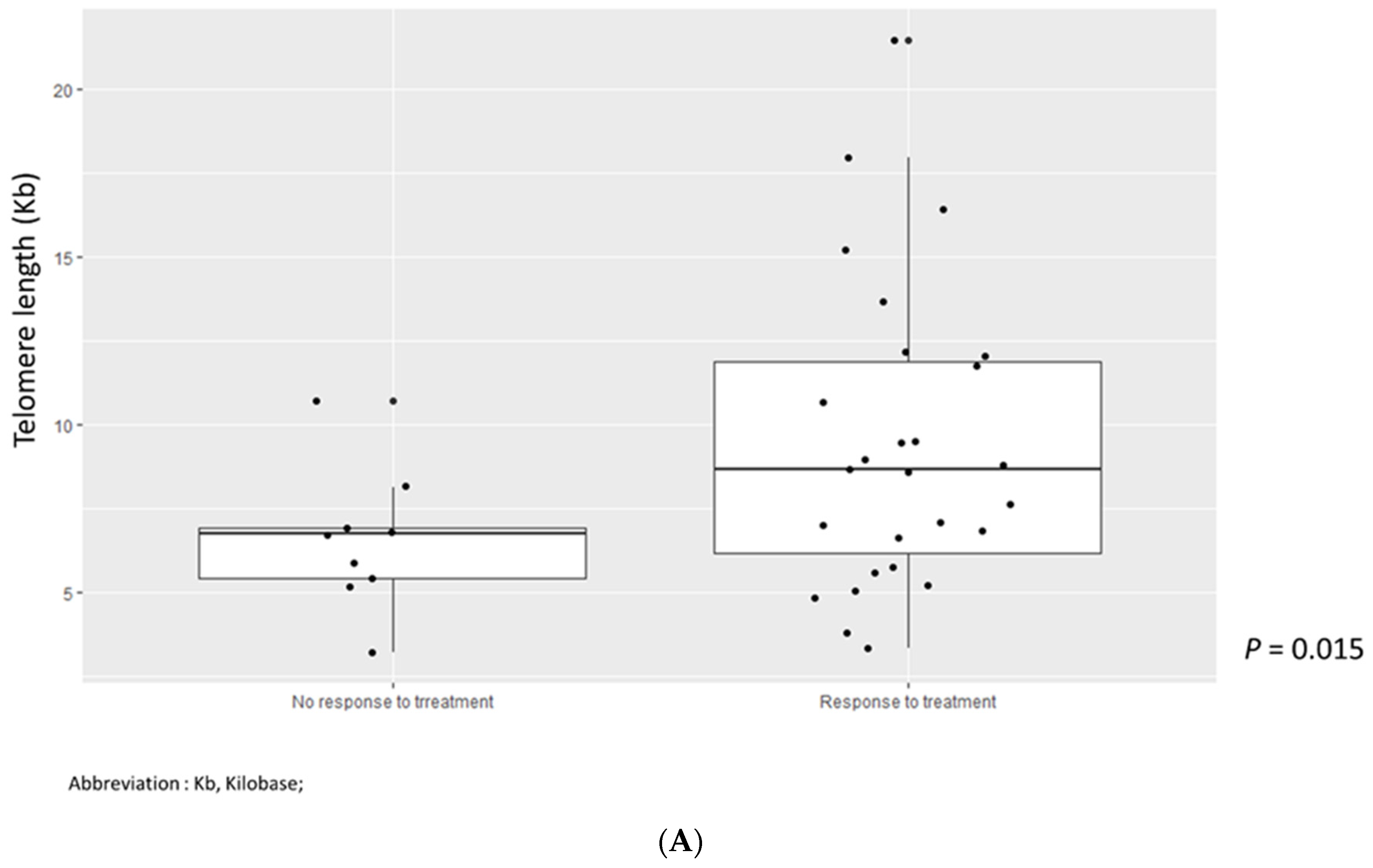
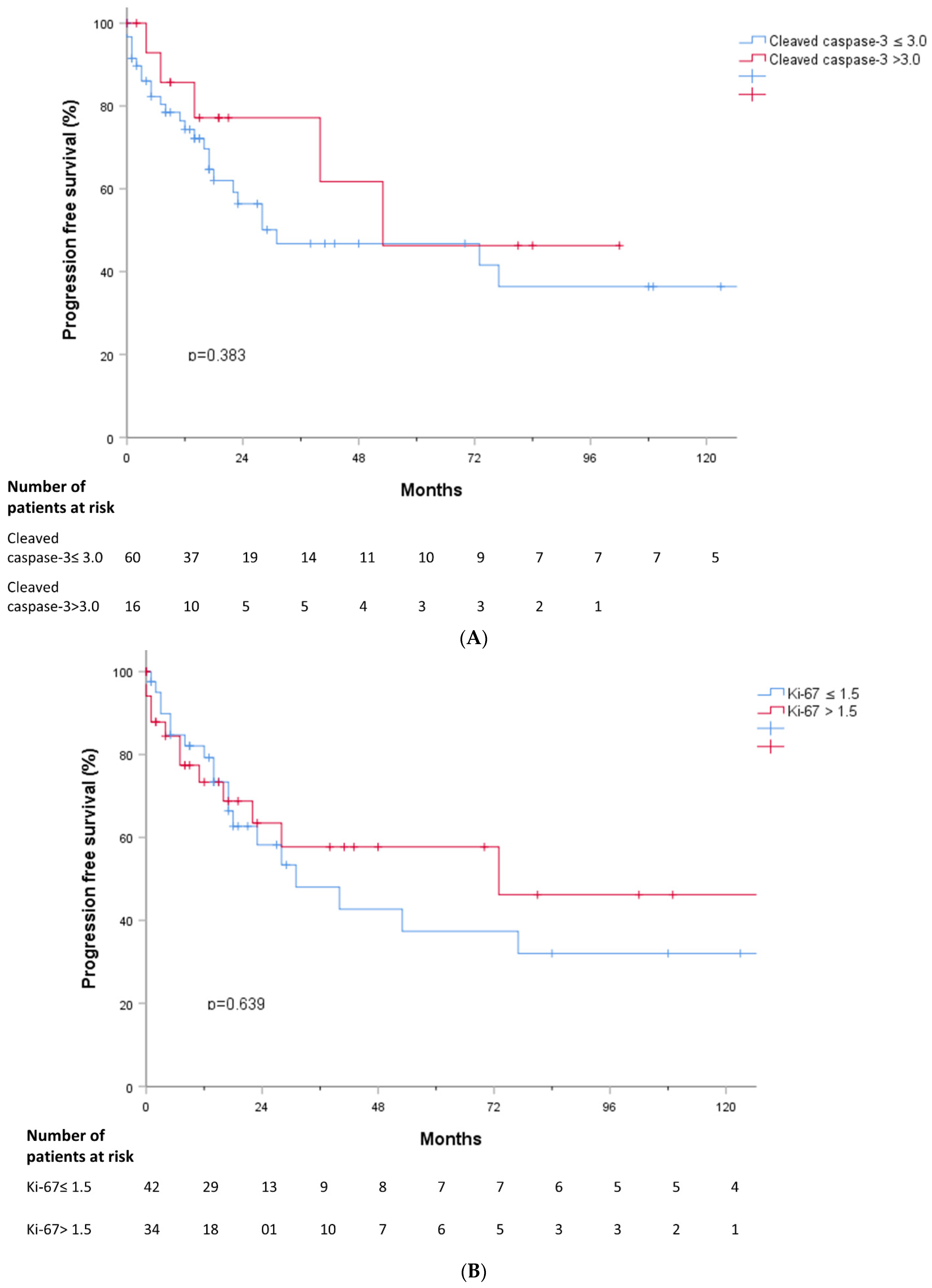
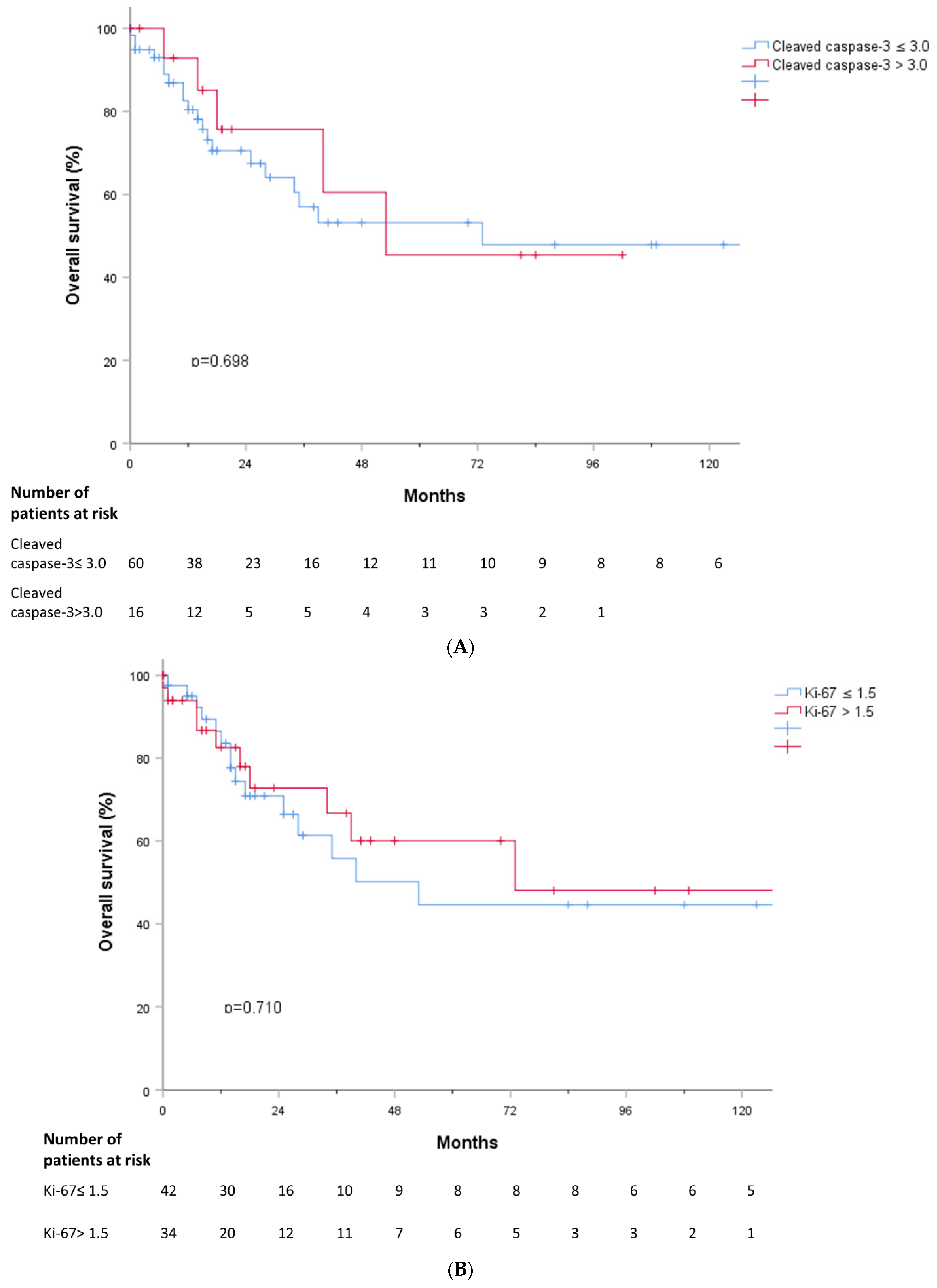
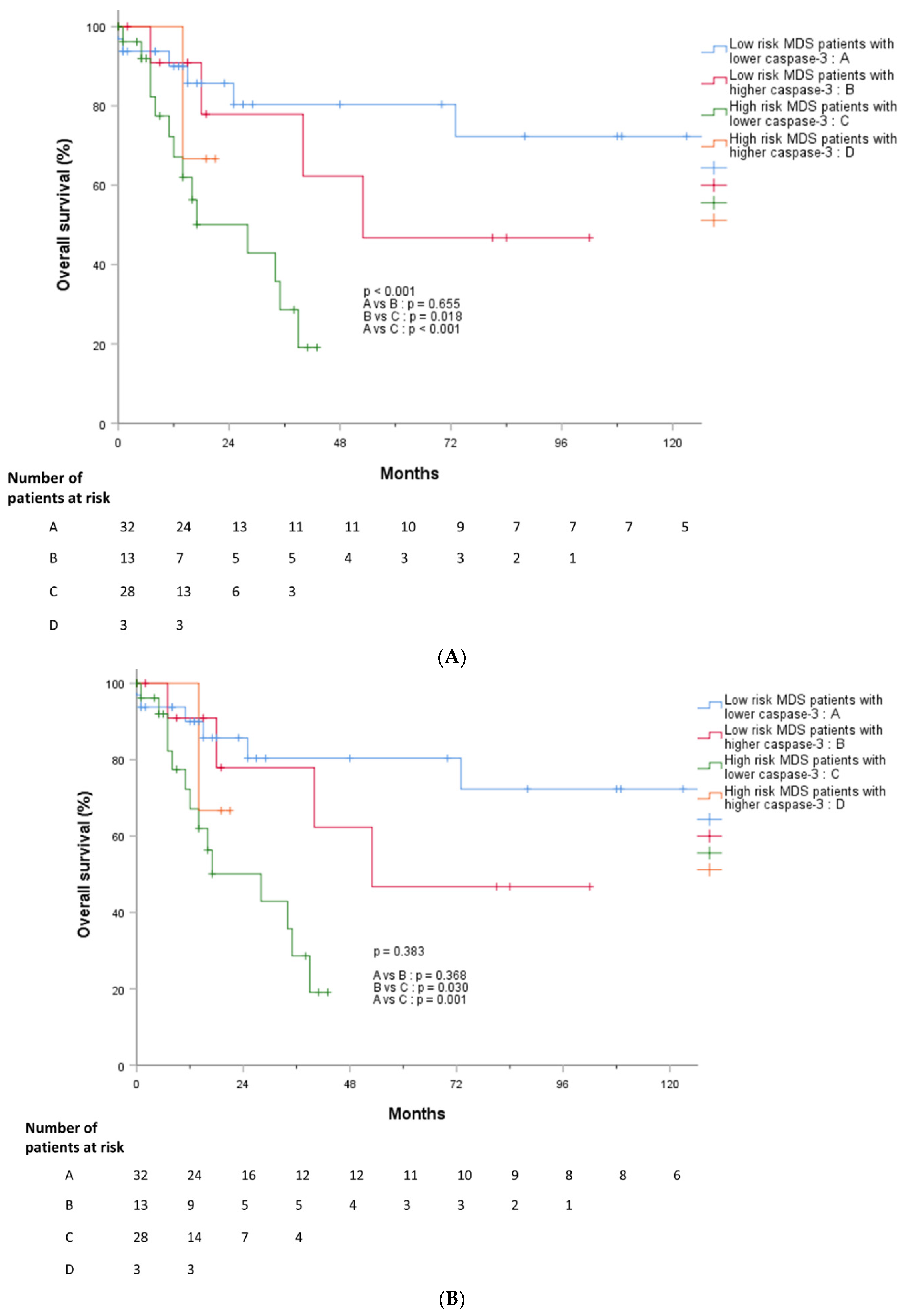
3.5. Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tefferi, A.; Vardiman, J.W. Myelodysplastic Syndromes. New Engl. J. Med. 2009, 361, 1872–1885. [Google Scholar] [CrossRef]
- Schanz, J.; Tüchler, H.; Solé, F.; Mallo, M.; Luño, E.; Cervera, J.; Granada, I.; Hildebrandt, B.; Slovak, M.L.; Ohyashiki, K.; et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J. Clin. Oncol. 2012, 30, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, B.B.; Kadam, N.N. Mutations of myelodysplastic syndromes (MDS): An update. Mutat. Res. Rev. Mutat. Res. 2016, 769, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Attar, E.; Bennett, J.M.; Bloomfield, C.D.; Borate, U.; De Castro, C.M.; Deeg, H.J.; Frankfurt, O.; Gaensler, K.; Garcia-Manero, G.; et al. Myelodysplastic syndromes: Clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2013, 11, 838–874. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.-Y.; Park, J.K.; Li, C.C.; Park, H.S.; Moon, S.Y.; Kim, S.-M.; Im, K.; Chang, Y.H.; Yoon, S.-S.; Lee, D.-S. Replicative senescence of hematopoietic cells in patients with idiopathic cytopenia of undetermined significance. Leuk. Res. 2019, 79, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Heppel, N.H.; Britt-Compton, B.; Grimstead, J.W.; Jones, R.E.; Tauro, S.; Bowen, D.T.; Knapper, S.; Groves, M.; Hills, R.K.; et al. Telomere length is an independent prognostic marker in MDS but not in de novo AML. Br. J. Haematol. 2017, 178, 240–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, P.L. Apoptosis and its role in the myelodysplastic syndromes: Implications for disease natural history and treatment. Leuk. Res. 1998, 22, 1123–1136. [Google Scholar] [CrossRef]
- Clark, D.M.; Lampert, I.A. Apoptosis is a Common Histopathological Finding in Myelodysplasia: The Correlate of Ineffective Haematopoiesis. Leuk. Lymphoma 1990, 2, 415–418. [Google Scholar] [CrossRef]
- Invernizzi, R.; Travaglino, E. Increased Apoptosis as a Mechanism of Ineffective Erythropoiesis in Myelodysplastic Syndromes. Clin. Leuk. 2008, 2, 113–120. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ezra, J.M.; Trinh, K.; Harris, A.C.; Kornstein, M.J. Ploidy Analysis and Ki-67 Expression in Myelodysplastic Syndromes. Hematology 1998, 3, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, H.D.; Bergmann, A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Peng, J.; Liu, W.; He, X.; Cui, L.; Chen, X.; Yang, M.; Liu, H.; Liu, S.; Wang, H. Elevated cleaved caspase-3 is associated with shortened overall survival in several cancer types. Int. J. Clin. Exp. Pathol. 2014, 7, 5057–5070. [Google Scholar]
- Wang, R.-A.; Li, Q.-L.; Li, Z.-S.; Zheng, P.-J.; Zhang, H.-Z.; Huang, X.-F.; Chi, S.-M.; Yang, A.-G.; Cui, R. Apoptosis drives cancer cells proliferate and metastasize. J. Cell Mol. Med. 2013, 17, 205–211. [Google Scholar] [CrossRef]
- Acar, V.; Couto Fernandez, F.L.; Buscariolo, F.F.; Novais, A.A.; Matheus Pereira, R.A.; de Campos Zuccari, D.A.P. Immunohistochemical Evaluation of PARP and Caspase-3 as Prognostic Markers in Prostate Carcinomas. Clin. Med. Res. 2021, 19, 183–191. [Google Scholar] [CrossRef]
- Huang, Q.; Li, F.; Liu, X.; Li, W.; Shi, W.; Liu, F.F.; O’Sullivan, B.; He, Z.; Peng, Y.; Tan, A.C.; et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011, 17, 860–866. [Google Scholar] [CrossRef]
- Donato, A.L.; Huang, Q.; Liu, X.; Li, F.; Zimmerman, M.A.; Li, C.Y. Caspase 3 promotes surviving melanoma tumor cell growth after cytotoxic therapy. J. Investig. Dermatol. 2014, 134, 1686–1692. [Google Scholar] [CrossRef] [Green Version]
- Mao, P.; Smith, L.; Xie, W.; Wang, M. Dying endothelial cells stimulate proliferation of malignant glioma cells via a caspase 3-mediated pathway. Oncol. Lett. 2013, 5, 1615–1620. [Google Scholar] [CrossRef] [Green Version]
- Warlick, E.D.; Hirsch, B.A.; Nguyen, P.L.; Cioc, A.; Roesler, M.A.; Fonstad, R.; Miller, J.S.; Weisdorf, D.J.; Ross, J.A. Comparison of IPSS and IPSS-R Scoring in a Population Based Myelodysplastic Syndromes (MDS) Study. Blood 2012, 120, 3841. [Google Scholar] [CrossRef]
- Neukirchen, J.; Lauseker, M.; Blum, S.; Giagounidis, A.; Lübbert, M.; Martino, S.; Siragusa, S.; Schlenk, R.F.; Platzbecker, U.; Hofmann, W.-K.; et al. Validation of the revised International Prognostic Scoring System (IPSS-R) in patients with myelodysplastic syndrome: A multicenter study. Leuk. Res. 2014, 38, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, S.; Alpermann, T.; Grossmann, V.; Kowarsch, A.; Nadarajah, N.; Eder, C.; Dicker, F.; Fasan, A.; Haferlach, C.; Haferlach, T.; et al. Landscape of TET2 mutations in acute myeloid leukemia. Leukemia 2012, 26, 934–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variables | Total (n = 76) | Low Cleaved Caspase-3 (n = 60, 78.9%) | High Cleaved Caspase-3 (n = 16, 21.1%) | p-Value |
|---|---|---|---|---|
| Age at diagnosis, median (range) | 70 (22–86) | 70 (25–86) | 72 (22–86) | 0.656 |
| Sex, n (%) | 0.037 | |||
| Male Female | 54 (71.1) 22 (28.9) | 46 (76.7) 14 (23.3) | 8 (50.0) 8 (50.0) | |
| BM, median (range) | ||||
| Cellularity, % Blast at diagnosis, % | 55 (5–95) 2.40 (0.0–19.1) | 55 (5–95) 2.40 (0.0–19.1) | 45 (15–95) 2.75 (0.0–19.0) | 0.223 0.463 |
| Cytopenia, n (%) | ||||
| Neutropenia (ANC < 1800/mm3) | 52 (68.4) | 40 (66.7) | 12 (75.0) | 0.524 |
| Anemia (Hb < 10 g/dL) | 59 (77.6) | 47 (78.3) | 12 (75.0) | 0.776 |
| Thrombocytopenia (Platelet <100,000/mm3) | 46 (60.5) | 40 (66.7) | 6 (37.5) | 0.034 |
| Lineage involvement of cytopenia, n (%) | 0.816 | |||
| Uni-lineage | 26 (34.2) | 21 (35.0) | 5 (31.3) | |
| Bi-lineage | 27 (35.5) | 22 (36.7) | 5 (31.3) | |
| Tri-lineage | 17 (22.4) | 12 (20.0) | 5 (31.3) | |
| Cytopenia grade, n (%) | 0.513 | |||
| 1 | 19 (25.0) | 15 (25.0) | 4 (25.0) | |
| 2 | 24 (31.6) | 17 (28.3) | 7 (43.8) | |
| 3 | 32 (39.5) | 26 (86.7) | 4 (25.0) | |
| IPSS risk, n (%) | 0.082 | |||
| Low | 10 (13.2) | 6 (10.0) | 4 (25.0) | |
| Intermediate-1 | 43 (56.6) | 34 (56.7) | 9 (56.3) | |
| Intermediate-2 | 17 (22.4) | 16 (26.7) | 1 (6.3) | |
| High | 5 (6.6) | 4 (6.7) | 1 (6.3) | |
| Revised IPSS risk, n (%) | 0.090 | |||
| Very low | 3 (3.9) | 2 (3.3) | 1 (6.3) | |
| Low | 22 (28.9) | 15 (25.0) | 7 (43.8) | |
| Intermediate | 20 (26.3) | 15 (25.0) | 5 (31.3) | |
| High | 18 (23.7) | 17 (28.3) | 1 (6.3) | |
| Very high | 12 (15.8) | 11 (18.3) | 1 (6.3) | |
| Karyotype, n (%) | 0.086 | |||
| Abnormal karyotype | 38 (50.0) | 18 (30.0) | 7 (43.8) | |
| Complex karyotype (>3 abnormalities) | 13 (17.1) | 13 (21.7) | 0 (0.0) | |
| Del(5q)/−5 | 7 (9.2) | 6 (10.0) | 1 (6.25) | 0.559 |
| Del(7q)/−7 | 12 (15.8) | 11 (18.3) | 1 (6.25) | 0.179 |
| Trisomy 8 | 12 (15.8) | 10 (16.7) | 2 (12.5) | 0.539 |
| Del(20q) | 6 (7.9) | 5 (8.3) | 1 (6.25) | 0.770 |
| Telomere length, mean kb (range) | 8.65 (3.2–21.47) | 8.35 (3.2–19.04) | 10.77 (5.74–21.47) | 0.169 |
| Ki-67, mean grade (range) | 1.5 (0.5–3.5) | 1.5 (0.5–3.5) | 1.75 (0.5–2.5) | 0.700 |
| Gene Mutation | Mean Age (Years) | p-Value | Mean Cleaved Caspase-3 (Grade) | p-Value | Mean Ki-67 (Grade) | p-Value | Mean Telomere Length (kb) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Any mutation | + (n = 60) | 71 (27–86) | 0.165 | 3.0 (1.0–3.5) | 0.294 | 1.5 (0.5–3.5) | 0.109 | 8.73 (3.33–21.47) | 0.914 |
| − (n = 16) | 68.5 (22–86) | 2.5 (1.5–3.5) | 1.75 (1.0–3.0) | 6.99 (3.20–17.79) | |||||
| ASXL1 | + (n = 17) | 72 (46–86) | 0.065 | 3.0 (1.0–3.5) | 0.680 | 2.0 (1.0–3.5) | 0.194 | 8.65 (3.33–2147) | 0.963 |
| − (n = 59) | 70 (22–86) | 3.0 (1.5–3.5) | 1.5 (0.5–3.0) | 8.68 (3.20–19.04) | |||||
| TP53 | + (n = 11) | 71 (61–84) | 0.101 | 3.0 (1.0–3.5) | 0.897 | 2.0 (1.0–3.0) | 0.398 | 10.65 (3.33–16.0) | 0.914 |
| − (n = 65) | 70 (22–86) | 3.0 (1.0–3.5) | 1.5 (0.5–3.5) | 8.61 (3.20–21.47) | |||||
| EZH2 | + (n = 5) | 72 (64–86) | 0.274 | 3.0 (1.0–3.5) | 0.730 | 1.0 (1.0–2.5) | 0.511 | 11.75 (8.35–21.47) | 0.081 |
| − (n = 71) | 70 (22–86) | 3.0(1.0–3.5) | 1.5 (0.5–3.5) | 8.62 (3.20–19.04) | |||||
| U2AF1 | + (n = 13) | 60 (27–84) | 0.057 | 3.0 (1.5–3.5) | 0.688 | 1.0 (0.5–3.0) | 0.259 | 8.80 (3.79–19.04) | 0.989 |
| − (n = 63) | 71 (22–86) | 3.0 (1.0–3.5) | 1.5 (0.5–3.5) | 8.35 (3.20–21.47) | |||||
| RUNX1 | + (n = 5) | 64 (33–78) | 0.223 | 2.5 (1.5–3.0) | 0.145 | 1.5 (0.5–3.0) | 0.717 | 8.91 (5.03–17.96) | 0.794 |
| − (n = 71) | 71 (22–86) | 3.0 (1.0–3.5) | 1.5 (0.5–3.5) | 8.65 (3.20–21.47) | |||||
| TET2 | + (n = 10) | 74 (67–86) | 0.030 | 2.25 (1.0–3.5) | 0.031 | 1.75 (0.5–3.0) | 0.751 | 11.0 (8.14–16.22) | 0.228 |
| − (n = 66) | 70 (22–86) | 3.0 (1.0–3.5) | 1.5 (0.5–3.5) | 8.35 (3.20–21.47) | |||||
| DNMT3A | + (n = 6) | 76 (56–86) | 0.186 | 3.0 (2.5–3.0) | 0.399 | 1.25 (0.5–2.0) | 0.122 | 8.14 (4.83–9.97) | 0.343 |
| − (n = 70) | 70 (22–86) | 2.75 (1.0–3.5) | 1.5 (0.5–3.5) | 8.67 (3.20–21.47) | |||||
| SRSF2 | + (n = 6) | 72 (65–86) | 0.186 | 3.0 (1.0–3.5) | 0.140 | 1.5 (0.5–2.0) | 0.204 | 9.08 (5.33–19.04) | 0.284 |
| − (n = 70) | 70 (22–86) | 2.5 (1.5–3.0) | 1.5 (0.5–3.5) | 8.58 (3.20–21.47) | |||||
| BCOR | + (n = 3) | 68 (47–83) | 0.982 | 3.0 (1.0–3.5) | 0.576 | 2.0 (0.5–3.0) | 0.806 | 8.78 (2.19–17.96) | 0.685 |
| − (n = 73) | 70 (22–86) | 2.5 (2.0–3.0) | 1.5 (0.5–3.5) | 8.62 (3.20–21.47) | |||||
| SF3B1 | + (n = 5) | 70 (46–73) | 0.739 | 2.5 (1.5–3.5) | 0.464 | 2.5 (1.0–3.5) | 0.159 | 9.97 (5.84–14.16) | 0.909 |
| − (n = 71) | 71 (22–86) | 3.0 (1.0–3.5) | 1.25 (0.5–3.5) | 8.62 (3.20–21.47) | |||||
| STAG2 | + (n = 4) | 68 (27–75) | 0.395 | 2.75 (2.0–3.5) | 0.883 | 1.75 (1.5–2.0) | 0.945 | 8.69 (5.59–18.00) | 0.651 |
| − (n = 72) | 70 (22–86) | 3.0 (1.0–3.5) | 1.5 (0.5–3.5) | 8.62 (3.20–21.47) | |||||
| WT1 | + (n = 4) | 60 (43–67) | 0.262 | 2.5 (1.5–3.5) | 0.515 | 1.5 (1.0–2.5) | 0.797 | 7.81 (3.79–8.69) | 0.206 |
| − (n = 72) | 71 (22–86) | 3.0 (1.0–3.5) | 1.5 (0.5–3.5) | 8.77 (3.20–21.47) | |||||
| Chromosomal abnormality | Mean age (years) | p-value | Mean cleaved caspase-3 (grade) | p-value | Mean Ki-67 (grade) | p-value | Mean telomere length (kb) | p-value | |
| IPSS | |||||||||
| Good | n = 44 (57.9%) | 70.5 (22–86) | 0.240 | 2.5 (1.0–3.5) | 0.919 | 1.5 (0.5–3.5) | 0.541 | 8.67 (3.20–21.47) | 0.960 |
| Intermediate | n = 16 (21.1%) | 70 (27–84) | 3.0 (1.5–3.5) | 1.75 (0.5–3.0) | 7.64 (3.79–17.96) | ||||
| Poor | n = 15 (19.7%) | 71 (55–83) | 3.0 (1.0–3.5) | 2.0 (0.5–3.0) | 9.03 (3.33–16.0) | ||||
| IPSS-R | |||||||||
| Very good | n = 3 (3.9%) | 61 (56–75) | 0.564 | 3.5 (3.0–3.5) | 0.404 | 2.0 (1.5–3.5) | 0.508 | 9.41 (6.78–12.05) | 0.680 |
| Good | n = 43 (56.6%) | 71 (22–86) | 2.5 (1.0–3.5) | 1.5 (0.5–3.5) | 8.69 (3.20–21.47) | ||||
| Intermediate | n = 15 (19.7%) | 70 (27–84) | 3.0 (1.5–3.5) | 1.5 (0.5–3.0) | 7.64 (3.79–17.96) | ||||
| Poor | n = 13 (17.1%) | 71 (55–83) | 3.0 (1.0–3.5) | 2.0 (0.5–3.0) | 8.47 (3.33–16.00) | ||||
| Very poor | n = 1 (1.3%) | 63 | 3.0 | 1.5 | 15.18 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Shin, D.-Y.; Park, J.S.; Park, H.S.; Moon, S.Y.; Yoon, S.-S.; Lee, D.-S. Increased Apoptotic Activity in Low-Risk Myelodysplastic Syndrome. J. Clin. Med. 2022, 11, 4604. https://doi.org/10.3390/jcm11154604
Park S, Shin D-Y, Park JS, Park HS, Moon SY, Yoon S-S, Lee D-S. Increased Apoptotic Activity in Low-Risk Myelodysplastic Syndrome. Journal of Clinical Medicine. 2022; 11(15):4604. https://doi.org/10.3390/jcm11154604
Chicago/Turabian StylePark, Songyi, Dong-Yeop Shin, Junseo Steve Park, Hee Sue Park, Soo Young Moon, Sung-Soo Yoon, and Dong-Soon Lee. 2022. "Increased Apoptotic Activity in Low-Risk Myelodysplastic Syndrome" Journal of Clinical Medicine 11, no. 15: 4604. https://doi.org/10.3390/jcm11154604
APA StylePark, S., Shin, D.-Y., Park, J. S., Park, H. S., Moon, S. Y., Yoon, S.-S., & Lee, D.-S. (2022). Increased Apoptotic Activity in Low-Risk Myelodysplastic Syndrome. Journal of Clinical Medicine, 11(15), 4604. https://doi.org/10.3390/jcm11154604






