Prognostic Impact of the HFA-PEFF Score in Patients with Acute Myocardial Infarction and an Intermediate to High HFA-PEFF Score
Abstract
:1. Introduction
2. Materials and Methods
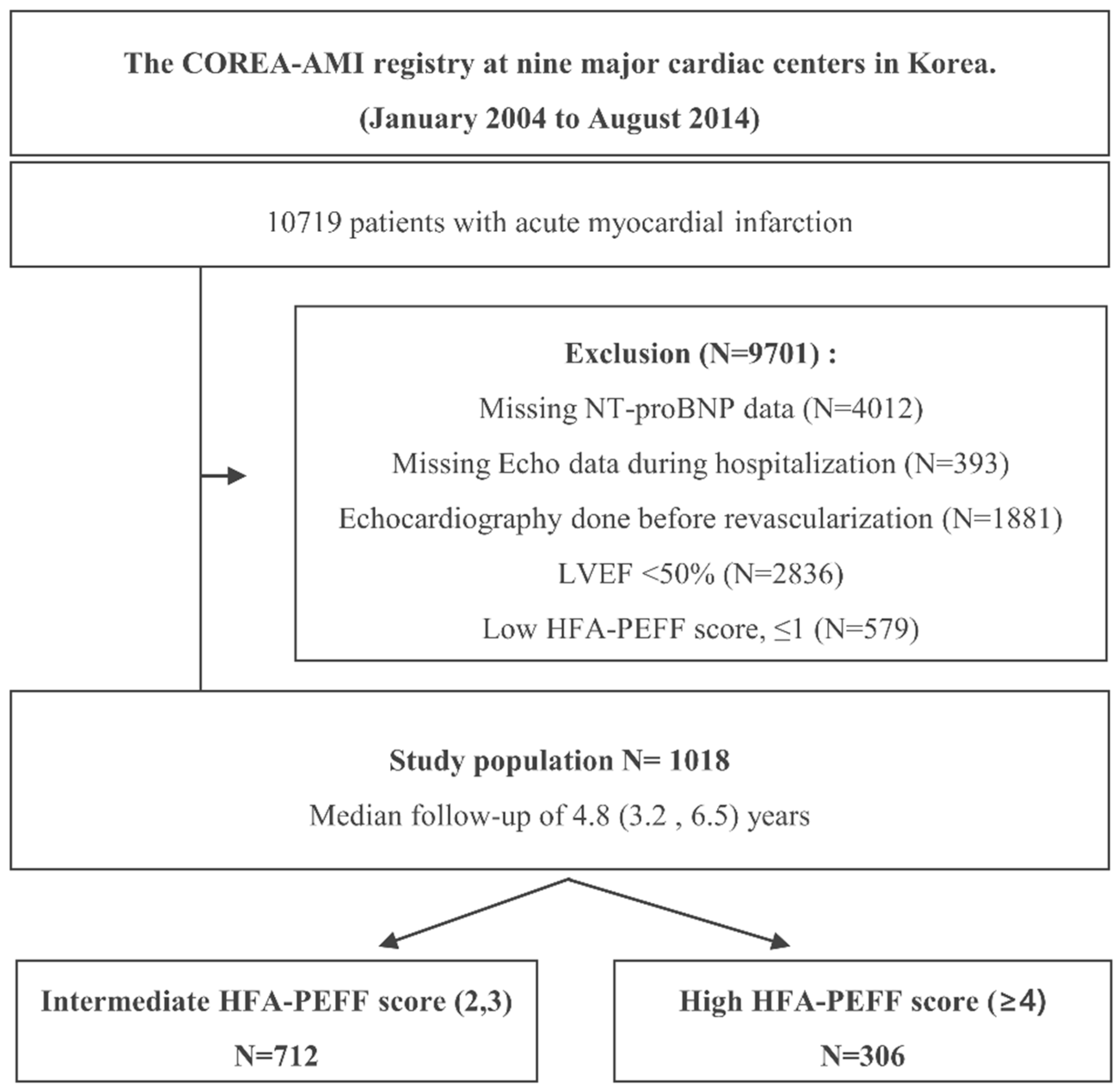
2.1. Study Protocols and Population Selection
2.2. PCI Procedure and Medical Treatment
2.3. Study Endpoints and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
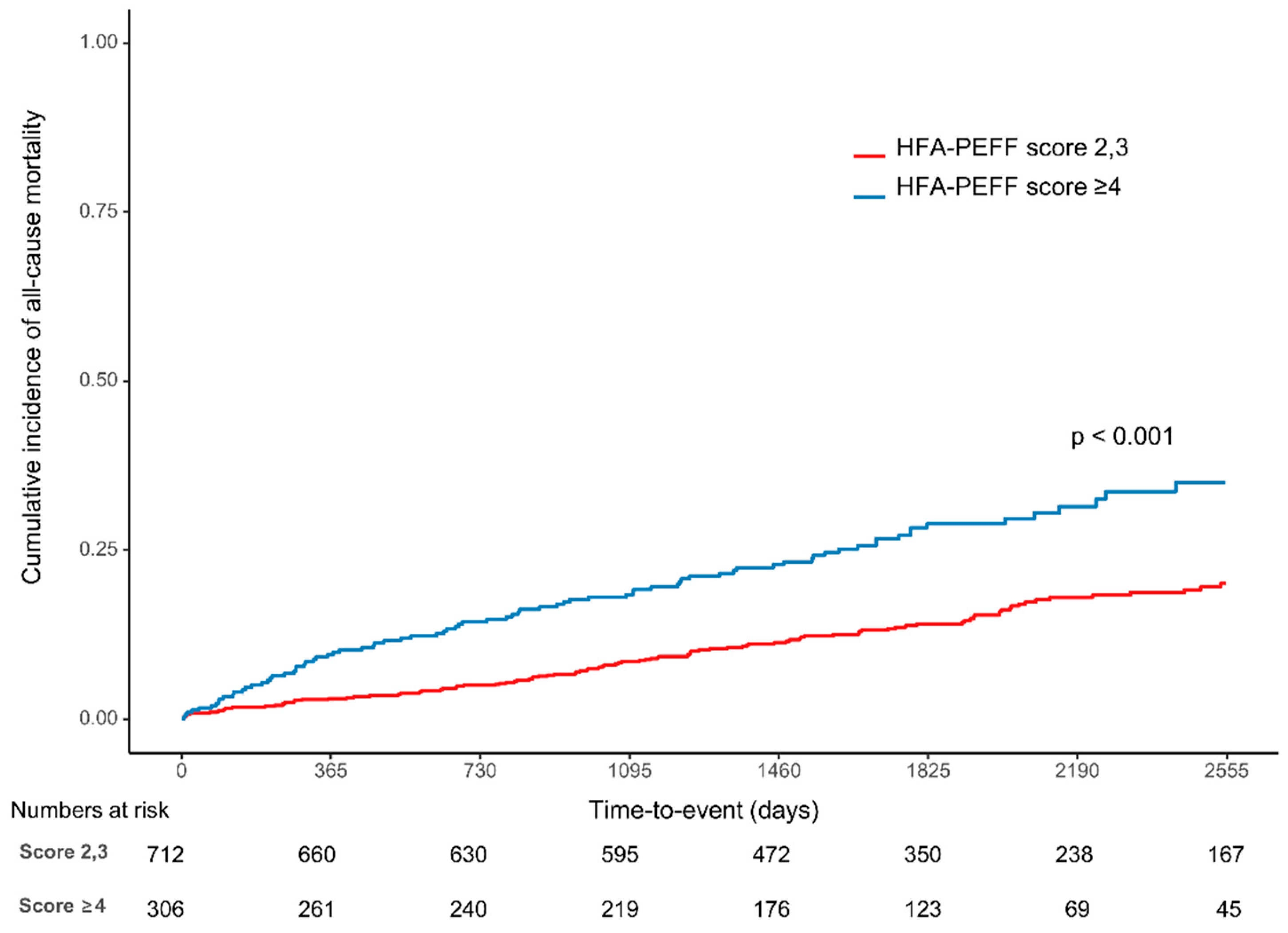
3.2. Clinical Outcomes
4. Discussion
4.1. Pathophysiology and Comorbidities of HFpEF
4.2. Predictive Value of the HFA-PEFF Score in Patients with an HFA-PEFF Score ≥ 2
4.3. Components of the HFA-PEFF Score and Its Meaning
4.4. Appropriateness of the HFA-PEFF Score in the AMI Population
4.5. Treatment of HFpEF
4.6. Future Perspectives
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Yoo, B.S. Current Prevalence, Incidence, and Outcomes of Heart Failure with Preserved Ejection Fraction. Heart Fail Clin. 2021, 17, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Oktay, A.A.; Rich, J.D.; Shah, S.J. The emerging epidemic of heart failure with preserved ejection fraction. Curr. Heart Fail Rep. 2013, 10, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, C.S.P.; Gamble, G.D.; Ling, L.H.; Sim, D.; Leong, K.T.G.; Yeo, P.S.D.; Ong, H.Y.; Jaufeerally, F.; Ng, T.P.; Cameron, V.A.; et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi-ethnic cohort study. Eur. Heart J. 2018, 39, 1770–1780. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [Green Version]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef] [Green Version]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.H.; Choe, W.S.; Cho, H.J.; Lee, H.Y.; Jang, J.; Lee, S.E.; Choi, J.O.; Jeon, E.S.; Kim, M.S.; Hwang, K.K.; et al. Comparison of Characteristics and 3-Year Outcomes in Patients With Acute Heart Failure With Preserved, Mid-Range, and Reduced Ejection Fraction. Circ. J. 2019, 83, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, J.W.; Vaduganathan, M.; Claggett, B.L.; John, J.E.; Desai, A.S.; Lewis, E.F.; Zile, M.R.; Carson, P.; Jhund, P.S.; Kober, L.; et al. Myocardial Infarction in Heart Failure With Preserved Ejection Fraction: Pooled Analysis of 3 Clinical Trials. JACC Heart Fail 2020, 8, 618–626. [Google Scholar] [CrossRef]
- Tam, M.C.; Lee, R.; Cascino, T.M.; Konerman, M.C.; Hummel, S.L. Current Perspectives on Systemic Hypertension in Heart Failure with Preserved Ejection Fraction. Curr. Hypertens. Rep. 2017, 19, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Heerebeek, L.; Paulus, W.J. Understanding heart failure with preserved ejection fraction: Where are we today? Neth. Heart J. 2016, 24, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Salah, K.; Stienen, S.; Pinto, Y.M.; Eurlings, L.W.; Metra, M.; Bayes-Genis, A.; Verdiani, V.; Tijssen, J.G.P.; Kok, W.E. Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 2019, 105, 1182–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myhre, P.L.; Vaduganathan, M.; O’Meara, E.; Claggett, B.L.; de Denus, S.; Jarolim, P.; Anand, I.S.; Pitt, B.; Rouleau, J.L.; Solomon, S.D.; et al. Mechanistic Effects of Spironolactone on Cardiovascular and Renal Biomarkers in Heart Failure With Preserved Ejection Fraction: A TOPCAT Biorepository Study. Circ. Heart Fail. 2020, 13, e006638. [Google Scholar] [CrossRef]
- Arcari, L.; Luciani, M.; Cacciotti, L.; Musumeci, M.B.; Spuntarelli, V.; Pistella, E.; Martolini, D.; Manzo, D.; Pucci, M.; Marone, C.; et al. Incidence and determinants of high-sensitivity troponin and natriuretic peptides elevation at admission in hospitalized COVID-19 pneumonia patients. Intern. Emerg. Med. 2020, 15, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Arcari, L.; Ciavarella, G.M.; Altieri, S.; Limite, L.R.; Russo, D.; Luciani, M.; De Biase, L.; Mené, P.; Volpe, M. Longitudinal changes of left and right cardiac structure and function in patients with end-stage renal disease on replacement therapy. Eur. J. Intern. Med. 2020, 78, 95–100. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Borlaug, B.A. Diastolic Dysfunction and Heart Failure With Preserved Ejection Fraction: Understanding Mechanisms by Using Noninvasive Methods. JACC Cardiovasc. Imaging 2020, 13, 245–257. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Caravita, S.; Paolillo, S.; Ghio, S.; Tocchetti, C.G.; Ruocco, G.; Correale, M.; Ambrosio, G.; Perrone Filardi, P.; Senni, M. Current gaps in HFpEF trials: Time to reconsider patients’ selection and to target phenotypes. Prog. Cardiovasc. Dis. 2021, 67, 89–97. [Google Scholar] [CrossRef]
- Wachter, R.; Shah, S.J.; Cowie, M.R.; Szecsödy, P.; Shi, V.; Ibram, G.; Zhao, Z.; Gong, J.; Klebs, S.; Pieske, B. Angiotensin receptor neprilysin inhibition versus individualized RAAS blockade: Design and rationale of the PARALLAX trial. ESC Heart Fail. 2020, 7, 856–864. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.V.; Jackson, A.M.; Lam, C.S.P.; Redfield, M.M.; Anand, I.S.; Ge, J.; Lefkowitz, M.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; et al. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared With Men With Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF. Circulation 2020, 141, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martinez, S.; Duchateau, N.; Erdei, T.; Kunszt, G.; Aakhus, S.; Degiovanni, A.; Marino, P.; Carluccio, E.; Piella, G.; Fraser, A.G.; et al. Machine Learning Analysis of Left Ventricular Function to Characterize Heart Failure With Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2018, 11, e007138. [Google Scholar] [CrossRef] [Green Version]
- Omar, A.M.S.; Narula, S.; Abdel Rahman, M.A.; Pedrizzetti, G.; Raslan, H.; Rifaie, O.; Narula, J.; Sengupta, P.P. Precision Phenotyping in Heart Failure and Pattern Clustering of Ultrasound Data for the Assessment of Diastolic Dysfunction. JACC Cardiovasc. Imaging 2017, 10, 1291–1303. [Google Scholar] [CrossRef]
- Shah, S.J.; Katz, D.H.; Selvaraj, S.; Burke, M.A.; Yancy, C.W.; Gheorghiade, M.; Bonow, R.O.; Huang, C.C.; Deo, R.C. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015, 131, 269–279. [Google Scholar] [CrossRef] [Green Version]

| Total | Intermediate HFA-PEFF Score (N = 712) | High HFA-PEFF Score (N = 306) | p-Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, yr | 65.0 ± 12.3 | 63.0 ± 12.3 | 69.7 ± 11.0 | <0.001 |
| Female | 336 (33.0) | 197 (27.7) | 139 (45.4) | <0.001 |
| DM | 322 (31.6) | 209 (29.4) | 113 (36.9) | 0.021 |
| Hypertension | 604 (59.3) | 389 (54.6) | 215 (70.3) | <0.001 |
| Dyslipidemia | 167 (16.4) | 117 (16.4) | 50 (16.3) | 1 |
| History of stroke | 72 (7.1) | 43 (6.0) | 29 (9.5) | 0.067 |
| Previous MI | 27 (2.7) | 19 (2.7) | 8 (2.6) | 1 |
| Previous PCI | 60 (5.9) | 36 (5.1) | 24 (7.8) | 0.113 |
| Previous CABG | 5 (0.5) | 2 (0.3) | 3 (1.0) | 0.163 |
| Atrial fibrillation on baseline ECG | 16 (1.6) | 10 (1.4) | 6 (2.0) | 0.584 |
| Cancer | 52 (5.1) | 34 (4.8) | 18 (5.9) | 0.562 |
| Chronic liver disease | 11 (1.1) | 6 (0.8) | 5 (1.6) | 0.321 |
| Chronic lung disease | 20 (2.0) | 14 (2.0) | 6 (2.0) | 1 |
| Chronic kidney disease | 21 (2.1) | 10 (1.4) | 11 (3.6) | 0.044 |
| KILLIP III or IV | 112 (11.0) | 70 (9.8) | 42 (13.7) | 0.087 |
| Cardiogenic shock | 37 (3.6) | 24 (3.4) | 13 (4.2) | 0.618 |
| 2nd drug-eluting stents | 801 (78.7) | 552 (77.5) | 249 (81.4) | 0.197 |
| SBP | 130.0 (110.0, 145.0) | 130.0 (110.0, 145.0) | 130.0 (110.0, 145.3) | 0.636 |
| DBP | 80.0 (70.0, 90.0) | 80.0 (70.0, 90.0) | 76.0 (63.8, 86.0) | 0.011 |
| HR | 76.0 (65.0, 88.0) | 76.0 (65.0, 87.0) | 78.0 (65.0, 88.0) | 0.400 |
| Laboratory findings | ||||
| NT-proBNP, ng/mL | 499.0 (226.7, 1434.0) | 380.0 (172.2, 978.0) | 1081.0 (409.0, 2703.0) | <0.001 |
| NT-proBNP in AF, ng/mL | 2048.0 (677.2, 5547.5) | 1504.3 (414.6, 2950.0) | 3219.0 (1249.0, 10734.0) | 0.193 |
| NT-proBNP in sinus rhythm, ng/mL | 481.0 (225.0, 1424.0) | 372.4 (170.8, 970.0) | 1063.5 (407.4, 2674.0) | <0.001 |
| Elevated troponin | 322 (31.6) | 223 (31.3) | 99 (32.4) | 0.802 |
| CK-MB, peak, ng/mL | 73.5 (20.3, 176.7) | 78.8 (23.9, 177.6) | 58.5 (14.0, 167.4) | 0.040 |
| Hemoglobin, mg/dL | 13.9 (12.5, 15.2) | 14.3 (13.0, 15.4) | 13.0 (11.4, 14.3) | <0.001 |
| Platelet, mg/dL | 226.0 (186.0, 268.0) | 229.5 (189.0, 267.0) | 218.5 (184.0, 271.0) | 0.201 |
| Creatinine, mg/dL | 1.0 (0.8, 1.2) | 0.9 (0.8, 1.1) | 1.0 (0.8, 1.3) | <0.001 |
| HbA1c, mg/dL | 6.1 (5.6, 7.1) | 6.0 (5.6, 6.9) | 6.3 (5.7, 7.3) | 0.078 |
| high-sensitivity CRP, mg/dL | 0.5 (0.1, 2.0) | 0.4 (0.1, 1.5) | 0.8 (0.2, 3.8) | <0.001 |
| Total cholesterol, mg/dL | 171.0 (143.0, 201.5) | 175.0 (147.0, 206.0) | 161.0 (134.3, 191.0) | <0.001 |
| Triglyceride, mg/dL | 96.0 (62.0, 138.0) | 96.0 (63.0, 143.0) | 96.0 (61.0, 133.0) | 0.239 |
| High-density lipoprotein, mg/dL | 39.0 (33.0, 46.0) | 39.7 (34.0, 47.0) | 37.0 (31.0, 45.0) | <0.001 |
| Low-density lipoprotein, mg/dL | 107.0 (84.0, 133.0) | 108.6 (87.0, 136.0) | 97.5 (75.5, 128.0) | <0.001 |
| Medication at discharge | ||||
| Antiplatelet agent | 1009 (99.1) | 708 (99.4) | 301 (98.4) | 0.138 |
| Potent P2Y12 inhibitor | 160 (15.7) | 118 (16.6) | 42 (13.7) | 0.149 |
| Beta-blocker | 873 (87.4) | 610 (87.5) | 263 (87.1) | 0.322 |
| ACEi or ARB | 504 (49.5) | 346 (48.6) | 158 (51.6) | 0.412 |
| Aldosterone antagonist | 42 (4.6) | 24 (3.7) | 18 (6.7) | 0.07 |
| Other Diuretics | 193 (21.1) | 119 (18.4) | 74 (27.6) | 0.002 |
| Oral anticoagulant | 12 (1.2) | 5 (0.7) | 7 (2.3) | 0.051 |
| Statin | 929 (95.6) | 653 (95.7) | 276 (95.2) | 0.328 |
| DAPT duration, month | 21.3 (13.2) | 21.7 (13.1) | 20.3 (13.6) | 0.112 |
| Total | Intermediate HFA-PEFF Score (N = 712) | High HFA-PEFF Score (N = 306) | p-Value | |
|---|---|---|---|---|
| Echocardiographic parameters | ||||
| LVEF (%) | 58.0 (54.0, 62.8) | 58.0 (54.0, 63.3) | 57.0 (53.0, 61.0) | <0.001 |
| Left atrial volume index (ml/m2) | 29.9 (22.5, 39.0) | 25.0 (18.7, 31.9) | 37.4 (30.5, 45.3) | <0.001 |
| Left ventricular end-systolic diameter (mm) | 31.6 (27.2, 35.5) | 31.0 (27.0, 35.0) | 32.5 (28.3, 36.5) | 0.002 |
| Left ventricular end-diastolic diameter (mm) | 48.0 (44.0, 52.0) | 47.9 (44.0, 51.4) | 48.4 (43.6, 53.0) | 0.064 |
| Left ventricular end-systolic volume (mL) | 32.2 (25.0, 40.3) | 32.0 (25.0, 40.0) | 33.2 (26.0, 41.1) | 0.140 |
| Left ventricular end-diastolic volume (mL) | 77.0 (63.3, 94.6) | 76.0 (62.4, 94.0) | 77.5 (64.4, 96.0) | 0.422 |
| E/e’ | 12.3 (9.8, 16.0) | 11.2 (9.1, 13.4) | 17.0 (15.0, 20.4) | <0.001 |
| Estimated PASP (mmHg) | 28.0 (24.5, 36.0) | 27.0 (24.0, 34.0) | 31.5 (26.0, 41.0) | <0.001 |
| Angiographic characteristics | ||||
| 3VD | 246 (24.2) | 147 (20.6) | 99 (32.4) | <0.001 |
| Left main | 59 (5.8) | 42 (5.9) | 17 (5.6) | 0.945 |
| Left anterior descending | 738 (72.5) | 507 (71.2) | 231 (75.5) | 0.185 |
| Left circumflex | 500 (49.1) | 325 (45.6) | 175 (57.2) | 0.001 |
| Right coronary artery | 604 (59.3) | 403 (56.6) | 201 (65.7) | 0.008 |
| Total stent number | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 0.210 |
| Total stent length | 28.0 (20.0, 38.0) | 28.0 (20.0, 38.0) | 28.0 (20.0, 38.8) | 0.644 |
| Bifurcation PCI | 25 (2.5) | 18 (2.5) | 7 (2.3) | 0.995 |
| Long stenting >60 mm | 160 (15.7) | 105 (14.7) | 55 (18.0) | 0.229 |
| CTO | 40 (3.9) | 33 (4.6) | 7 (2.3) | 0.111 |
| Restenosis lesion | 16 (1.6) | 11 (1.5) | 5 (1.6) | 1 |
| Ostial lesion | 36 (3.5) | 25 (3.5) | 11 (3.6) | 1 |
| Unadjusted | Multivariable-Adjusted | ||||||
|---|---|---|---|---|---|---|---|
| Total (N = 1018) | No Event (N = 817) | Event (N = 201) | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Echocardiographic scores and parameters | |||||||
| High HFA-PEFF score group (≥4 points) | 306 (30.1) | 219 (26.8) | 87 (43.3) | 2.12 (1.6, 2.8) | <0.001 | 1.53 (1.15, 2.04) | 0.004 |
| High biomarker score: 2 | 774 (76.0) | 598 (73.2) | 176 (87.6) | 2.39 (1.57, 3.63) | <0.001 | 1.98 (1.3, 3.03) | 0.002 |
| High functional score: 2 | 327 (32.1) | 242 (29.6) | 85 (42.3) | 1.64 (1.24, 2.18) | <0.001 | 1.15 (0.86, 1.53) | 0.356 |
| High morphological score: 2 | 130 (37.6) | 111 (38.3) | 19 (34.5) | 0.91 (0.52, 1.59) | 0.737 | 1.01 (0.56, 1.83) | 0.963 |
| Conventional clinical risk factors | |||||||
| Age ≥ 75 | 245 (24.1) | 144 (17.6) | 101 (50.2) | 4.33 (3.27, 5.73) | <0.001 | 4.33 (3.23, 5.8) | <0.001 |
| Chronic kidney disease | 21 (2.1) | 10 (1.2) | 11 (5.5) | 4.73 (2.57, 8.71) | <0.001 | 3.97 (2.11, 7.5) | <0.001 |
| Atrial fibrillation | 16 (1.6) | 8 (1.0) | 8 (4.0) | 2.98 (1.47, 6.05) | 0.003 | 2.12 (1.04, 4.33) | 0.039 |
| Diabetes | 322 (31.6) | 234 (28.6) | 88 (43.8) | 1.9 (1.43, 2.51) | <0.001 | 1.81 (1.35, 2.42) | <0.001 |
| Hypertension | 604 (59.3) | 458 (56.1) | 146 (72.6) | 1.97 (1.44, 2.69) | <0.001 | 1.4 (1.01, 1.92) | 0.042 |
| Female | 336 (33.0) | 258 (31.6) | 78 (38.8) | 1.35 (1.02, 1.8) | 0.038 | 0.92 (0.69, 1.24) | 0.591 |
| Intermediate HFA-PEFF Score (N = 712) | High HFA-PEFF Score (N = 306) | p-Value | Unadjusted | Multivariable-Adjusted | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||||
| All-cause death | 114 (16.0) | 87 (28.4) | <0.001 | 2.12 (1.6, 2.8) | <0.001 | 1.53 (1.15, 2.04) | 0.004 |
| Cardiovascular death | 86 (12.1) | 66 (21.6) | <0.001 | 2.15 (1.55, 2.96) | <0.001 | 1.54 (1.11, 2.15) | 0.01 |
| Non-cardiovascular death | 28 (3.9) | 21 (6.9) | 0.065 | 2.03 (1.15, 3.58) | 0.014 | 1.48 (0.82, 2.65) | 0.19 |
| Readmission due to heart failure | 11 (1.5) | 20 (6.5) | <0.001 | 4.87 (2.33, 10.21) | <0.001 | 3.63 (1.69, 7.82) | <0.001 |
| Readmission due to unstable angina | 64 (9.0) | 23 (7.5) | 0.517 | 0.92 (0.57, 1.48) | 0.717 | 0.77 (0.47, 1.26) | 0.299 |
| MI | 20 (2.8) | 15 (4.9) | 0.135 | 1.04 (0.54, 1.97) | 0.914 | 0.88 (0.45, 1.71) | 0.705 |
| Definite or probable ST | 39 (5.5) | 13 (4.2) | 0.508 | 1.69 (0.55, 5.22) | 0.358 | 1.34 (0.42, 4.24) | 0.62 |
| Revascularization | 92 (12.9) | 38 (12.4) | 0.906 | 1.1 (0.75, 1.61) | 0.622 | 1.06 (0.72, 1.57) | 0.759 |
| Ischemic stroke | 20 (2.8) | 15 (4.9) | 0.135 | 2.02 (1.03, 3.96) | 0.041 | 1.59 (0.8, 3.2) | 0.189 |
| BARC 3, or 5 bleeding | 53 (7.4) | 34 (11.1) | 0.072 | 1.59 (1.03, 2.44) | 0.036 | 1.18 (0.76, 1.85) | 0.466 |
| Model | C-Index | 95% CI | p-Value | NRI | 95% CI | p-Value | IDI | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| For predicting mortality | ||||||||||
| Model A | Old age, female, HTN, DM, AF, CKD | 0.742 | 0.702–0.781 | |||||||
| Model B | Old age, female, HTN, DM, AF, CKD, High HFA-PEFF score (≥4) | 0.750 | 0.712–0.789 | 0.049 | 0.330 | 0.180–0.479 | <0.001 | 0.004 | −0.002–0.010 | 0.161 |
| For predicting mortality and readmission due to heart failure | ||||||||||
| Model A | Old age, female, HTN, DM, AF, CKD | 0.740 | 0.701–0.779 | |||||||
| Model B | Old age, female, HTN, DM, AF, CKD, High HFA-PEFF score (≥4) | 0.754 | 0.716–0.791 | 0.033 | 0.372 | 0.227–0.518 | <0.001 | 0.007 | 0–0.014 | 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.Y.; Hwang, B.-H.; Kim, C.J.; Sa, Y.K.; Choi, Y.; Kim, J.-J.; Choo, E.-H.; Lim, S.; Choi, I.J.; Park, M.-W.; et al. Prognostic Impact of the HFA-PEFF Score in Patients with Acute Myocardial Infarction and an Intermediate to High HFA-PEFF Score. J. Clin. Med. 2022, 11, 4589. https://doi.org/10.3390/jcm11154589
Lee KY, Hwang B-H, Kim CJ, Sa YK, Choi Y, Kim J-J, Choo E-H, Lim S, Choi IJ, Park M-W, et al. Prognostic Impact of the HFA-PEFF Score in Patients with Acute Myocardial Infarction and an Intermediate to High HFA-PEFF Score. Journal of Clinical Medicine. 2022; 11(15):4589. https://doi.org/10.3390/jcm11154589
Chicago/Turabian StyleLee, Kwan Yong, Byung-Hee Hwang, Chan Jun Kim, Young Kyoung Sa, Young Choi, Jin-Jin Kim, Eun-Ho Choo, Sungmin Lim, Ik Jun Choi, Mahn-Won Park, and et al. 2022. "Prognostic Impact of the HFA-PEFF Score in Patients with Acute Myocardial Infarction and an Intermediate to High HFA-PEFF Score" Journal of Clinical Medicine 11, no. 15: 4589. https://doi.org/10.3390/jcm11154589
APA StyleLee, K. Y., Hwang, B.-H., Kim, C. J., Sa, Y. K., Choi, Y., Kim, J.-J., Choo, E.-H., Lim, S., Choi, I. J., Park, M.-W., Oh, G. C., Yang, I.-H., Yoo, K. D., Chung, W. S., & Chang, K. (2022). Prognostic Impact of the HFA-PEFF Score in Patients with Acute Myocardial Infarction and an Intermediate to High HFA-PEFF Score. Journal of Clinical Medicine, 11(15), 4589. https://doi.org/10.3390/jcm11154589






