Is Fetal Hydrops in Turner Syndrome a Risk Factor for the Development of Maternal Mirror Syndrome?
Abstract
:1. Introduction
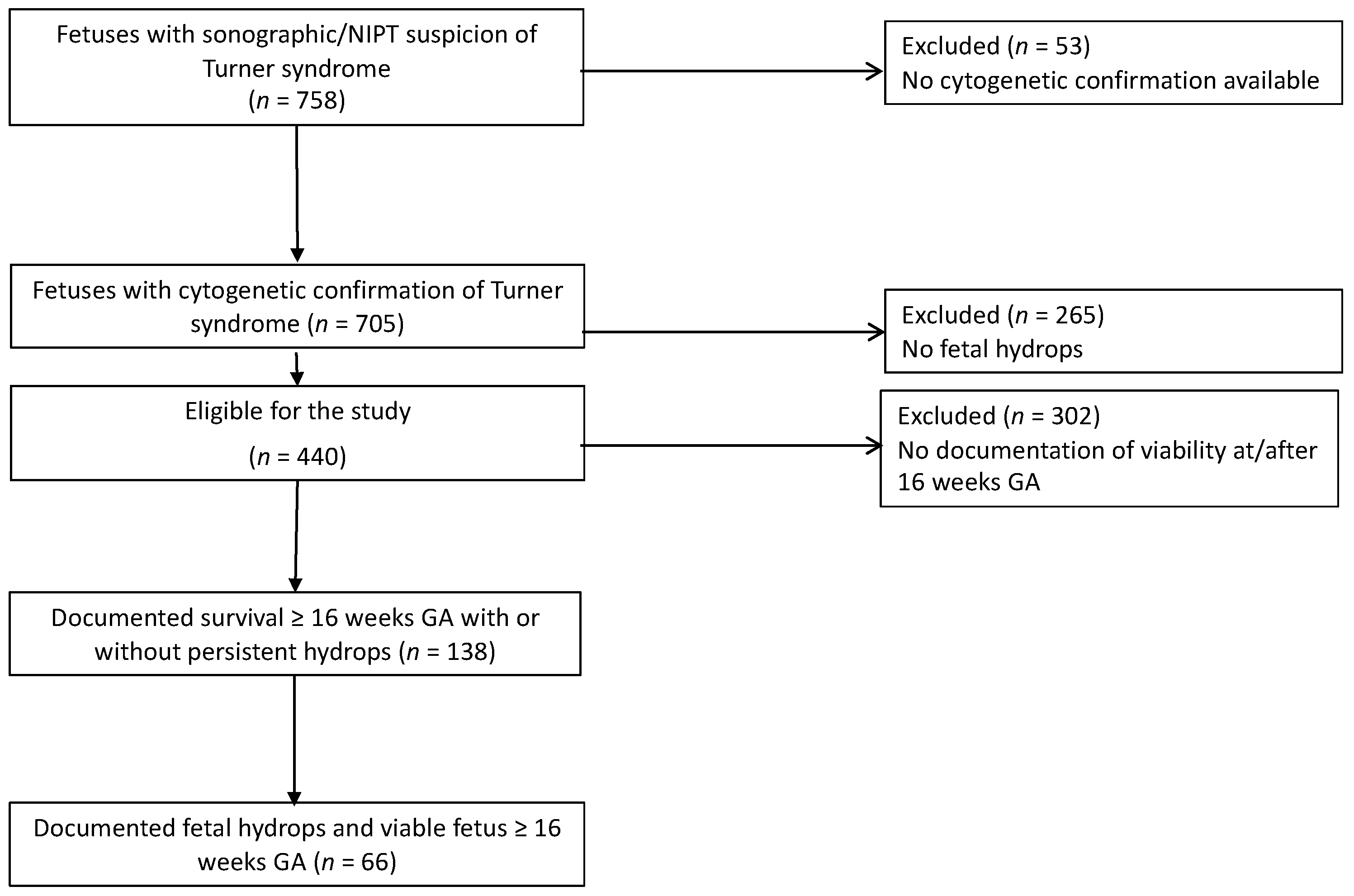
2. Materials and Methods
2.1. Inclusion Criteria
- Fetal hydrops, defined as an abnormal fluid collection in two or more body compartments (ascites, pleural effusions, pericardial effusion, skin edema) at the time of diagnosis/first presentation. In the first trimester, generalized skin edema with or without cystic hygroma was also considered fetal hydrops
- Karyotype resulting in Turner syndrome (45,X or cytogenetic variants)
2.2. Exclusion Criteria
- No cytogenetic confirmation of Turner syndrome by karyotype on chorionic villous sampling or genetic amniocentesis.
- Intrauterine demise before 16 weeks or, no documented cardiac activity at or after 16 weeks.
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaiser, I.H. Ballantyne and triple edema. Am. J. Obstet. Gynecol. 1971, 110, 115–120. [Google Scholar] [CrossRef]
- Espinoza, J.; Romero, R.; Nien, J.K.; Kusanovic, J.P.; Richani, K.; Gomez, R.; Kim, C.J.; Mittal, P.; Gotsh, F.; Erez, O.; et al. A role of the anti-angiogenic factor sVEGFR-1 in the “mirror syndrome” (Ballantyne’s syndrome). J. Matern.-Fetal Neonatal Med. 2006, 19, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Perez, S.F.; Corona-Fernandez, K.; Rodriguez-Chavez, J.L.; Bañuelos-Franco, A.; Zavala-Cerna, M.G. Significant Clinical Manifestations in Ballantyne Syndrome, after a Case Report and Literature Review: Recognizing Preeclampsia as a Differential Diagnosis. Case Rep. Obstet. Gynecol. 2019, 2019, 2013506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valsky, D.V.; Daum, H.; Yagel, S. Reversal of mirror syndrome after prenatal treatment of Diamond-Blackfan anemia. Prenat. Diagn. 2007, 27, 1161–1164. [Google Scholar] [CrossRef]
- Mathias, C.R.; Rizvi, C. The diagnostic conundrum of maternal mirror syndrome progressing to pre-eclampsia—A case report. Case Rep. Women’s Health 2019, 23, e00122. [Google Scholar] [CrossRef]
- Heinonen, S.; Ryynänen, M.; Kirkinen, P. Etiology and outcome of second trimester non-immunologic fetal hydrops. Acta Obstet. Gynecol. Scand. 2000, 79, 15–18. [Google Scholar]
- Braun, T.; Brauer, M.; Fuchs, I.; Czernik, C.; Dudenhausen, J.W.; Henrich, W.; Sarioglu, N. Mirror syndrome: A systematic review of fetal associated conditions, maternal presentation and perinatal outcome. Fetal Diagn. Ther. 2010, 27, 191–203. [Google Scholar] [CrossRef]
- Bixel, K.; Silasi, M.; Zelop, C.M.; Lim, K.H.; Zsengeller, Z.; Stillman, I.E.; Rana, S. Placental Origins of Angiogenic Dysfunction in Mirror Syndrome. Hypertens. Pregnancy 2012, 31, 211–217. [Google Scholar] [CrossRef]
- Llurba, E.; Crispi, F.; Verlohren, S. Update on the Pathophysiological Implications and Clinical Role of Angiogenic Factors in Pregnancy. Fetal Diagn. Ther. 2015, 37, 81–92. [Google Scholar] [CrossRef]
- Graham, N.; Garrod, A.; Bullen, P.; Heazell, A.E.P. Placental expression of anti-angiogenic proteins in mirror syndrome: A case report. Placenta 2012, 33, 528–531. [Google Scholar] [CrossRef]
- Katoh, Y.; Seyama, T.; Mimura, N.; Furuya, H.; Nakayama, T.; Iriyama, T.; Nagamatsu, T.; Osuga, Y.; Fujii, T. Elevation of Maternal Serum sFlt-1 in Pregnancy with Mirror Syndrome Caused by Fetal Cardiac Failure. Oxf. Med Case Rep. 2018, 2018, omx112. [Google Scholar] [CrossRef] [Green Version]
- Hirata, G.; Aoki, S.; Sakamaki, K.; Takahashi, T.; Hirahara, F.; Ishikawa, H. Clinical characteristics of mirror syndrome: A comparison of 10 cases of mirror syndrome with non-mirror syndrome fetal hydrops cases. J. Matern.-Fetal Neonatal Med. 2016, 29, 2630–2634. [Google Scholar] [CrossRef] [PubMed]
- Gherman, R.B.; Incerpi, M.H.; Wing, D.A.; Goodwin, T.M. Ballantyne syndrome: Is placental ischemia the etiology? J. Matern.-Fetal Med. 1998, 7, 227–229. [Google Scholar]
- Gedikbasi, A.; Oztarhan, K.; Gunenc, Z.; Yildirim, G.; Arslan, O.; Yildirim, D.; Ceylan, Y. Preeclampsia Due to Fetal Non-immune Hydrops: Mirror Syndrome and Review of Literature. Hypertens. Pregnancy 2011, 30, 322–330. [Google Scholar] [CrossRef]
- Han, Z.; Chen, X.; Wang, Q.; Zhou, J.; Guo, Y.; Hou, H.; Zhang, Y. Clinical characteristics and risk factors of mirror syndrome: A retrospective case-control study. BMC Pregnancy Childbirth 2021, 21, 660. [Google Scholar] [CrossRef]
- Norton, M.E.; Chauhan, S.P.; Dashe, J.S. Society for Maternal-Fetal Medicine (SMFM) Clinical Guideline #7: Nonimmune hydrops fetalis. Am. J. Obstet. Gynecol. 2015, 212, 127–139. [Google Scholar]
- Sparks, T.N.; Thao, K.; Lianoglou, B.R.; Boe, N.M.; Bruce, K.G.; Datkhaeva, I.; Field, N.T.; Fratto, V.M.; Jolley, J.; Laurent, L.C.; et al. Nonimmune hydrops fetalis: Identifying the underlying genetic etiology. Genet. Med. 2019, 21, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Bellini, C.; Donarini, G.; Paladini, D.; Calevo, M.G.; Bellini, T.; Ramenghi, L.A.; Hennekam, R.C. Etiology of non-immune hydrops fetalis: An update. Am. J. Med Genet. Part A 2015, 167, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Santo, S.; Mansour, S.; Thilaganathan, B.; Homfray, T.; Papageorghiou, A.; Calvert, S.; Bhide, A. Prenatal diagnosis of non-immune hydrops fetalis: What do we tell the parents? Prenat. Diagn. 2011, 31, 186–195. [Google Scholar] [CrossRef]
- Gravholt, C.H. Epidemiological, endocrine and metabolic features in Turner syndrome. Eur. J. Endocrinol. 2004, 151, 657–688. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.E.; Prakash, S.K.; Andersen, N.H.; Viuff, M.H.; Levitsky, L.L.; Rivera-Davila, M.; Crenshaw, M.L.; Hansen, L.; Colvin, M.K.; Hayes, F.J.; et al. Recognition and management of adults with Turner syndrome: From the transition of adolescence through the senior years. Am. J. Med. Genet. Part A 2019, 179, 1987–2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hook, E.B.; Warburton, D. The distribution of chromosomal genotypes associated with Turner’s syndrome: Livebirth prevalence rates and evidence for diminished fetal mortality and severity in genotypes associated with structural X abnormalities or mosaicism. Hum. Genet. 1983, 64, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Viuff, M.H.; Stochholm, K.; Uldbjerg, N.; Nielsen, B.B.; the Danish Fetal Medicine Study Group; Gravholt, C.H. Only a minority of sex chromosome abnormalities are detected by a national prenatal screening program for Down syndrome. Hum. Reprod. 2015, 30, 2419–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surerus, E.; Huggon, I.C.; Allan, L.D. Turner’s syndrome in fetal life. Ultrasound Obstet. Gynecol. 2003, 22, 264–267. [Google Scholar] [CrossRef]
- Bellini, C.; Hennekam, R.C.; Fulcheri, E.; Rutigliani, M.; Morcaldi, G.; Boccardo, F.M.; Bonioli, E. Etiology of Nonimmune Hydrops Fetalis: A Systematic Review. Am. J. Med. Genet. Part A 2009, 149, 844–851. [Google Scholar] [CrossRef]
- Alvarez-Nava, F.; Soto, M.; Lanes, R.; Pons, H.; Morales-Machin, A.; Bracho, A. Elevated second-trimester maternal serum β-human chorionic gonadotropin and amniotic fluid alpha-fetoprotein as indicators of adverse obstetric outcomes in fetal Turner syndrome: Biochemical screening in fetal TS. J. Obstet. Gynaecol. Res. 2015, 41, 1891–1898. [Google Scholar] [CrossRef]
- Lambert-Messerlian, G.M.; Saller, D.N.; Tumber, M.B.; French, C.A.; Peterson, C.J.; Canick, J.A. Second-trimester maternal serum progesterone levels in Turner syndrome with and without hydrops and in trisomy 18. Prenat. Diagn. 1999, 19, 476–479. [Google Scholar] [CrossRef]
- Benirschke, K.; Kaufmann, P. Pathology of the Human Placenta; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; 891p. [Google Scholar]
- Wegrzyn, P.; Faro, C.; Falcon, O.; Peralta, C.F.A.; Nicolaides, K.H. Placental volume measured by three-dimensional ultrasound at 11 to 13 + 6 weeks of gestation: Relation to chromosomal defects. Ultrasound Obstet. Gynecol. 2005, 26, 28–32. [Google Scholar] [CrossRef]
- Jeon, K.C.; Chen, L.S.; Goodson, P. Decision to abort after a prenatal diagnosis of sex chromosome abnormality: A systematic review of the literature. Genet. Med. 2012, 14, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Burwick, R.M.; Pilliod, R.A.; Dukhovny, S.E.; Caughey, A.B. Fetal hydrops and the risk of severe preeclampsia. J. Matern.-Fetal Neonatal Med. 2019, 32, 961–965. [Google Scholar] [CrossRef]
- Torres-Gómez, L.G.; Silva-González, M.E.; González-Hernández, R. Ballantyne syndrome or mirror syndrome. Ginecol. Obstet. Mex. 2010, 78, 621–625. [Google Scholar] [PubMed]
- Vogel, M. Atlas der Morphologischen Plazentadiagnostik, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996; 263p. [Google Scholar]
- Chimenea, A.; García-Díaz, L.; Calderón, A.M.; Heras, M.M.D.L.; Antiñolo, G. Resolution of maternal Mirror syndrome after succesful fetal intrauterine therapy: A case series. BMC Pregnancy Childbirth 2018, 18, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xi, M.; Peng, B.; You, Y. Mirror syndrome associated with Fetal Hemoglobin Bart’s disease: A case report. Arch. Gynecol. Obstet. 2013, 288, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Hermyt, E.; Zmarzły, N.; Jęda-Golonka, A. Mirror syndrome: A literature review. Pediatr. Med. Rodz. 2019, 15, 246–251. [Google Scholar] [CrossRef]
- Llurba, E.; Marsal, G.; Sanchez, O.; Dominguez, C.; Alijotas-Reig, J.; Carreras, E.; Cabero, L. Angiogenic and antiangiogenic factors before and after resolution of maternal mirror syndrome. Ultrasound Obstet. Gynecol. 2012, 40, 367–369. [Google Scholar] [CrossRef]
- Chervenak, F.A.; Isaacson, G.; Blakemore, K.J.; Breg, W.R.; Hobbins, J.C.; Berkowitz, R.L.; Tortora, M.; Mayden, K.; Mahoney, M.J. Fetal Cystic Hygroma: Cause and Natural History. N. Engl. J. Med. 1983, 309, 822–825. [Google Scholar] [CrossRef]
- Jauniaux, E. Diagnosis and management of early non-immune hydrops fetalis. Prenat. Diagn. 1997, 17, 1261–1268. [Google Scholar] [CrossRef]
- Atton, G.; Gordon, K.; Brice, G.; Keeley, V.; Riches, K.; Ostergaard, P.; Mortimer, P.S.; Mansour, S. The lymphatic phenotype in Turner syndrome: An evaluation of nineteen patients and literature review. Eur. J. Hum. Genet. 2015, 23, 1634–1639. [Google Scholar] [CrossRef] [Green Version]
- Mostello, D.J.; Bofinger, M.K.; Siddiqi, T.A. Spontaneous resolution of fetal cystic hygroma and hydrops in Turner syndrome. Obstet. Gynecol. 1989, 73 Pt 2, 862–865. [Google Scholar]
| Maternal Characteristics n = 138 | |
|---|---|
| Age (years) | 30 (17–45) |
| BMI (kg/m2) | 24.9 (17.8–46.3) |
| Mode of conception | |
| Spontaneous | 111 (80.4%) |
| IVF/ICSI | 8 (5.8%) |
| Unknown | 19 (13.8%) |
| Fetal karyotype n = 138 | |
| 45,X | 134 (97.1%) |
| mos 45,X/46,XX | 4 (2.9%) |
| First diagnosis of/presentation with fetal hydrops (weeks) (n = 133) | 15.4 (10.57–25.43) |
| GA at delivery in case of live birth (n = 20) | 38 (31–42) |
| Median (Weeks) | Minimum and Maximum (Weeks) | |
|---|---|---|
| GA at fetal death (n = 29) | 23 | 16.14–38 |
| Interval from diagnosis of hydrops to live birth (n = 18) | 25.4 | 17.14–27.86 |
| Interval from diagnosis of hydrops to fetal death (n = 28) | 7.4 | 0.28–25.14 |
| n (%) | |
|---|---|
| None | 105 (76.1) |
| IUGR | 27 (19.6) |
| PPROM | 2 (1.4) |
| Preexisting hypertonus | 1 (0.7) |
| Preeclampsia/HELLP | 2 (1.4) |
| Preterm birth <34 weeks GA | 1 (0.7) |
| Mirror syndrome | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedei, I.A.; Graf, A.; Gloning, K.-P.; Meyer-Wittkopf, M.; Willner, D.; Krapp, M.; Hentze, S.; Scharf, A.; Degenhardt, J.; Heling, K.-S.; et al. Is Fetal Hydrops in Turner Syndrome a Risk Factor for the Development of Maternal Mirror Syndrome? J. Clin. Med. 2022, 11, 4588. https://doi.org/10.3390/jcm11154588
Bedei IA, Graf A, Gloning K-P, Meyer-Wittkopf M, Willner D, Krapp M, Hentze S, Scharf A, Degenhardt J, Heling K-S, et al. Is Fetal Hydrops in Turner Syndrome a Risk Factor for the Development of Maternal Mirror Syndrome? Journal of Clinical Medicine. 2022; 11(15):4588. https://doi.org/10.3390/jcm11154588
Chicago/Turabian StyleBedei, Ivonne Alexandra, Alexander Graf, Karl-Philipp Gloning, Matthias Meyer-Wittkopf, Daria Willner, Martin Krapp, Sabine Hentze, Alexander Scharf, Jan Degenhardt, Kai-Sven Heling, and et al. 2022. "Is Fetal Hydrops in Turner Syndrome a Risk Factor for the Development of Maternal Mirror Syndrome?" Journal of Clinical Medicine 11, no. 15: 4588. https://doi.org/10.3390/jcm11154588
APA StyleBedei, I. A., Graf, A., Gloning, K.-P., Meyer-Wittkopf, M., Willner, D., Krapp, M., Hentze, S., Scharf, A., Degenhardt, J., Heling, K.-S., Kozlowski, P., Trautmann, K., Jahns, K., Geipel, A., Tekesin, I., Elsässer, M., Wilhelm, L., Gottschalk, I., Baumüller, J.-E., ... Axt-Fliedner, R. (2022). Is Fetal Hydrops in Turner Syndrome a Risk Factor for the Development of Maternal Mirror Syndrome? Journal of Clinical Medicine, 11(15), 4588. https://doi.org/10.3390/jcm11154588







