Test Performance Characteristics of Dynamic Liver Enzyme Trends in the Prediction of Choledocholithiasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patients, Variables, and Outcomes
2.3. Statistical Analyses
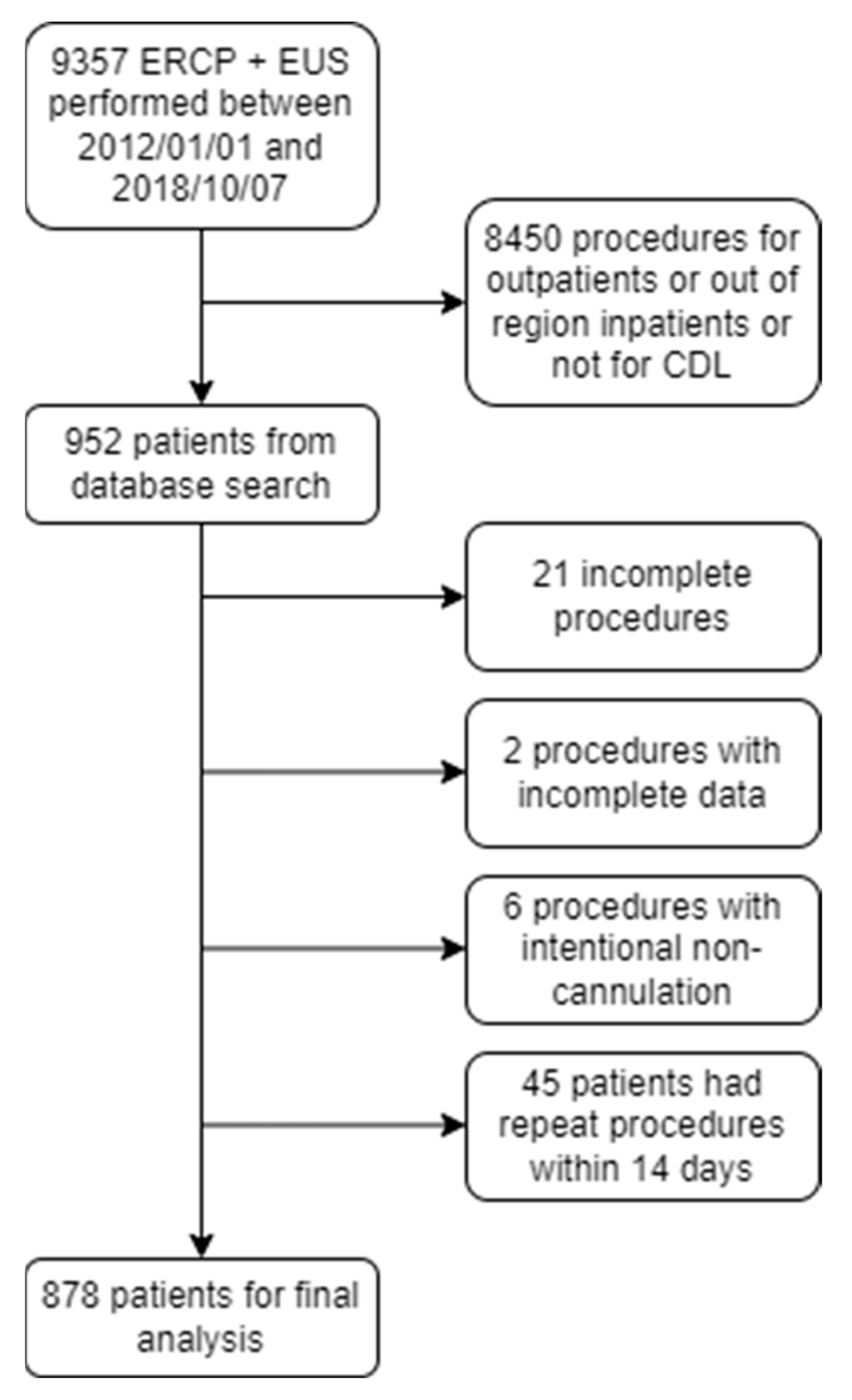
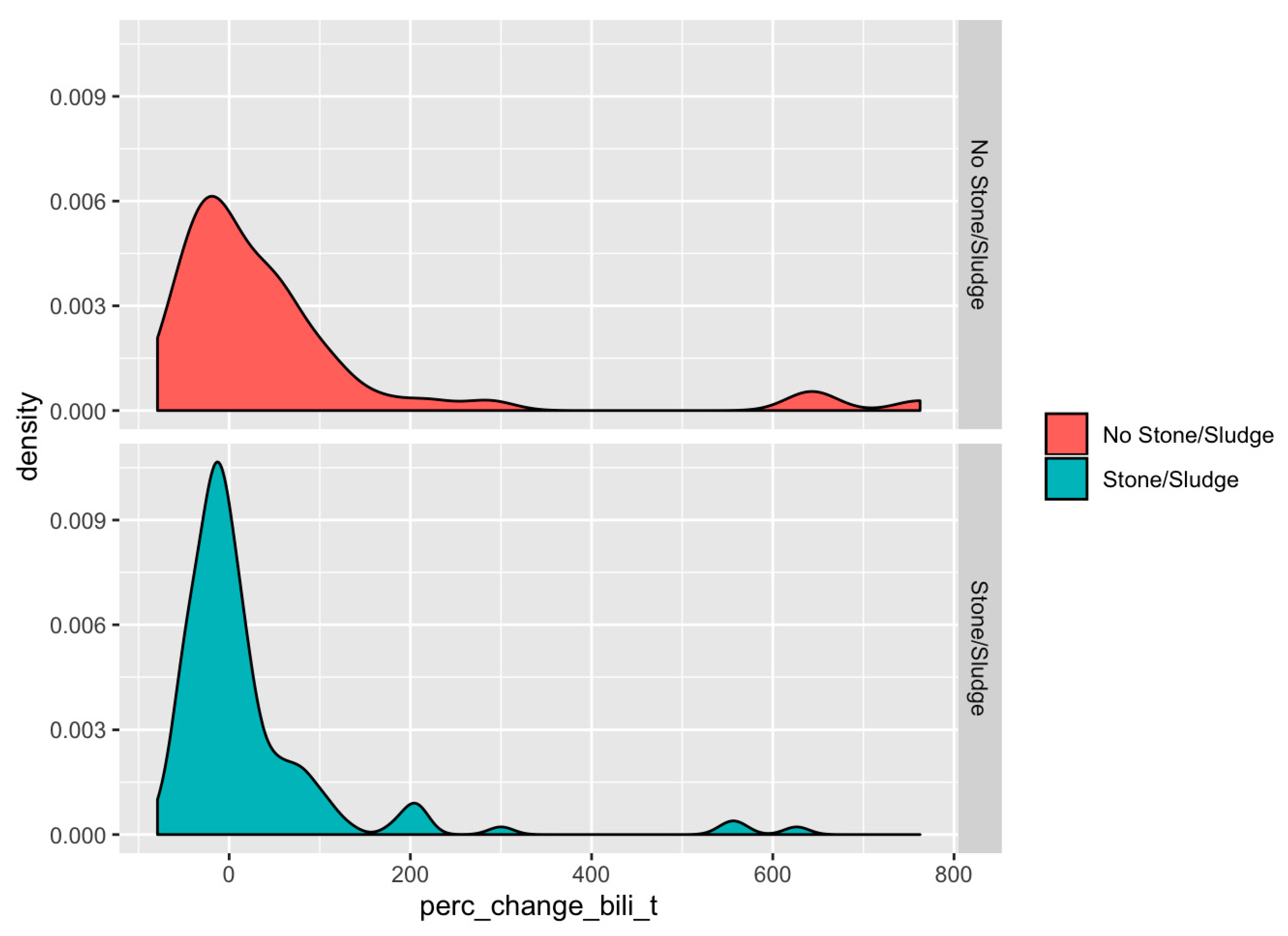
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | adverse event |
| ALP | alkaline phosphatase |
| ALT | alanine transaminase |
| ASGE | American Society for Gastrointestinal Endoscopy |
| AST | aspartate transaminase |
| CBD | common bile duct |
| CDL | choledocholithiasis |
| CT | computed tomography |
| ERCP | endoscopic retrograde cholangiopancreatography |
| ESGE | European Society of Gastrointestinal Endoscopy |
| EUS | endoscopic ultrasound |
| GGT | gamma-glutamyltransferase |
| MRCP | magnetic resonance cholangiopancreatography |
| NPV | negative predictive value |
| PPV | positive predictive value |
References
- Frossard, J.L.; Morel, P.M. Detection and management of bile duct stones. Gastrointest. Endosc. 2010, 72, 808–816. [Google Scholar] [CrossRef]
- Gurusamy, K.S.; Giljaca, V.; Takwoingi, Y.; Higgie, D.; Poropat, G.; Štimac, D.; Davidson, B.R. Endoscopic retrograde cholangiopancreatography versus intraoperative cholangiography for diagnosis of common bile duct stones. Cochrane Database Syst. Rev. 2015, 2, CD010339. [Google Scholar] [CrossRef]
- Maple, J.T.; Ben-Menachem, T.; Anderson, M.A.; Appalaneni, V.; Banerjee, S.; Cash, B.D.; Fisher, L.; Harrison, M.E.; Fanelli, R.D.; Fukami, N.; et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest. Endosc. 2010, 71, 1–9. [Google Scholar] [CrossRef]
- Chandrasekhara, V.; Khashab, M.A.; Muthusamy, V.R.; Acosta, R.D.; Agrawal, D.; Bruining, D.H.; Eloubeidi, M.A.; Fanelli, R.D.; Faulx, A.L.; Gurudu, S.R.; et al. Adverse events associated with ERCP. Gastrointest. Endosc. 2017, 85, 32–47. [Google Scholar] [CrossRef]
- Meeralam, Y.; Al-Shammari, K.; Yaghoobi, M. Diagnostic accuracy of EUS compared with MRCP in detecting choledocholithiasis: A meta-analysis of diagnostic test accuracy in head-to-head studies. Gastrointest. Endosc. 2017, 86, 986–993. [Google Scholar] [CrossRef]
- Buxbaum, J.L.; Fehmi, S.M.A.; Sultan, S.; Fishman, D.S.; Qumseya, B.J.; Cortessis, V.K.; Schilperoort, H.; Kysh, L.; Matsuoka, L.; Yachimski, P.; et al. ASGE guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointest. Endosc. 2019, 89, 1075–1105.e15. [Google Scholar] [CrossRef]
- Manes, G.; Paspatis, G.; Aabakken, L.; Anderloni, A.; Arvanitakis, M.; Ah-Soune, P.; Barthet, M.; Domagk, D.; Dumonceau, J.-M.; Gigot, J.-F.; et al. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2019, 51, 472–491. [Google Scholar] [CrossRef]
- Sherman, J.L.; Shi, E.W.; Ranasinghe, N.E.; Sivasankaran, M.T.; Prigoff, J.G.; Divino, C.M. Validation and improvement of a proposed scoring system to detect retained common bile duct stones in gallstone pancreatitis. Surgery 2015, 157, 1073–1079. [Google Scholar] [CrossRef]
- Jovanovic, P.; Salkic, N.N.; Zerem, E. Artificial neural network predicts the need for therapeutic ERCP in patients with suspected choledocholithiasis. Gastrointest. Endosc. 2014, 80, 260–268. [Google Scholar] [CrossRef]
- Chandran, A.; Rashtak, S.; Patil, P.; Gottlieb, A.; Bernstam, E.; Guha, S.; Ramireddy, S.; Badillo, B.; DaVee, R.T.; Kao, L.S.; et al. Comparing diagnostic accuracy of current practice guidelines in predicting choledocholithiasis: Outcomes from a large healthcare system comprising both academic and community settings. Gastrointest. Endosc. 2021, 2, 1–9. [Google Scholar] [CrossRef]
- Kuzu, U.B.; Ödemiş, B.; Dişibeyaz, S.; Parlak, E.; Öztaş, E.; Saygılı, F.; Yıldız, H.; Kaplan, M.; Coskun, O.; Aksoy, A.; et al. Management of suspected common bile duct stone: Diagnostic yield of current guidelines. HPB 2017, 19, 126–132. [Google Scholar] [CrossRef][Green Version]
- Jagtap, N.; Yashavanth, H.S.; Tandan, M.; Basha, J.; Chavan, R.; Nabi, Z.; Kalapala, R.; Reddy, P.M.; Ramchandani, M.; Gupta, R.; et al. Clinical utility of ESGE and ASGE guidelines for prediction of suspected choledocholithiasis in patients undergoing cholecystectomy. Endoscopy 2020, 52, 569–573. [Google Scholar] [CrossRef]
- Narváez-Rivera, R.M.; González-González, J.A.; Monreal-Robles, R.; García-Compean, D.; Paz-Delgadillo, J.; Garza-Galindo, A.A.; Maldonado-Garza, H.J. Accuracy of ASGE criteria for the prediction of choledocholithiasis. Rev. Española De Enferm. Dig. 2016, 108, 309–314. [Google Scholar] [CrossRef]
- Adams, M.A.; Hosmer, A.E.; Wamsteker, E.J.; Anderson, M.A.; Elta, G.H.; Kubiliun, N.M.; Kwon, R.S.; Piraka, C.R.; Scheiman, J.M.; Waljee, A.K.; et al. Predicting the likelihood of a persistent bile duct stone in patients with suspected choledocholithiasis: Accuracy of existing guidelines and the impact of laboratory trends. Gastrointest. Endosc. 2015, 82, 88–93. [Google Scholar] [CrossRef][Green Version]
- He, H.; Tan, C.; Wu, J.; Dai, N.; Hu, W.; Zhang, Y.; Laine, L.; Scheiman, J.; Kim, J.J. Accuracy of ASGE high-risk criteria in evaluation of patients with suspected common bile duct stones. Gastrointest. Endosc. 2017, 86, 525–532. [Google Scholar] [CrossRef]
- Forbes, N.; Koury, H.F.; Bass, S.; Cole, M.; Mohamed, R.; Turbide, C.; Gonzalez-Moreno, E.; Kayal, A.; Chau, M.; Lethebe, B.C.; et al. Characteristics and Outcomes of ERCP at a Canadian Tertiary Centre: Initial Results from a Prospective High-Fidelity Biliary Endoscopy Registry. J. Can. Assoc. Gastroenterol. 2021, 4, 78–83. [Google Scholar] [CrossRef]
- Panda, N.; Chang, Y.; Chokengarmwong, N.; Martinez, M.; Yu, L.; Fagenholz, P.J.; Kaafarani, H.A.; King, D.R.; DeMoya, M.A.; Velmahos, G.C.; et al. Gallstone Pancreatitis and Choledocholithiasis: Using Imaging and Laboratory Trends to Predict the Likelihood of Persistent Stones at Cholangiography. World J. Surg. 2018, 42, 3143–3149. [Google Scholar] [CrossRef]
- Gillaspie, D.B.; Davis, K.A.; Schuster, K.M. Total bilirubin trend as a predictor of common bile duct stones in acute cholecystitis and symptomatic cholelithiasis. Am. J. Surg. 2019, 217, 98–102. [Google Scholar] [CrossRef]
- Yu, C.Y.; Roth, N.; Jani, N.; Cho, J.; Van Dam, J.; Selby, R.; Buxbaum, J. Dynamic liver test patterns do not predict bile duct stones. Surg. Endosc. 2019, 33, 3300–3313. [Google Scholar] [CrossRef]
- Suarez, A.L.; LaBarre, N.T.; Cotton, P.B.; Payne, K.M.; Coté, G.A.; Elmunzer, B.J. An assessment of existing risk stratification guidelines for the evaluation of patients with suspected choledocholithiasis. Surg. Endosc. 2016, 30, 4613–4618. [Google Scholar] [CrossRef]
- Jacob, J.S.; Lee, M.E.; Chew, E.Y.; Thrift, A.P.; Sealock, R.J. Evaluating the revised American society for gastrointestinal endoscopy guidelines for common bile duct stone diagnosis. Clin. Endosc. 2021, 54, 269–274. [Google Scholar] [CrossRef] [PubMed]
| Probability | ASGE 2010 [3] | ASGE 2019 [6] | ESGE 2019 [7] |
|---|---|---|---|
| High (>50%) | (1) CBD stone on US and/or (2) Clinical ascending cholangitis and/or (3) Bilirubin > 4 mg/dL and/or (4) Bilirubin 1.8–4 mg/dL AND CBD > 6 mm on US | (1) CBD stone on US or cross-sectional imaging and/or (2) Clinical ascending cholangitis and/or (3) Bilirubin > 4 mg/dL AND CBD > 6 mm on US | (1) Clinical ascending cholangitis and/or (2) CBD stone on US |
| Intermediate (10–50%) | (1) Bilirubin 1.8–4 mg/dL and/or (2) CBD > 6 mm on US and/or (3) Abnormal liver biochemical tests other than bilirubin and/or (4) Clinical gallstone pancreatitis and/or (5) Age > 55 years | (1) Bilirubin ≥ 1.8 mg/dL and/or (2) CBD > 6 mm on US and/or (3) Abnormal liver biochemical tests other than bilirubin and/or (4) Clinical gallstone pancreatitis and/or (5) Age > 55 years | (1) Any abnormal liver biochemical tests and/or (2) CBD > 6 mm on US |
| Low (<10%) | None of the above predictors present | None of the above predictors present | None of the above predictors present |
| Patients with Positive Diagnosis of Choledocholithiasis (n = 622) | Patients with Negative Diagnosis of Choledocholithiasis (n = 257) | p-Value | |
|---|---|---|---|
| Sex | 0.20 | ||
| Female % (n) | 60.0 (373) | 55.3 (142) | |
| Male % (n) | 40.0 (249) | 44.7 (115) | |
| Mean age (SD) | 59.3 (21.2) | 66.0 (18.2) | <0.001 |
| Procedure performed | <0.001 | ||
| ERCP % (n) | 97.7 (608) | 86.0 (221) | |
| EUS % (n) | 2.3 (14) | 14.0 (36) | |
| Pre-Procedure Imaging | |||
| Yes % (n) | 78.9 (472) | 82.9 (213) | |
| No % (n) | 24.1 (150) | 17.1 (44) | |
| Stone seen on imaging | <0.001 | ||
| Yes % (n) | 54.7 (340) | 0.0 (0) | |
| No % (n) | 45.3 (282) | 100.0 (257) | |
| CBD > 6 mm | 0.04 | ||
| Yes % (n) | 55.3 (344) | 47.9 (123) | |
| No % (n) | 44.7 (278) | 52.1 (134) | |
| Mean CBD size in mm, of those >6 mm (SD) | 12.4 (4.5) | 11.6 (3.8) | 0.01 |
| Mean total bilirubin in µmol/L (SD) | 64.9 (62.1) | 60.6 (63.3) | 0.35 |
| Mean ALT in U/L (SD) | 292.4 (346.6) | 283.6 (267.0) | 0.72 |
| Mean ALP in U/L (SD) | 278.2 (256.5) | 261.1 (191.4) | 0.75 |
| 2010 ASGE guideline risk category | 0.01 | ||
| High % (n) | 67.7 (421) | 57.6 (148) | |
| Intermediate % (n) | 27.7 (172) | 33.5 (86) | |
| Low % (n) | 4.7 (29) | 8.9 (23) |
| Accuracy % (95% CI) | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | Patients Used in Models | |
|---|---|---|---|---|---|---|
| Bilirubin increase of at least 30% | 33.5 | 24.1 | 57.7 | 59.3 | 22.9 | |
| (26.8–40.8) | (17.1–32.2) | (43.2–71.3) | (45.0–72.4) | (16.0–31.1) | 190 | |
| Bilirubin increase of at least 50% | 31.9 | 19.5 | 63.5 | 57.8 | 23.6 | |
| (25.2–39.1) | (13.2–27.3) | (49.0–76.4) | (42.2–72.3) | (16.8–31.5) | 190 | |
| ALP increase of at least 30% | 32.2 | 12.2 | 82.7 | 64.0 | 27.2 | |
| (25.5–39.5) | (7.1–19.1) | (69.7–91.8) | (42.5–82.0) | (20.4–34.9) | 187 | |
| ALP increase of at least 50% | 29.5 | 6.1 | 88.5 | 57.1 | 27.2 | |
| (23.0–36.7) | (2.7–11.7) | (76.6–95.6) | (28.9–82.3) | (20.7–34.6) | 187 | |
| ALT increase of at least 30% | 34.6 | 19.8 | 72.5 | 65.0 | 26.1 | |
| (27.7–42.0) | (13.4–27.7) | (58.3–84.1) | (48.3–79.4) | (19.1–34.1) | 186 | |
| ALT increase of at least 50% | 34.1 | 16.0 | 80.4 | 67.7 | 27.2 | |
| (27.2–41.4) | (10.2–23.5) | (66.9–90.2) | (48.6–83.3) | (20.2–35.0) | 186 | |
| Bilirubin OR ALT increase of at least 30% | 37.4 | 32.0 | 51.0 | 62.1 | 23.0 | |
| (30.3–45.0) | (24.1–40.9) | (36.6–65.2) | (49.3–73.8) | (15.6–31.9) | 183 | |
| Bilirubin OR ALT increase of at least 50% | 34.6 | 25.0 | 58.8 | 60.4 | 23.8 | |
| (27.7–42.1) | (17.8–33.4) | (44.2–72.4) | (46.0–73.5) | (16.7–32.2) | 183 | |
| Bilirubin AND ALT increase of at least 30% | 31.3 | 12.5 | 78.4 | 59.3 | 26.3 | |
| (24.6–38.6) | (7.3–19.5) | (64.7–88.7) | (38.8–77.6) | (19.5–34.1) | 183 | |
| Bilirubin AND ALT increase of at least 50% | 31.8 | 10.9 | 84.3 | 63.6 | 27.4 | |
| (25.1–39.2) | (6.1–17.7) | (71.4–93.0) | (40.7–82.8) | (20.6–35.1) | 183 | |
| Any enzyme increase of at least 30% | 37.9 | 34.7 | 46.0 | 61.4 | 22.1 | |
| (30.7–45.6) | (26.4–43.7) | (31.8–60.7) | (49.0–72.8) | (14.6–31.3) | 177 | |
| Any enzyme increase of at least 50% | 35.1 | 25.8 | 80.4 | 60.4 | 24.0 | |
| (28.0–42.6) | (18.4–34.4) | (66.9–90.2) | (46.0–73.5) | (16.7–32.6) | 177 |
| Accuracy % (95% CI) | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | Patients Used in Models | |
|---|---|---|---|---|---|---|
| Bilirubin decrease of at least 30% | 61.6 | 21.2 | 77.4 | 26.8 | 71.5 | |
| (54.2–68.7) | (11.1–34.7) | (69.4–84.2) | (14.2–42.9) | (63.4–78.7) | 190 | |
| Bilirubin decrease of at least 50% | 68.6 | 9.6 | 91.7 | 31.2 | 72.2 | |
| (61.4–75.3) | (3.2–21.0) | (85.7–95.8) | (11.0–58.7) | (64.8–78.8) | 190 | |
| ALP decrease of at least 30% | 67.2 | 1.9 | 93.1 | 10.0 | 70.5 | |
| (59.9–74.0) | (0.0–10.3) | (87.4–96.8) | (0.3–44.5) | (63.1–77.2) | 187 | |
| ALP decrease of at least 50% | 71.6 | 0.0 | 100.0 | N/R | 71.6 | |
| (64.5–78.0) | (0.0–6.8) | (97.2–100.0) | (64.5–78.0) | 187 | ||
| ALT decrease of at least 30% | 55.5 | 19.6 | 69.5 | 20.0 | 68.9 | |
| (48.0–62.8) | (9.8–33.1) | (60.8–77.2) | (10.0–33.7) | (60.3–76.7) | 186 | |
| ALT decrease of at least 50% | 64.8 | 3.9 | 88.5 | 11.8 | 70.3 | |
| (57.4–71.8) | (0.5–13.5) | (81.8- 93.4) | (1.5–36.4) | (62.7–77.2) | 186 | |
| Bilirubin OR ALT decrease of at least 30% | 52.0 | 35.3 | 58.6 | 25.4 | 69.4 | |
| (44.4–59.5) | (22.4–49.9) | (49.6–67.2) | (15.8–37.1) | (59.8–77.9) | 183 | |
| Bilirubin OR ALT decrease of at least 50% | 62.0 | 11.8 | 82.0 | 20.7 | 69.7 | |
| (54.5–69.1) | (4.4–23.9) | (74.3–88.3) | (8.0–39.7) | (61.5–77.0) | 183 | |
| Bilirubin AND ALT decrease of at least 30% | 64.8 | 3.9 | 89.1 | 12.5 | 69.9 | |
| (57.3–71.8) | (0.5–13.5) | (82.3–93.9) | (1.6–38.3) | (62.3–76.9) | 183 | |
| Bilirubin AND ALT decrease of at least 50% | 70.4 | 0.0 | 98.4 | 0.0 | 71.2 | |
| (63.1–77.0) | (0.0–7.0) | (94.5–99.8) | (0.0–8.4) | (63.9–77.7) | 183 | |
| Any enzyme decrease of at least 30% | 51.1 | 36.0 | 57.3 | 25.4 | 68.9 | |
| (43.5–58.8) | (22.9–50.8) | (48.1–66.1) | (15.8–37.1) | (59.1–77.7) | 177 | |
| Any enzyme decrease of at least 50% | 61.5 | 12.0 | 81.5 | 20.7 | 69.7 | |
| (53.8–68.8) | (4.5–24.3) | (73.5–87.9) | (8.0–39.7) | (61.5–77.0) | 177 |
| Patients with Choledocholithiasis/Total Patients in Risk Category | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | |
|---|---|---|---|---|---|
| ASGE 2010—high risk [3] | 67.7 | 57.6 | 74.0 | 35.2 | |
| 421/569 | (64–71.4) | (36.4–48.5) | (70.4–77.6) | (29.8–40.5) | |
| ASGE 2010—intermediate risk [3] | 27.7 | 33.5 | 66.7 | 27.5 | |
| 172/258 | (24.1–31.2) | (60.8–72.3) | (60.9–72.4) | (24–31.1) | |
| ASGE 2019—high risk [6] | 59.5 | 39.7 | 78.4 | 38.1 | |
| 370/472 | (55.6–63.3) | (54.3–66.3) | (74.7–82.1) | (33.3–42.8) | |
| ASGE 2019—intermediate risk [6] | 35.9 | 54.1 | 61.6 | 22.8 | |
| 223/362 | (32.1–39.6) | (39.8–52) | (56.6–66.6) | (19.2–26.4) | |
| ESGE 2019—high risk [7] | 46.3 | 19.5 | 85.2 | 38.3 | |
| 288/338 | (42.4–50.2) | (75.7–85.4) | (81.4–89) | (34.2–42.4) | |
| ESGE 2019—intermediate risk [7] | 37.6 | 66.1 | 57.9 | 18.3 | |
| 243/404 | (33.8–41.4) | (28.1–39.6) | (53.1–62.7) | (14.8–21.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Lethebe, B.C.; Wishart, E.; Bazerbachi, F.; Elmunzer, B.J.; Thosani, N.; Buxbaum, J.L.; Chen, Y.-I.; Bass, S.; Cole, M.J.; et al. Test Performance Characteristics of Dynamic Liver Enzyme Trends in the Prediction of Choledocholithiasis. J. Clin. Med. 2022, 11, 4575. https://doi.org/10.3390/jcm11154575
Lei Y, Lethebe BC, Wishart E, Bazerbachi F, Elmunzer BJ, Thosani N, Buxbaum JL, Chen Y-I, Bass S, Cole MJ, et al. Test Performance Characteristics of Dynamic Liver Enzyme Trends in the Prediction of Choledocholithiasis. Journal of Clinical Medicine. 2022; 11(15):4575. https://doi.org/10.3390/jcm11154575
Chicago/Turabian StyleLei, Yang, B. Cord Lethebe, Erin Wishart, Fateh Bazerbachi, B. Joseph Elmunzer, Nirav Thosani, James L. Buxbaum, Yen-I Chen, Sydney Bass, Martin J. Cole, and et al. 2022. "Test Performance Characteristics of Dynamic Liver Enzyme Trends in the Prediction of Choledocholithiasis" Journal of Clinical Medicine 11, no. 15: 4575. https://doi.org/10.3390/jcm11154575
APA StyleLei, Y., Lethebe, B. C., Wishart, E., Bazerbachi, F., Elmunzer, B. J., Thosani, N., Buxbaum, J. L., Chen, Y.-I., Bass, S., Cole, M. J., Turbide, C., Brenner, D. R., Heitman, S. J., Mohamed, R., & Forbes, N. (2022). Test Performance Characteristics of Dynamic Liver Enzyme Trends in the Prediction of Choledocholithiasis. Journal of Clinical Medicine, 11(15), 4575. https://doi.org/10.3390/jcm11154575





