Impact of Sleep-Disordered Breathing Treatment on Ventricular Tachycardia in Patients with Heart Failure
Abstract
:1. Introduction
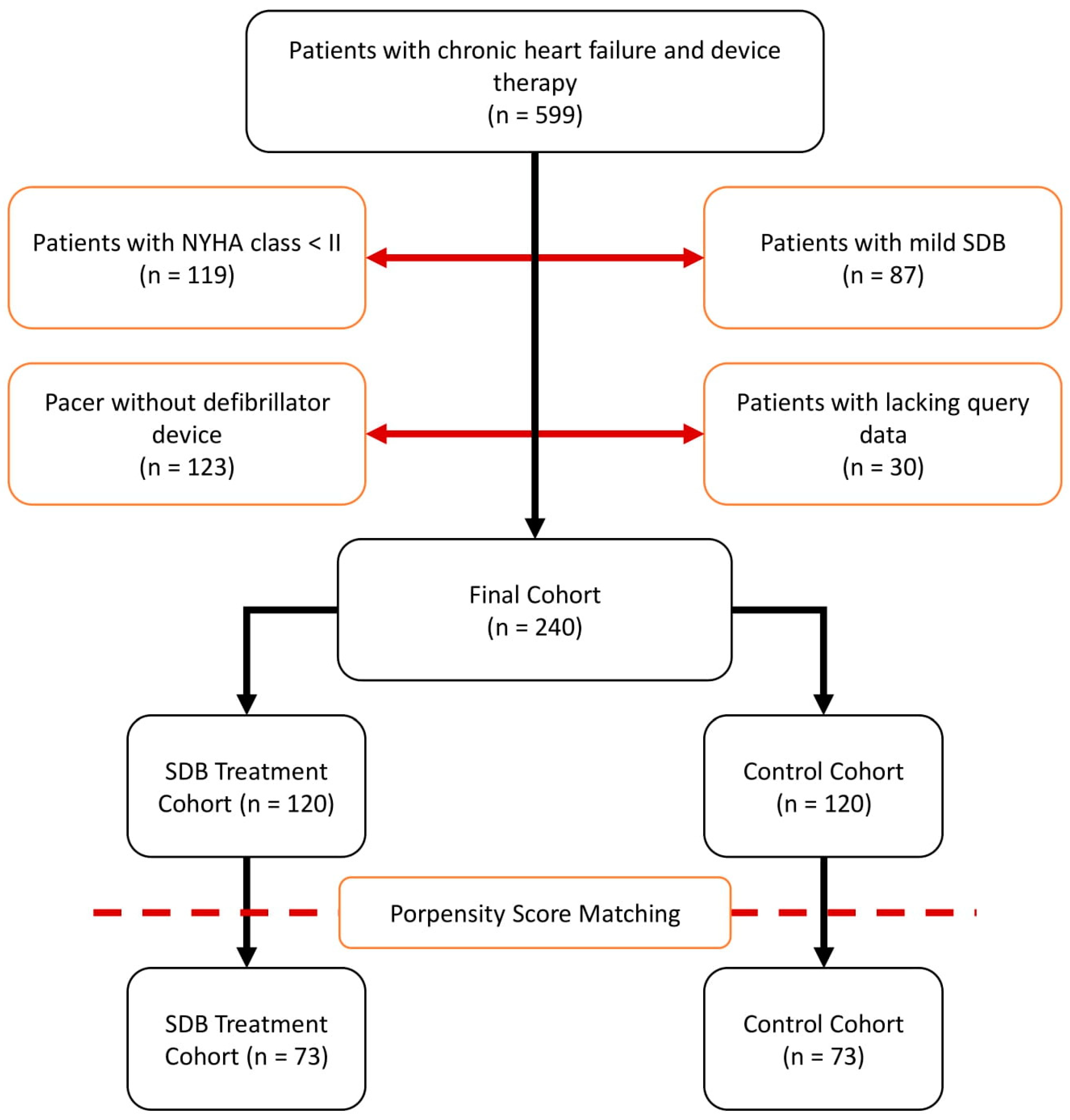
2. Patients and Methods
2.1. Patient Recruitment
2.2. Multichannel Cardiorespiratory Polygraphy (PG)
2.3. Device Interrogations
2.4. Statistical Analysis
3. Results
3.1. Treatment of SDB
3.2. Device Interrogation
3.3. Changes of Pacer Stimulation and Events
4. Discussion
4.1. Arrhythmic Events in Patients with Heart Failure and SDB
4.2. SDB Treatment Reduces the Total Burden of Ventricular Arrhythmia
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oldenburg, O.; Lamp, B.; Faber, L.; Teschler, H.; Horstkotte, D.; Töpfer, V. Sleep-disordered breathing in patients with symptomatic heart failure: A contemporary study of prevalence in and characteristics of 700 patients. Eur. J. Heart Fail. 2007, 9, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Arzt, M.; Woehrle, H.; Oldenburg, O.; Graml, A.; Suling, A.; Erdmann, E.; Teschler, H.; Wegscheider, K. Prevalence and Predictors of Sleep-Disordered Breathing in Patients with Stable Chronic Heart Failure: The SchlaHF Registry. JACC Heart Fail. 2016, 4, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.; Purucker, H.C.; Holzhacker, I.; Tebtmann, U.; Bitter, T.; Horstkotte, D.; Graml, A.; Woehrle, H.; Oldenburg, O. Prevalence of Sleep-Disordered Breathing and Patient Characteristics in a Coronary Artery Disease Cohort Undergoing Cardiovascular Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2016, 36, 421–429. [Google Scholar] [CrossRef]
- Sharma, S.; Oldenburg, O.; Fox, H. Sleep apnea and pulmonary hypertension: Connecting the dots. J. Clin. Sleep Med. 2021, 17, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Tafelmeier, M.; Weizenegger, T.; Ripfel, S.; Fauser, M.; Floerchinger, B.; Camboni, D.; Zausig, Y.; Wittmann, S.; Drzymalski, M.A.; Zeman, F.; et al. Postoperative complications after elective coronary artery bypass grafting surgery in patients with sleep-disordered breathing. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2018, 107, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Gerçek, M.; Oldenburg, O.; Gerçek, M.; Fox, H.; Rudolph, V.; Puehler, T.; Omran, H.; Wolf, L.K.; Hakim-Meibodi, K.; Zeiher, A.M.; et al. Prevalence of Sleep Disordered Breathing in Patients with Primary Mitral Regurgitation Undergoing Mitral Valve Surgery. J. Clin. Med. 2021, 10, 2039. [Google Scholar] [CrossRef]
- Omran, H.; Bitter, T.; Horstkotte, D.; Oldenburg, O.; Fox, H. Characteristics and circadian distribution of cardiac arrhythmias in patients with heart failure and sleep-disordered breathing. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2018, 107, 965–974. [Google Scholar] [CrossRef]
- Coniglio, A.C.; Mentz, R.J. Sleep Breathing Disorders in Heart Failure. Heart Fail. Clin. 2020, 16, 45–51. [Google Scholar] [CrossRef]
- Kadhim, K.; Middeldorp, M.E.; Elliott, A.D.; Agbaedeng, T.; Gallagher, C.; Malik, V.; Wong, C.X.; McEvoy, R.D.; Kalman, J.M.; Lau, D.H.; et al. Prevalence and Assessment of Sleep-Disordered Breathing in Patients with Atrial Fibrillation: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2021, 37, 1846–1856. [Google Scholar] [CrossRef]
- Lavergne, F.; Morin, L.; Armitstead, J.; Benjafield, A.; Richards, G.; Woehrle, H. Atrial fibrillation and sleep-disordered breathing. J. Thorac. Dis. 2015, 7, E575–E584. [Google Scholar] [CrossRef]
- Sohns, C.; Marrouche, N.F.; Costard-Jackle, A.; Sossalla, S.; Bergau, L.; Schramm, R.; Fuchs, U.; Omran, H.; Rubarth, K.; Dumitrescu, D.; et al. Catheter ablation for atrial fibrillation in patients with end-stage heart failure and eligibility for heart transplantation. ESC Heart Fail. 2021, 8, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.; Bitter, T.; Horstkotte, D.; Oldenburg, O. Sleep-Disordered Breathing and Arrhythmia in Heart Failure Patients. Sleep Med. Clin. 2017, 12, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.; Arzt, M.; Bergmann, M.W.; Bitter, T.; Linz, D.; Oldenburg, O.; Penzel, T.; Rillig, A.; Schöbel, C.; Sinha, A.-M.; et al. Positionspapier “Schlafmedizin in der Kardiologie”, Update 2021. Der. Kardiol. 2021, 15, 429–461. [Google Scholar] [CrossRef]
- Oldenburg, O.; Lamp, B.; Horstkotte, D. Cardiorespiratory screening for sleep-disordered breathing. Eur. Respir. J. 2006, 28, 1065–1067. [Google Scholar] [CrossRef] [Green Version]
- Cowie, M.R.; Woehrle, H.; Wegscheider, K.; Angermann, C.; d’Ortho, M.P.; Erdmann, E.; Levy, P.; Simonds, A.K.; Somers, V.K.; Zannad, F.; et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N. Engl. J. Med. 2015, 373, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [Green Version]
- Buxton, A.E.; Calkins, H.; Callans, D.J.; DiMarco, J.P.; Fisher, J.D.; Greene, H.L.; Haines, D.E.; Hayes, D.L.; Heidenreich, P.A.; Miller, J.M.; et al. ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). Circulation 2006, 114, 2534–2570. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, C.T.; Kay, G.N.; Kalman, J.; Borggrefe, M.; Della-Bella, P.; Dickfeld, T.; Dorian, P.; Huikuri, H.; Kim, Y.H.; Knight, B.; et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Europace 2014, 16, 1257–1283. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Austin, P.C. A comparison of 12 algorithms for matching on the propensity score. Stat. Med. 2014, 33, 1057–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Stansbury, R.; Hackett, B.; Fox, H. Sleep apnea and pulmonary hypertension: A riddle waiting to be solved. Pharmacol. Ther. 2021, 227, 107935. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, N.; Yumino, D.; Kajimoto, K.; Tagawa, Y.; Takagi, A.; Shoda, M.; Kasanuki, H.; Hagiwara, N. Impact of sleep-disordered breathing on life-threatening ventricular arrhythmia in heart failure patients with implantable cardioverter-defibrillator. Am. J. Cardiol. 2008, 102, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.B.; Anstrom, K.J.; Sun, J.L.; Clapp-Channing, N.E.; Tsiatis, A.A.; Davidson-Ray, L.; Lee, K.L.; Bardy, G.H. Quality of life with defibrillator therapy or amiodarone in heart failure. N. Engl. J. Med. 2008, 359, 999–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghuram, A.; Clay, R.; Kumbam, A.; Tereshchenko, L.G.; Khan, A. A systematic review of the association between obstructive sleep apnea and ventricular arrhythmias. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2014, 10, 1155–1160. [Google Scholar] [CrossRef] [Green Version]
- Oldenburg, O.; Wellmann, B.; Bitter, T.; Fox, H.; Buchholz, A.; Freiwald, E.; Horstkotte, D.; Wegscheider, K. Adaptive servo-ventilation to treat central sleep apnea in heart failure with reduced ejection fraction: The Bad Oeynhausen prospective ASV registry. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2018, 107, 719–728. [Google Scholar] [CrossRef]
- Ryan, C.M.; Usui, K.; Floras, J.S.; Bradley, T.D. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax 2005, 60, 781–785. [Google Scholar] [CrossRef] [Green Version]
- Craig, S.; Pepperell, J.C.; Kohler, M.; Crosthwaite, N.; Davies, R.J.; Stradling, J.R. Continuous positive airway pressure treatment for obstructive sleep apnoea reduces resting heart rate but does not affect dysrhythmias: A randomised controlled trial. J. Sleep Res. 2009, 18, 329–336. [Google Scholar] [CrossRef]
- Bitter, T.; Westerheide, N.; Prinz, C.; Hossain, M.S.; Vogt, J.; Langer, C.; Horstkotte, D.; Oldenburg, O. Cheyne-Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur. Heart J. 2011, 32, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, M.R.; Javaheri, S.; Ponikowski, P.; Oldenburg, O.; Augostini, R.; Goldberg, L.R.; Stellbrink, C.; Fox, H.; Schwartz, A.R.; Gupta, S.; et al. Transvenous Phrenic Nerve Stimulation for Treatment of Central Sleep Apnea: Five-Year Safety and Efficacy Outcomes. Nat. Sci. Sleep 2021, 13, 515–526. [Google Scholar] [CrossRef]
- Fox, H.; Oldenburg, O.; Javaheri, S.; Ponikowski, P.; Augostini, R.; Goldberg, L.R.; Stellbrink, C.; McKane, S.; Meyer, T.E.; Abraham, W.T.; et al. Long-term efficacy and safety of phrenic nerve stimulation for the treatment of central sleep apnea. Sleep 2019, 42, zsz158. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Before PS-Matching | After PS-Matching | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control n = 120 | Treated n = 120 | SMD | p-Value | Control n = 73 | Treated n = 73 | SMD | p-Value | |||
| Baseline Characteristics | Baseline Characteristics used for Propensity Score Matching | Age [years] | 66.97 ± 11.14 | 66.72 ± 10.20 | −0.02 | 0.86 | 67.67 ± 10.78 | 67.2 ± 10.10 | −0.05 | 0.79 |
| Genderfemale [n (%)] | 21 (17.50) | 12 (10.00) | 0.25 | 0.92 | 11 (15.06) | 8 (10.95) | 0.14 | 0.46 | ||
| Height [cm] | 173.70 ± 12.49 | 175.70 ± 8.20 | 0.24 | 0.14 | 173.73 ± 14.99 | 175.9 ± 7.92 | 0.26 | 0.28 | ||
| Weight [kg] | 85.16 ± 18.00 | 92.22 ± 18.46 | 0.38 | <0.01 | 87.63 ± 17.61 | 88.89 ± 18.42 | 0.07 | 0.67 | ||
| BMI [kg/m²] | 27.78 ± 4.93 | 29.89 ± 5.51 | 0.38 | <0.01 | 28.38 ± 4.60 | 28.57 ± 5.32 | 0.03 | 0.82 | ||
| NYHA [class] | 2.74 ± 0.54 | 2.51 ± 0.70 | −0.32 | 0.01 | 2.63 ± 0.57 | 2.69 ± 0.57 | 0.10 | 0.47 | ||
| II [n (%)] | 28 (25.83) | 36 (40.00) | - | - | 24 (3288) | 20 (27.40) | ||||
| III [n (%)] | 86 (71.66) | 70 (58.33) | - | - | 49 (67.12) | 52 (71.23) | ||||
| IV [n (%)] | 3 (2.50) | 2 (1.66) | - | - | 0 (0.00) | 1 (1.37) | ||||
| Rhythm baseline | 1.93 ± 1.18 | 1.86 ± 1.24 | −0.05 | 0.67 | 1.9 ± 1.16 | 1.91 ± 1.28 | 0.01 | 0.95 | ||
| (I) SR [n (%)] | 71 (59.16) | 79 (65.83) | - | - | 44 (60.27) | 47 (64.38) | ||||
| (II) AFlut [n (%)] | 2 (1.66) | 0 (0.00) | - | - | 0 (0.00) | 0 (0.00) | ||||
| (III) AFib [n (%)] | 31 (25.83) | 19 (15.83) | - | - | 21 (28.76) | 11 (15.06) | ||||
| (IV) Pacemaker [n (%)] | 22 (18.33) | 16 (13.33) | - | - | 8 (10.95) | 15 (20.54) | ||||
| History of CPR [n (%)] | 2 (1.66) | 2 (1.66) | 0.00 | 1.00 | 1 (1.36) | 2 (2.73) | 0.11 | 0.56 | ||
| Hypertension [n (%)] | 72 (60.00) | 85 (70.83) | 0.24 | 0.08 | 46 (63.01) | 51 (69.86) | 0.15 | 0.38 | ||
| CAD [n (%)] | 86 (71.66) | 62 (51.66) | −0.40 | <0.01 | 47 (64.38) | 49 (67.12) | 0.05 | 0.73 | ||
| DCM [n (%)] | 28 (23.33) | 56 (46.66) | 0.47 | 0.00 | 22 (30.13) | 23 (31.50) | 0.03 | 0.86 | ||
| HCM [n (%)] | 4 (3.33) | 3 (2.50) | −0.05 | 0.70 | 1 (1.36) | 1 (1.36) | 0.00 | 1.00 | ||
| other CM [n (%)] | 11 (9.16) | 6 (5.00) | −0.19 | 0.21 | 7 (9.58) | 5 (6.84) | −0.13 | 0.55 | ||
| Diabetes [n (%)] | 42 (35.00) | 56 (46.66) | 0.23 | 0.07 | 24 (32.87) | 28 (38.35) | 0.11 | 0.49 | ||
| CKD [n (%)] | 59 (49.16) | 64 (53.33) | 0.08 | 0.52 | 38 (52.05) | 42 (57.53) | 0.11 | 0.51 | ||
| CKD [stage] | 1.21 ± 1.35 | 1.34 ± 1.38 | 0.09 | 0.48 | 1.26 ± 1.32 | 1.46 ± 1.41 | 0.15 | 0.37 | ||
| 0 [n (%)] | 62 (51.66) | 56 (46.66) | - | - | 35 (47.94) | 31 (42.46) | - | - | ||
| 1 [n (%)] | 2 (1.66) | 3 (2.50) | - | - | 2 (2.73) | 2 (2.73) | - | - | ||
| 2 [n (%)] | 28 (23.33) | 33 (27.5) | - | - | 21 (28.76) | 21 (28.76) | - | - | ||
| 3 [n (%)] | 24 (20.00) | 20 (16.66) | - | - | 12 (16.43) | 13 (17.80) | - | - | ||
| 4 [n (%)] | 4 (3.33) | 8 (6.66) | - | - | 3 (4.10) | 6 (8.21) | - | - | ||
| Dialysis [n (%)] | 3 (2.50) | 5 (4.16) | 0.08 | 0.47 | 3 (4.10) | 4 (5.47) | 0.07 | 0.70 | ||
| COPD [n (%)] | 10 (8.33) | 14 (11.66) | 0.10 | 0.39 | 9 (12.32) | 10 (13.69) | 0.04 | 0.81 | ||
| Device | 2.3 ± 0.89 | 2.46 ± 0.83 | 0.20 | 0.14 | 2.41 ± 0.85 | 2.3 ± 0.91 | −0.13 | 0.45 | ||
| (0) no [n (%)] | 2 (1.66) | 0 (0.00) | - | - | 1 (1.36) | 0 (0.00) | - | - | ||
| (I) 1-chamber ICD [n (%)] | 29 (24.16) | 26 (21.66) | - | - | 14 (19.17) | 22 (30.13) | - | - | ||
| (II) 2-chamber ICD [n (%)] | 20 (16.66) | 12 (10.00) | - | - | 12 (16.43) | 7 (9.58) | - | - | ||
| (III) CRT [n (%)] | 69 (57.50) | 82 (68.33) | - | - | 46 (63.01) | 44 (60.27) | - | - | ||
| ACE/AT1-Blocker [n (%)] | 111 (92.50) | 112 (93.33) | 0.03 | 0.80 | 64 (87.67) | 68 (93.15) | 0.22 | 0.26 | ||
| Diuretics [n (%)] | 110 (91.66) | 107 (89.16) | −0.08 | 0.51 | 65 (89.04) | 67 (91.78) | 0.09 | 0.57 | ||
| Beta Blocker [n (%)] | 111 (92.50) | 116 (96.66) | 0.23 | 0.15 | 69 (90.00) | 69 (94.52) | 0.00 | 1.00 | ||
| Digitalis [n (%)] | 11 (9.16) | 12 (10.00) | 0.03 | 0.83 | 7 (9.58) | 3 (4.10) | −0.18 | 0.19 | ||
| Amiodarone [n (%)] | 49 (40.83) | 42 (35.00) | −0.12 | 0.35 | 29 (39.72) | 28 (38.35) | −0.03 | 0.87 | ||
| Sotalol [n (%)] | 4 (3.33) | 5 (4.16) | 0.04 | 0.73 | 3 (4.10) | 3 (4.10) | 0.00 | >0.99 | ||
| other antiarrhythmic medication [n (%)] | 13 (10.83) | 6 (5.00) | −0.27 | 0.09 | 5 (6.84) | 6 (8.21) | 0.06 | 0.75 | ||
| MRA [n (%)] | 84 (70.00) | 98 (81.66) | 0.30 | 0.04 | 57 (78.08) | 55 (75.34) | −0.07 | 0.70 | ||
| LVEF [%] | 31.09 ± 9.94 | 30.81 ± 9.69 | −0.03 | 0.92 | 30.69 ± 9.94 | 30.04 ± 9.23 | −0.07 | 0.68 | ||
| SDB | ||||||||||
| CSA [n (%)] | 51 (42.50) | 47 (39.16) | - | - | 31 (42.46) | 30 (41.09) | - | - | ||
| OSA [n (%)] | 33 (27.50) | 41 (34.16) | - | - | 19 (26.02) | 23 (31.50) | - | - | ||
| Mixed [n (%)] | 36 (30.00) | 32 (26.67) | - | - | 23 (31.50) | 20 (27.40) | - | - | ||
| Variable | PS Matched Cohort | ||||
|---|---|---|---|---|---|
| Control (n = 73) | Treated (n = 73) | 95%-CI | p-Value | ||
| Pacemaker | HR min (PG) [/s] | 48.61 ± 10.58 | 55.80 ± 12.85 | −11.26; −3.13 | <0.01 |
| HR max (PG) [/s] | 92.04 ± 23.24 | 89.66 ± 24.39 | −5.80; 10.56 | 0.57 | |
| HR mean (PG) [/s] | 64.77 ± 9.14 | 65.23 ± 10.73 | −3.90; 2.97 | 0.79 | |
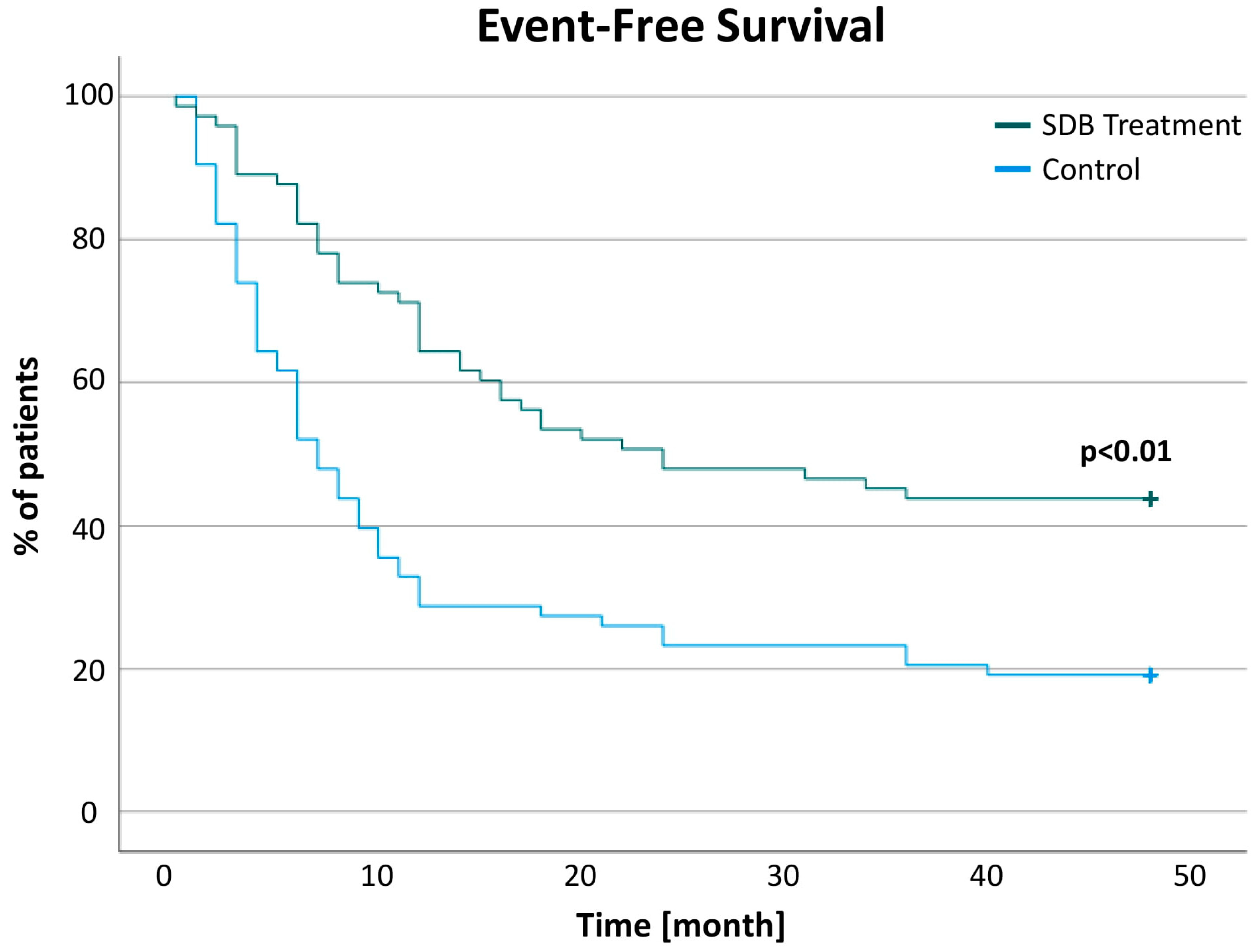
| Time to first rhythmic event since Embletta [month] | 8.06 ± 8.63 | 13.02 ± 10.94 | −8.83; −1.09 | 0.01 | |
| Pacemaker Queries (2010–2014) | 9.45 ± 5.12 | 11.06 ± 6.15 | −3.47; 0.23 | 0.09 | |
| Arrhythmic events 1 year before SDB treatment | 8.72 ± 16.51 | 18.73 ± 42.44 | −20.89; 0.85 | 0.07 | |
| VT events 1 year before SDB treatment | 1.97 ± 4.56 | 3.04 ± 8.65 | −3.37; 1.21 | 0.36 | |
| ATP events 1 year before SDB treatment | 1.50 ± 4.29 | 2.60 ± 8.21 | −3.28; 1.06 | 0.31 | |
| Defibrillation events 1 year before SDB treatment | 0.19 ± 0.86 | 0.59 ± 2.06 | −0.94; 0.13 | 0.14 | |
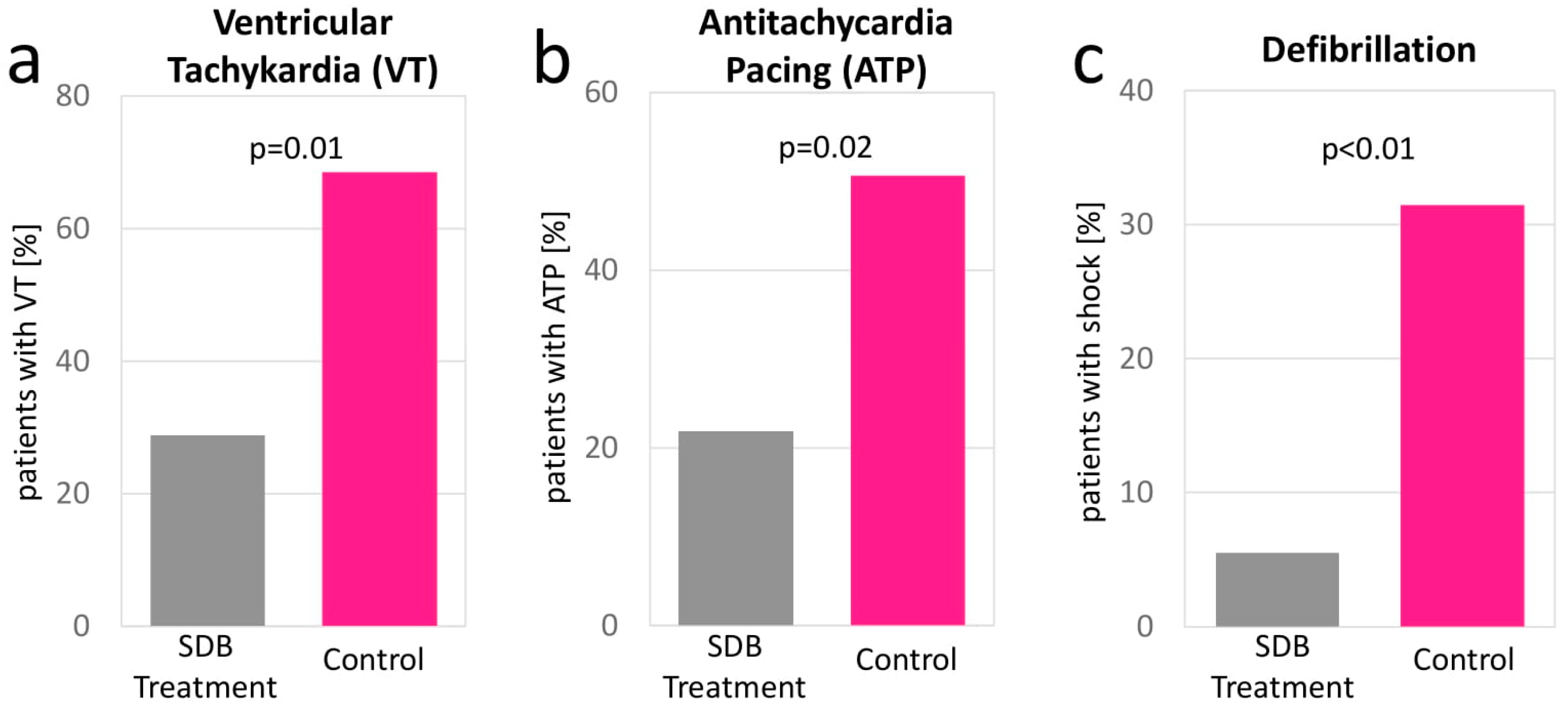
| VT events 1 year after SDB treatment | 3.78 ± 9.85 | 0.50 ± 1.62 | 0.94; 5.60 | 0.01 | |
| ATP events 1 year after SDB treatment | 3.02 ± 9.17 | 0.40 ± 1.43 | 0.45; 4.78 | 0.02 | |
| Defibrillation events 1 year after SDB treatment | 0.53 ± 1.30 | 0.05 ± 0.37 | 0.16; 0.79 | <0.01 | |
| AHI/h | 31.80 ± 14.55 | 37.60 ± 16.56 | −10.90; −0.70 | 0.03 | |
| ODI/h | 29.22 ± 15.54 | 35.56 ± 16.64 | −12.45; −0.25 | 0.04 | |
| AI/h | 17.97 ± 14.34 | 19.78 ± 18.44 | −8.10; 4.47 | 0.57 | |
| Hypopnea Duration (mean) [s] | 52.39 ± 24.38 | 67.37 ± 34.09 | −24.85; −5.11 | <0.01 | |
| Apnea Duration (max) [s] | 42.42 ± 18.65 | 47.34 ± 24.9 | −12.29; 2.45 | 0.19 | |
| Mean SaO2 (%) | 92.42 ± 2.46 | 91.76 ± 2.90 | −0.23; 1.53 | 0.14 | |
| Lowest SaO2 (%) | 80.23 ± 7.39 | 79.13 ± 10.17 | −1.82; 4.00 | 0.46 | |
| NT-proBNP [pg/mL] | 4712.04 ± 7423.21 | 3480.93 ± 4448.61 | −1598.1; 4060.3 | 0.39 | |
| PS-Matched Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Control | SDB Treatment | ||||||
| Baseline | 1 Year | 95%-CI | p-Value | Baseline | 1 Year | 95%-CI | p-Value | |
| Arrhythmic events | 8.72 ± 16.51 | 31.66 ± 103.92 | −46.92; 1.02 | 0.06 | 18.73 ± 42.44 | 6.56 ± 17.99 | 5.08; 19.26 | <0.01 |
| VT events | 1.97 ± 4.56 | 3.83 ± 9.91 | −4.17; 0.43 | 0.11 | 3.04 ± 8.65 | 0.52 ± 1.64 | 0.40; 4.63 | 0.02 |
| ATP events | 1.50 ± 4.29 | 3.06 ± 9.23 | −3.65; 0.50 | 0.14 | 2.60 ± 8.21 | 0.42 ± 1.44 | 0.20; 4.16 | 0.03 |
| Defibrillation events | 0.19 ± 0.86 | 0.54 ± 1.31 | −0.71; 0.01 | 0.06 | 0.59 ± 2.06 | 0.05 ± 0.37 | 0.03; 1.04 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerçek, M.; Gerçek, M.; Alzein, K.; Sciacca, V.; Sohns, C.; Sommer, P.; Rudolph, V.; Fox, H. Impact of Sleep-Disordered Breathing Treatment on Ventricular Tachycardia in Patients with Heart Failure. J. Clin. Med. 2022, 11, 4567. https://doi.org/10.3390/jcm11154567
Gerçek M, Gerçek M, Alzein K, Sciacca V, Sohns C, Sommer P, Rudolph V, Fox H. Impact of Sleep-Disordered Breathing Treatment on Ventricular Tachycardia in Patients with Heart Failure. Journal of Clinical Medicine. 2022; 11(15):4567. https://doi.org/10.3390/jcm11154567
Chicago/Turabian StyleGerçek, Muhammed, Mustafa Gerçek, Kanjo Alzein, Vanessa Sciacca, Christian Sohns, Philipp Sommer, Volker Rudolph, and Henrik Fox. 2022. "Impact of Sleep-Disordered Breathing Treatment on Ventricular Tachycardia in Patients with Heart Failure" Journal of Clinical Medicine 11, no. 15: 4567. https://doi.org/10.3390/jcm11154567
APA StyleGerçek, M., Gerçek, M., Alzein, K., Sciacca, V., Sohns, C., Sommer, P., Rudolph, V., & Fox, H. (2022). Impact of Sleep-Disordered Breathing Treatment on Ventricular Tachycardia in Patients with Heart Failure. Journal of Clinical Medicine, 11(15), 4567. https://doi.org/10.3390/jcm11154567







