1. Introduction
Tissue dissemination can occur during the process of morcellation. The last decade has seen many controversies concerning the method of morcellation, to answer the question whether the dissemination carries any significance or, on the other hand, if it is safe to use an in-bag containment system for morcellation. Handling larger size of myoma specimen with a bag may be challenging to many surgeons. Therefore, this video article’s objective is to demonstrate a step-by-step approach to contained multiple leiomyoma extirpation by in-bag laparoscopic electronic power morcellation. This is the largest organ-saving and fertility-sparing minimally invasive myomectomy, based on total weight, published in the world literature.
The main concern of morcellation is related with the risk of sarcoma and postoperative parasitic leyomioma secondary to tissue dissemination into the abdominal cavity during the procedure [
1,
2,
3,
4]. To avoid this, containment systems have been developed. Initial experience of this technique was described since 2014 and was also shown useful in terms of avoiding spillage of tissue or cells during extraction [
5]. Especially in cases such as myomectomy and supracervical hysterectomy, the only way to extract the specimen, especially when it is larger, is dividing it into smaller pieces. In-bag morcellation has shown to be feasible [
6]. Unsuspected leiomyosarcoma removal with the in-bag morcellation technique has been documented with good outcome [
7]. The FDA-2020 final guidelines state that, when morcellation is done appropriately, the contained technique may be adequate and allowed to be performed [
8]. Parasitic leiomyomas are a problematic finding after laparoscopic electronic power morcellation when containment is not practiced [
9]. Data also refer to patients presenting with adenomyosis masses post hysterectomy by uncontained morcellation [
10]. A meta-analysis in the Cochrane database found no significant differences in postoperative outcomes between uncontained and contained morcellation [
11].
Guidelines and recommendations, suggested by the FDA, line out that a morcellator can only be used after careful patient selection [
12]. Therefore, we are performing ultrasound and color doppler velocimetry for the large fibroids followed by MRI and, if indicated, along with serum markers CA125 to evaluate clinical suspicion of malignancy. Endometrial biopsy is reserved for cases with abnormally thickened endometrium.
Although some studies showed an increase in surgical time due to the morcellation within a containment system, this is only the case during the early learning curve [
13]; improvement in necessary operation time with increasing volume of cases and experience with the contained morcellation, even when compared with different types of containment, was published [
14]. Complications associated in long-term patient outcome from tissue spilling and dissemination are avoidable even if the containment system has an increased operating time in the beginning of the implementation in the surgical work [
15]. In leiomyoma, being the second most common disease for surgery to be performed in women, the possibility of adhesions is high; therefore, the importance of concomitant adhesion prophylaxis is important in all myomectomies [
16,
17]. Although laparoscopic surgery, being the gold standard in myomectomy, has diminished the incidence of adhesions, it has been demonstrated that the use of an adhesion barrier decreases this possibility significantly [
18,
19]. Here we present the step-by-step in-bag contained morcellation performed on a patient who presented with a giant myomatous uterus.
2. Materials and Methods
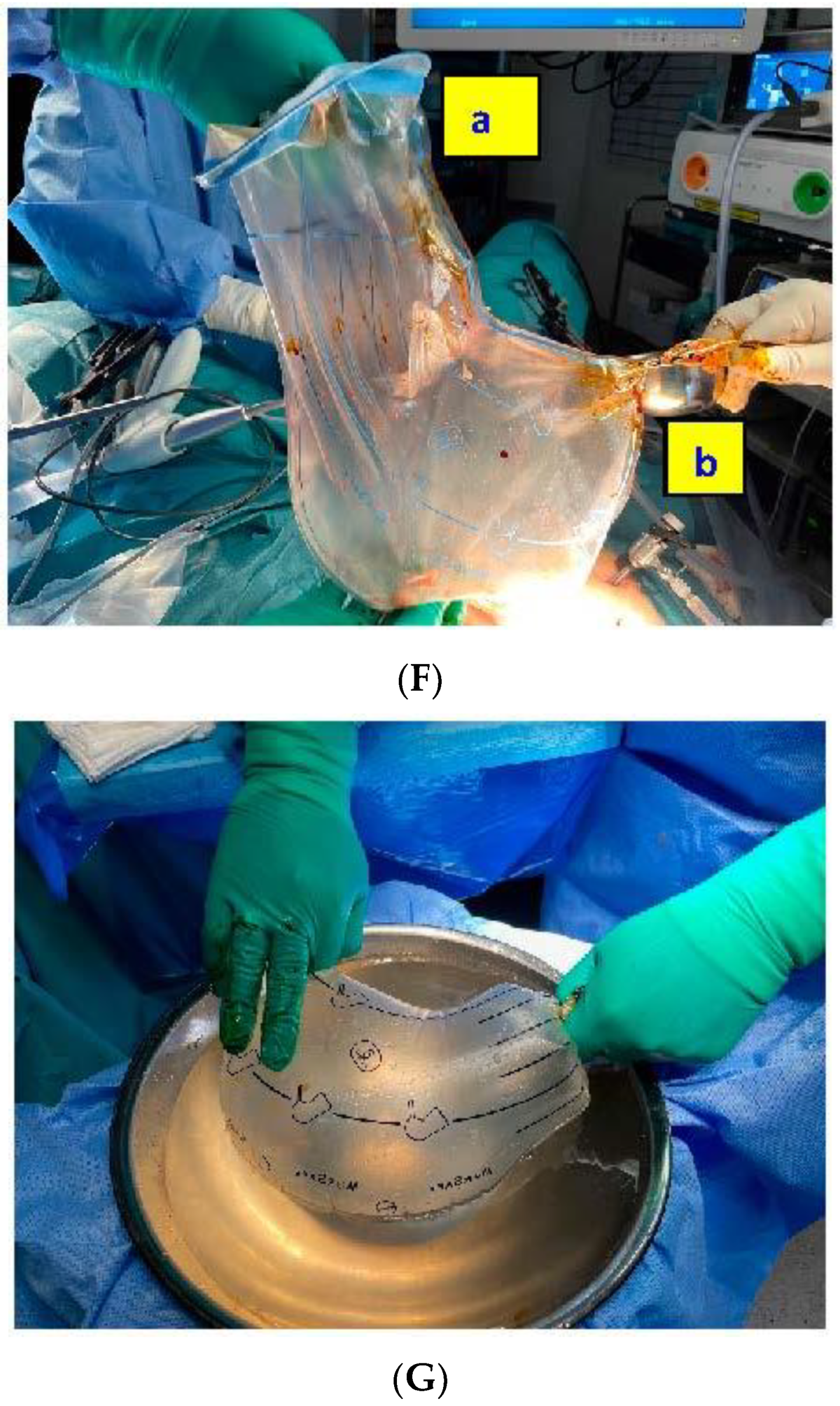
We produced a technical video demonstrating in step-by-step fashion a case of laparoscopic multiple myomectomy, utilizing contained in-bag electronic power morcellation. The MRI assessment described a 36-week gravid uteri of clinical size. The entire surgery included multiple leiomyomata enucleation, contained in-bag electronic power morcellation, uterine reconstruction and the application of an adhesion prophylactic medical product. The largest myoma was purely subserous (FIGO type 6), others were intramural myomas (FIGO types 2, 3 and 5). Vasopressin was applied pericapsular as an infiltration medium (0.3 dilution) for bleeding control. The uterine artery and the internal iliac artery were not clipped as they were not accessible due to the large size of the myoma. The uterine cavity was not entered. Myometrium layers were closed with 1-0 VLOC and serosa with 2-0 Vicryl and Monocryl. The blood loss intraoperatively was 1000 mL. The patient received three units of compatible red blood cells, as she was already anemic before the surgery. After morcellation, 1500 mL of bloody fluid was suctioned from the bags.
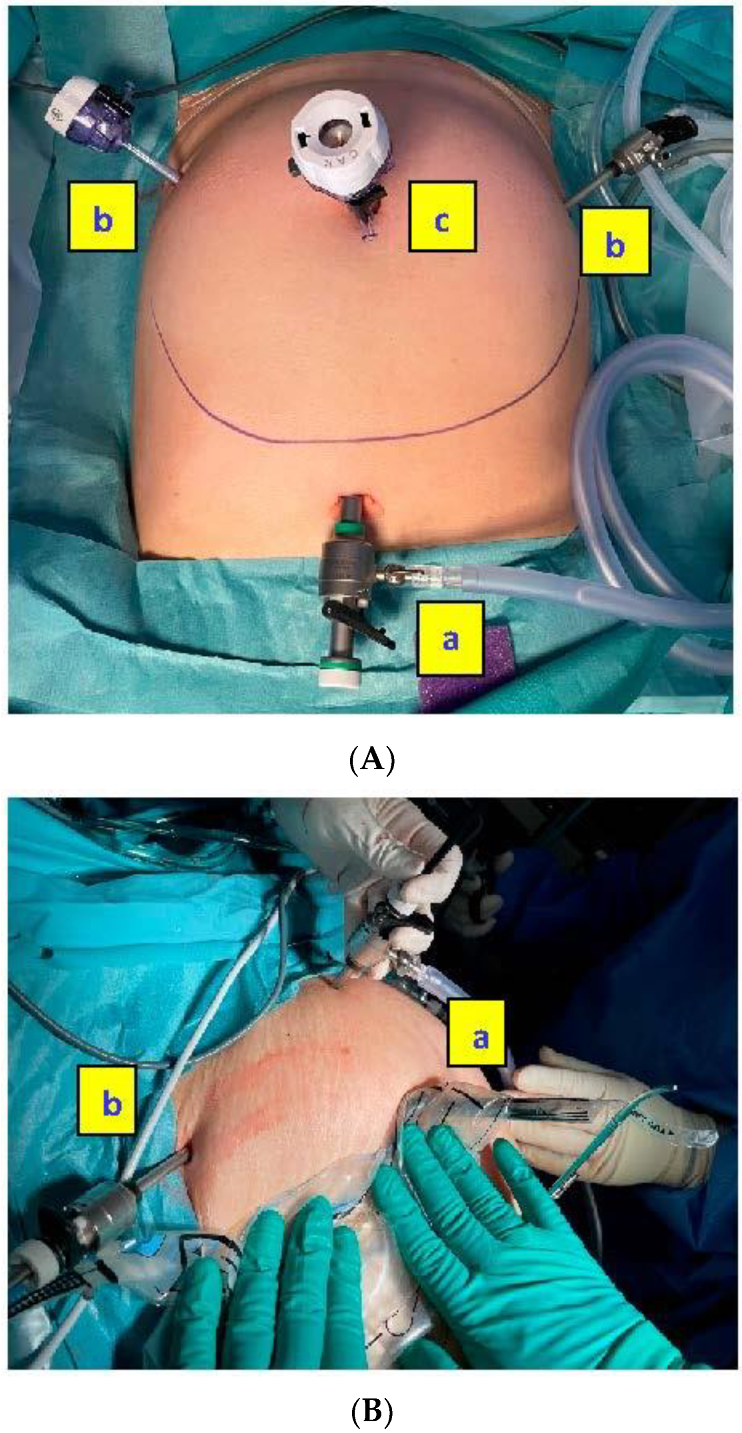
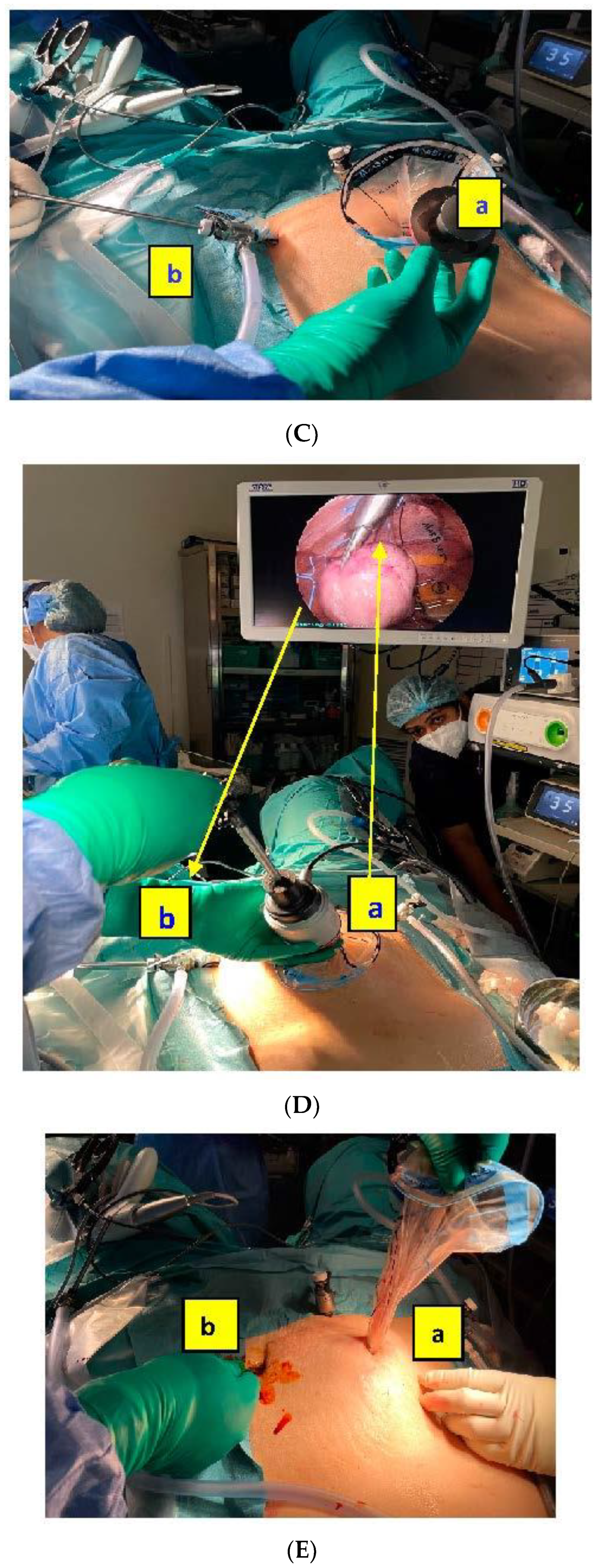
As represented in the
Figure 1, the camera port is inserted through the second sleeve situated on the end of the bag, opposite to the wider bag opening that corresponds to the morcellator port. It avoids air and potential tissue escapes and allows a direct visualization and control of the morcellation procedure.
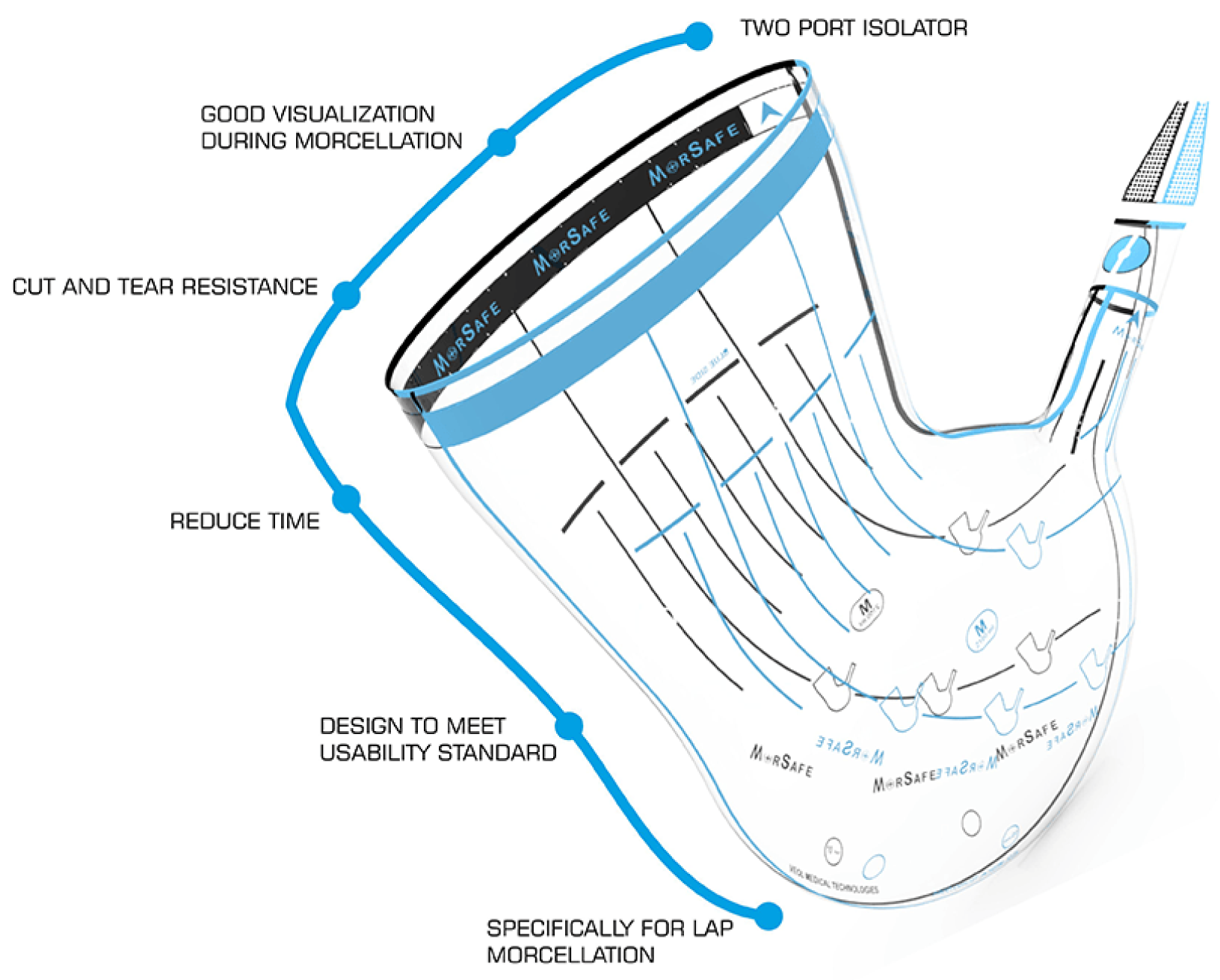
Because of the presence of a giant fibroid (20 × 30 cm), it was necessary to use two different specimen retrieval bags; Ecosac bag (Espiner Medical—Fannin U.K. Ltd., Stroud, UK) was used to retrieve the giant fibroid and the MorSafe
® bag (Veol Medical Technologies Pvt. Ltd., Mumbai, India) was used for the seven remaining tumors. These bags do not tear easily after inadvertent grasping with a non-sharp surgical tenaculum, as expressed by the manufacturers and as we observed throughout our own experience.
Figure 2 shows the main steps of the in-bag morcellation process using the MorSafe
® system, including a picture of the trocar placement that is recommended when the uteri are grown high up cranially in the abdomen (
Figure 2A).
3. Results
A 35-year-old nulliparous woman presented with a fast-growing uterus due to multiple leiomyomata, filling the entire and complete abdomen. Desiring pregnancy, in previous consultation at another center, the patient had refused hysterectomy, laparotomy, or non-contained electronic power morcellation, in fear of sarcoma tumor spread. The complete surgical procedure included multiple leiomyoma enucleation, contained in-bag electronic power morcellation, uterine reconstruction, as well as, the application of an adhesion prophylactic medical product (4DryField PH™; PlantTec GmBH, Lüneburg, Germany). Postsurgical peritoneal-washing cytology showed no tissue spillage and the bags showed any puncture at the post-usage insufflation and submersible test.
The patient’s intraoperative and postoperative course was uneventful and without complication. The postoperative pain was adequately controlled with diclofenac and paracetamol parenteral every eight hours until discharge from the hospital, continuing with the same therapy at home, orally, until the fifth postoperative day. The intraperitoneal drain was removed on the first postoperative day. Due to normal food intake and bowel function, the patient was discharged on the second postoperative day. The patient is a medical professional and returned for duty after 14 postoperative days.
The total removed specimen weight was 4781 g and the histopathological examination showed an atypical or bizarre leiomyoma, exhibiting foci of cellular atypia without evidence of tumor cell necrosis or malignancy. These abnormal cell collections are benign, but are risk for cancer, therefore close-follow up by means of MRI with contrast was suggested by the oncological board to be performed after three to six months; if normal, further ultrasound controls were recommended every six months over two years.
4. Discussion
Laparoscopic myomectomy could be a surgical challenge, especially when surgeons are removing big surgical specimens, which produce a larger amount of debris, requiring a time-consuming clearance after non-contained morcellation. Contained morcellation has given the opportunity for surgeons to increase the level of surgical standards in terms of safety, concerning the spillage of tissue, which can occur during and after the tumor excision [
20,
21]. Moreover, it is possible to perform the vaginal extraction of the specimen in a containment system, even without morcellation [
22]. According to the case here described and to the larger in-bag morcellation study published by us until now [
15], we observed effective and safe implementation of in-bag morcellation in all cases, irrespective of patient weight or specimen size. Neither the thinnest (44.0 Kg) nor the heaviest woman (127.6 Kg) presented complications during surgery or bag manipulation and it was possible to remove big surgical specimens up to 2805 g (mean 435.5 g) without technical problems.
Besides a careful examination of the patient, other factors are important to consider when opting for the minimally-invasive approach to treat big uterine tumors, like surgeon- and institute experience, equipment availability and related costs. Otherwise, surgeons would choose an open procedure for big fibroids. With this video-article, we show that laparoscopic in-bag morcellation is a feasible option even in cases of giant fibroids. We encourage endoscopic surgeons to continue the proper learning curve to perform safely this type of procedure.
It is a known fact that morcellation of an undetected sarcoma can worsen the prognosis of the patient. In 2014 the FDA reported that the incidence of unsuspected uterine sarcoma is 1:352 for any type of sarcoma and 1:498 for leiomyosarcoma [
1]. Thereafter, several studies report different incidences after hysterectomy, 0.056% (1:1784), 0.14% (1:700), 0.49% (1 in 204) [
19]. The overall pooled risk was reported to be higher 0.15% (1 in 650) after hysterectomy than after myomectomy, 0.08% (1:1306) [
19]. The risk appears to be age related with one study demonstrating a lower risk in patients under 45 years of age [
2,
3,
4]. Other long-term implications like parasitic adenomyosis or leiomyomatosis are infrequently reported. Currently, there are no available methods to preoperatively diagnose a potential malignancy in leiomyoma [
23,
24]. Therefore, a cautious manipulation of the tumor, bags and instruments minimizes the risk of intraoperative spillage, especially in cases of larger myomata. Moreover, there is a lack of evidence regarding how much of a bag puncture would provoke tissue spillage, as well as the meaning of micro-spillage occurring in inadvertent breach of bag integrity [
25]. In the presented case no tissue spillage or bag puncture occurred.
Irrespective of the low-risk of malignancy, careful presurgical image-based assessment, bag examination and the histological examination of all fibroids and peritoneal fluid samples (taken at surgery initiation and at end) should be the standard. The follow-up schedule should be individualized according to the histologic findings; re-examination is mandatory in case of suspected myoma recurrence or abnormal appearance of the peritoneal cavity at ultrasound.
On the other hand, and considering the size of this patient’s tumors and the fact that myomectomy is associated with profuse capillary bleeding and postoperative adhesions, we chose a barrier with dual properties of hemostasis and adhesion prophylaxis that can be applied evenly on all the serosal scars. The application of an adhesion prophylaxis agent is a strategy that could significantly optimize the surgical outcomes after myomectomy [
26,
27].
5. Conclusions
Contained in-bag electronic power morcellation, in the hands of an experienced and technically proficient minimally-invasive gynecologic surgeon, enables a purely laparoscopic approach for the treatment of a gigantic leiomyoma mass without enhanced risk of spillage compared to laparotomy. Postoperative complications by subsequent adhesion formation, with possible reduction of quality of life, fertility or even leading to bowel obstruction, can be reduced by intra-laparoscopic application of adhesion prophylactic agents.
Author Contributions
Conceptualization and methodology, R.L.D.W. and R.D.; formal analysis and data curation, R.D., J.S.J. and L.A.T.-d.l.R.; writing—original draft preparation, R.D., J.S.J. and L.A.T.-d.l.R.; writing—review and editing, All authors; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Dubai-London Clinic & Specialty Hospital (code: DLCEC15032022).
Informed Consent Statement
Because of the retrospective design of this study, patient consent was waived. The aforementioned Ethics Committee approved the data analysis.
Conflicts of Interest
Rajesh Devassy, Rudy Leon De Wilde and Harald Krentel have received previously financial grants from Plantech GmbH in adhesiolysis research.
References
- Quantitative Assessment of the Prevalence of Unsuspected Uterine Sarcoma in Women Undergoing Treatment of Uterine Fibroids: Summary and Key Yfindings. Available online: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/ObstetricianandGynecologyDevices/UCM404148.pdf (accessed on 25 June 2022).
- Tanos, V.; Brölmann, H.; DeWilde, R.L.; O’Donovan, P.; Campo, R. Myoma morcellation and leiomyosarcoma panic. Gynecol. Surg. 2015, 12, 17–19. [Google Scholar] [CrossRef] [Green Version]
- Bojahr, B.; De Wilde, R.L.; Tchartchian, G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch. Gynecol. Obstet. 2015, 292, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brölmann, H.; Tanos, V.; Grimbizis, G.; Ind, T.; Philips, K.; van den Bosch, T.; Sawalhe, S.; van den Haak, L.; Jansen, F.-W.; Pijnenborg, J.; et al. European Society of Gynaecological Endoscopy (ESGE) steering committee on fibroid morcellation. Options on fibroid morcellation: A literature review. Gynecol. Surg. 2015, 12, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenna, J.B.; Kanade, T.; Choi, S.; Tsai, B.P.; Rosen, D.M.; Cario, G.M.; Chou, D. The Sydney Contained in Bag Morcellation technique. J. Minim. Invasive Gynecol. 2014, 21, 984–985. [Google Scholar] [CrossRef]
- Einarsson, J.I.; Cohen, S.L.; Fuchs, N.; Wang, K.C. In-bag morcellation. J. Minim. Invasive Gynecol. 2014, 21, 951–953. [Google Scholar] [CrossRef]
- Krentel, H.; Wilde, R.L. Laparoscopic Supracervical Hysterectomy with In-Bag Morcellation in Very Large Uterus. Case Rep. Med. 2017, 2017, 9410571. [Google Scholar] [CrossRef] [Green Version]
- Boruta, D.M.; Shibley, T. Power Morcellation of Unsuspected High-grade Leiomyosarcoma Within an Inflated Containment Bag: 2-Year Follow-up. J. Minim. Invasive Gynecol. 2016, 23, 1009–1011. [Google Scholar] [CrossRef]
- UPDATE: Perform Only Contained Morcellation When Laparoscopic Power Morcellation Is Appropriate: FDA Safety Communication. 29 December 2020. Available online: https://www.fda.gov/medical-devices/safety-communications/update-perform-only-contained-morcellation-when-laparoscopic-power-morcellation-appropriate-fda (accessed on 25 June 2022).
- Takeda, A.; Mori, M.; Sakai, K.; Mitsui, T.; Nakamura, H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: Report of a case and review of the literature. J. Minim. Invasive Gynecol. 2007, 14, 770–775. [Google Scholar] [CrossRef]
- Donnez, O.; Squifflet, J.; Leconte, I.; Jadoul, P.; Donnez, J. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J. Minim. Invasive Gynecol. 2007, 14, 156–160. [Google Scholar] [CrossRef]
- Zullo, F.; Venturella, R.; Raffone, A.; Saccone, G. In-bag manual versus uncontained power morcellation for laparoscopic myomectomy. Cochrane Database Syst. Rev. 2020, 5, CD013352. [Google Scholar] [CrossRef]
- Murji, A.; Scott, S.; Singh, S.S.; Bougie, O.; Leyland, N.; Laberge, P.Y.; Vilos, G.A. No. 371-Morcellation During Gynaecologic Surgery: Its Uses, Complications, and Risks of Unsuspected Malignancy. J. Obstet. Gynaecol. Can. 2019, 41, 116–126. [Google Scholar] [CrossRef]
- Vargas, M.V.; Cohen, S.L.; Fuchs-Weizman, N.; Wang, K.C.; Manoucheri, E.; Vitonis, A.F.; Einarsson, J.I. Open power morcellation versus contained power morcellation within an insufflated isolation bag: Comparison of perioperative outcomes. J. Minim. Invasive Gynecol. 2015, 22, 433–438. [Google Scholar] [CrossRef]
- Devassy, R.; Cezar, C.; Krentel, H.; Verhoeven, H.C.; Devassy, R.; de Wilde, M.S.; Torres-de la Roche, L.A.; de Wilde, R.L. Feasibility of myomatous tissue extraction in laparoscopic surgery by contained in-bag morcellation: A retrospective single arm study. Int. J. Surg. 2019, 62, 22–27. [Google Scholar] [CrossRef]
- Thang, N.M.; Thien, D.H.; Huyen Anh, N.T.; Cuong, T.D. Leiomyomatosis peritonealis dissemianata five years after laparoscopic uterine myomectomy: A case report. Ann. Med. Surg. 2021, 66, 102377. [Google Scholar] [CrossRef]
- Torres-De La Roche, L.A.; Campo, R.; Devassy, R.; Di Spiezio Sardo, A.; Hooker, A.; Koninckx, P.; Urman, B.; Wallwiener, M.; De Wilde, R.L. Adhesions and Anti-Adhesion Systems Highlights. Facts Views Vis. Obgyn 2019, 11, 137–149. [Google Scholar]
- Herrmann, A.; Torres-de la Roche, L.A.; Krentel, H.; Cezar, C.; de Wilde, M.S.; Devassy, R.; De Wilde, R.L. Adhesions after Laparoscopic Myomectomy: Incidence, Risk Factors, Complications, and Prevention. Gynecol. Minim. Invasive Ther. 2020, 9, 190–197. [Google Scholar] [CrossRef]
- Ahmad, G.; O’Flynn, H.; Hindocha, A.; Watson, A. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst. Rev. 2015, 2015, CD000475. [Google Scholar] [CrossRef]
- De Wilde, R.L.; Bakkum, E.A.; Brölmann, H.; Crowe, A.; Koninckx, P.; Korell, M.; Lundorff, P.; Pistofidis, G.; Tchartchian, G.; Trew, G.; et al. Consensus recommendations on adhesions (version 2014) for the ESGE Adhesions Research Working Group (European Society for Gynecological Endoscopy): An expert opinion. Arch. Gynecol. Obstet. 2014, 290, 581–582. [Google Scholar] [CrossRef]
- Rimbach, S.; Holzknecht, A.; Nemes, C.; Offner, F.; Craina, M. A new in-bag system to reduce the risk of tissue morcellation: Development and experimental evaluation during laparoscopic hysterectomy. Arch. Gynecol. Obstet. 2015, 292, 1311–1320. [Google Scholar] [CrossRef]
- Srouji, S.S.; Kaser, D.J.; Gargiulo, A.R. Techniques for contained morcellation in gynecologic surgery. Fertil. Steril. 2015, 103, e34. [Google Scholar] [CrossRef]
- Kho, K.A.; Brown, D.N. Surgical Treatment of Uterine Fibroids Within a Containment System and Without Power Morcellation. Clin. Obstet. Gynecol. 2016, 59, 85–92. [Google Scholar] [CrossRef]
- Beckmann, M.W.; Juhasz-Böss, I.; Denschlag, D.; Gaß, P.; Dimpfl, T.; Harter, P.; Wallwiener, D. Surgical Methods for the Treatment of Uterine Fibroids—Risk of Uterine Sarcoma and Problems of Morcellation: Position Paper of the DGGG. Geburtshilfe Frauenheilkd. 2015, 75, 148–164. [Google Scholar] [CrossRef] [Green Version]
- Van den Haak, L.; van der Eijk, A.C.; Sandberg, E.M.; Frank, G.P.G.M.; Ansink, K.; Pelger, R.C.M.; de Kroon, C.D.; Jansen, F.W. Towards spill-free in-bag morcellation: A health failure mode and effects analysis. Surg. Endosc. 2018, 32, 4357–4362. [Google Scholar] [CrossRef] [Green Version]
- Hirschelmann, A.; Tchartchian, G.; Wallwiener, M.; Hackethal, A.; De Wilde, R.L. A review of the problematic adhesion prophylaxis in gynaecological surgery. Arch. Gynecol. Obstet. 2012, 285, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, N.; De Wilde, R.L. Reduction of adhesion formation after gynaecological adhesiolysis surgery with 4DryField PH—a retrospective, controlled study with second look laparoscopies. J. Obstet. Gynaecol. 2022, 42, 658–664. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).