Comparison between Automatic and Semiautomatic System for the 3D Echocardiographic Multiparametric Evaluation of RV Function and Dimension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Two-Dimensional Echocardiography
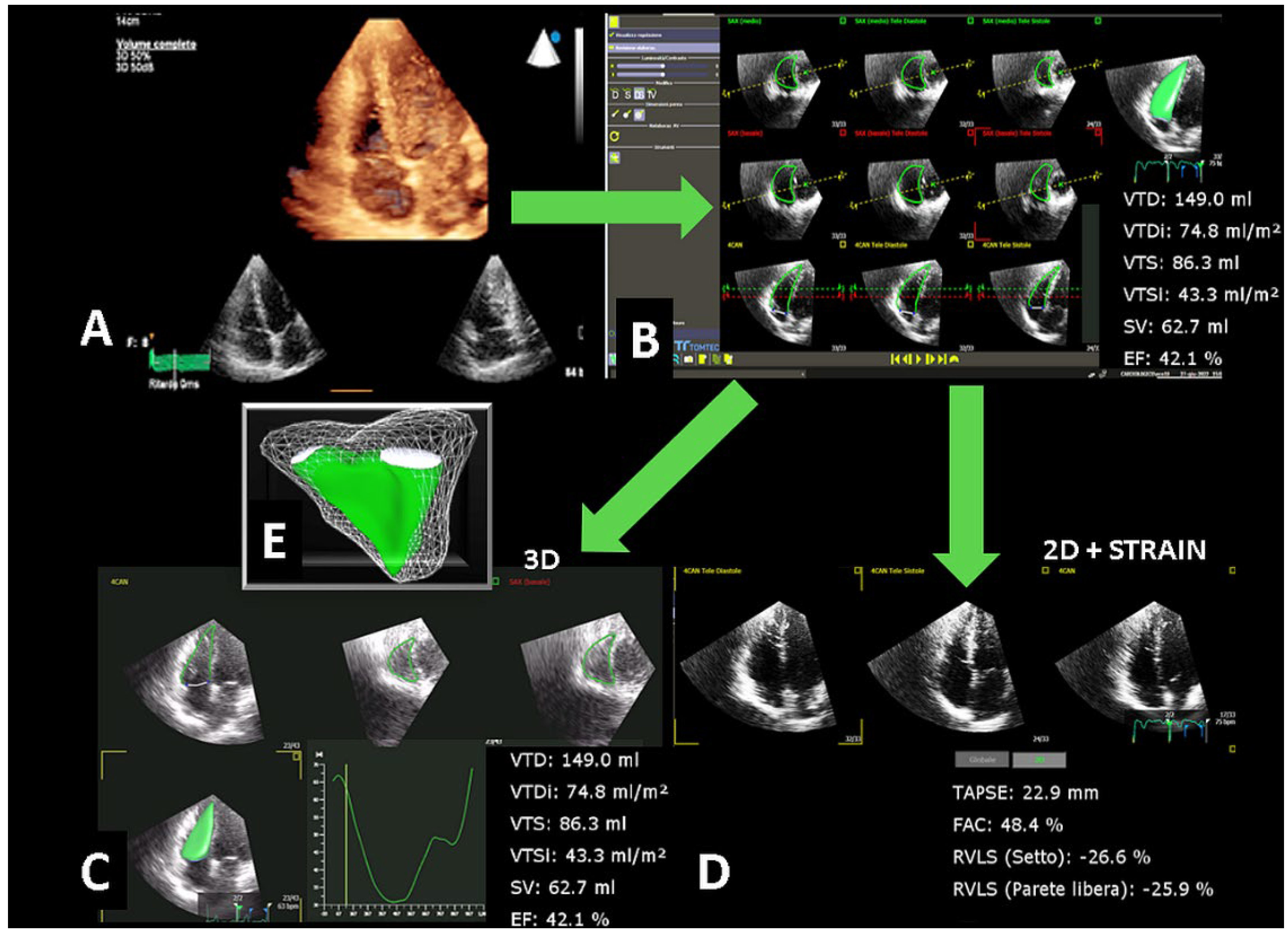
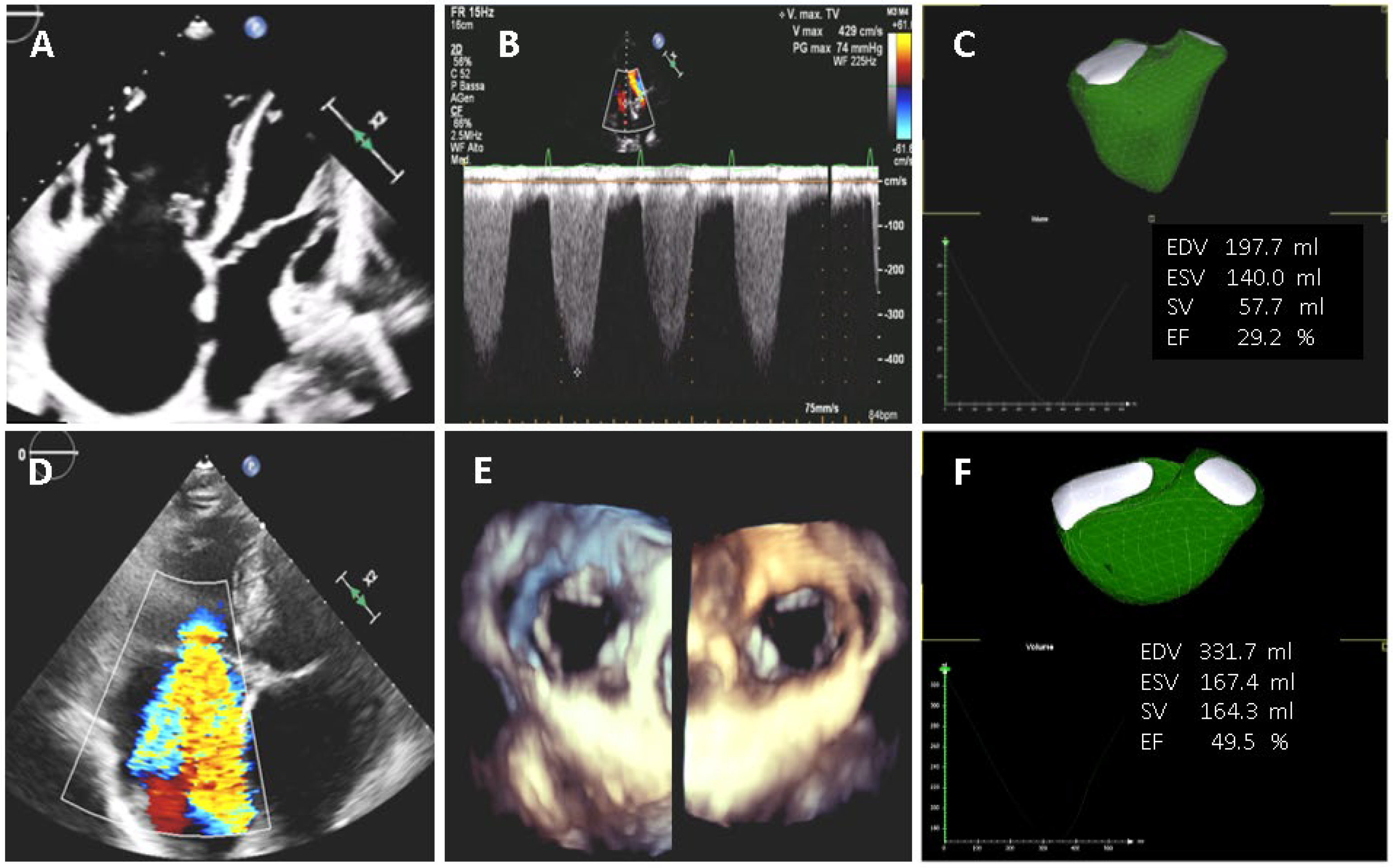
2.3. Three-Dimensional Echocardiography
2.4. Statistical Analysis
3. Results
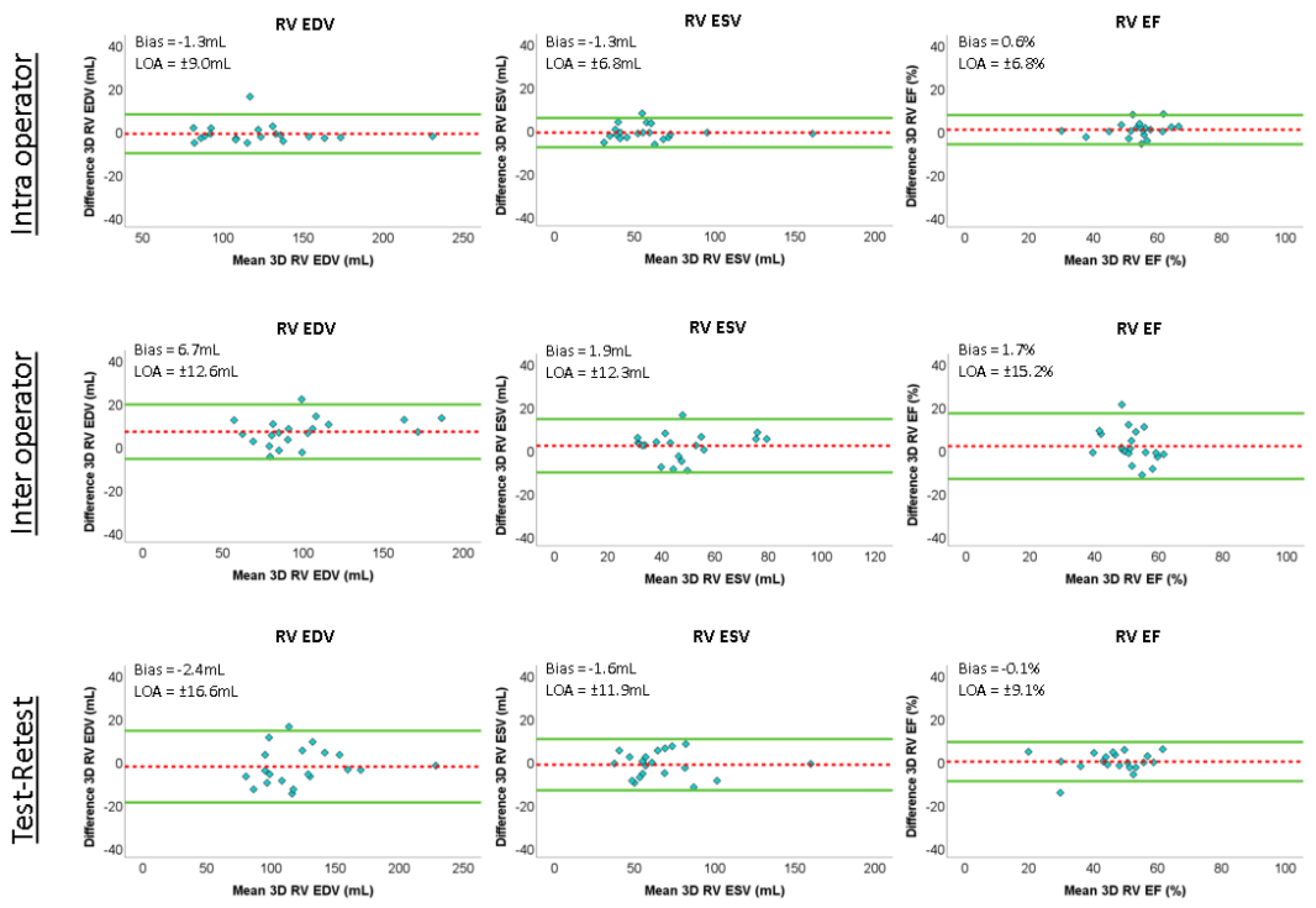
Reproducibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiota, T. 3D echocardiography: Evaluation of the right ventricle. Curr. Opin. Cardiol. 2009, 24, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, G.; Pepi, M.; Galli, C.A.; Maltagliati, A.; Celeste, F.; Muratori, M.; Rezvanieh, S.; Veglia, F. Feasibility and accuracy of a routine echocardiographic assessment of right ventricular function. Int. J. Cardiol. 2007, 115, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Addetia, K.; Miyoshi, T.; Citro, R.; Daimon, M.; Gutierrez Fajardo, P.; Kasliwal, R.R.; Kirkpatrick, J.N.; Monaghan, M.J.; Muraru, D.; Ogunyankin, K.O.; et al. Two-Dimensional Echocardiographic Right Ventricular Size and Systolic Function Measurements Stratified by Sex, Age, and Ethnicity: Results of the World Alliance of Societies of Echocardiography Study. J. Am. Soc. Echocardiogr. 2021, 34, 1148–1157.e1. [Google Scholar] [CrossRef]
- Muraru, D.; Spadotto, V.; Cecchetto, A.; Romeo, G.; Aruta, P.; Ermacora, D.; Jenei, C.; Cucchini, U.; Iliceto, S.; Badano, L.P. New speckle-tracking algorithm for right ventricular volume analysis from three-dimensional echocardiographic data sets: Validation with cardiac magnetic resonance and comparison with the previous analysis tool. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1279–1289. [Google Scholar] [CrossRef]
- Khoo, N.S.; Young, A.; Occleshaw, C.; Cowan, B.; Zeng, I.S.; Gentles, T.L. Assessments of right ventricular volume and function using three-dimensional echocardiography in older children and adults with congenital heart disease: Comparison with cardiac magnetic resonance imaging. J. Am. Soc. Echocardiogr. 2009, 22, 1279–1288. [Google Scholar] [CrossRef]
- Melenovsky, V.; Kotrc, M.; Borlaug, B.A.; Marek, T.; Kovar, J.; Malek, I.; Kautzner, J. Relationships between right ventricular function, body composition, and prognosis in advanced heart failure. J. Am. Coll. Cardiol. 2013, 62, 1660–1670. [Google Scholar] [CrossRef] [Green Version]
- Grapsa, J.; O’Regan, D.P.; Pavlopoulos, H.; Durighel, G.; Dawson, D.; Nihoyannopoulos, P. Right ventricular remodelling in pulmonary arterial hypertension with three-dimensional echocardiography: Comparison with cardiac magnetic resonance imaging. Eur. J. Echocardiogr. 2010, 11, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Tamborini, G.; Muratori, M.; Brusoni, D.; Celeste, F.; Maffessanti, F.; Caiani, E.G.; Alamanni, F.; Pepi, M. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur. J. Echocardiogr. 2009, 10, 630–634. [Google Scholar] [CrossRef] [Green Version]
- Tamborini, G.; Cefalù, C.; Celeste, F.; Fusini, L.; Garlaschè, A.; Muratori, M.; Ghulam Ali, S.; Gripari, P.; Berna, G.; Pepi, M. Multi-parametric “on board” evaluation of right ventricular function using three-dimensional echocardiography: Feasibility and comparison to traditional two-and three dimensional echocardiographic measurements. Int. J. Cardiovasc. Imaging 2019, 35, 275–284. [Google Scholar] [CrossRef]
- Genovese, D.; Rashedi, N.; Weinert, L.; Narang, A.; Addetia, K.; Patel, A.R.; Prater, D.; Gonçalves, A.; Mor-Avi, V.; Lang, R.M. Machine Learning-Based Three-Dimensional Echocardiographic Quantification of Right Ventricular Size and Function: Validation Against Cardiac Magnetic Resonance. J. Am. Soc. Echocardiogr. 2019, 32, 969–977. [Google Scholar] [CrossRef]
- Pepi, M.; Tamborini, G.; Galli, C.; Barbier, P.; Doria, E.; Berti, M.; Guazzi, M.; Fiorentini, C. A new formula for echo-Doppler estimation of right ventricular systolic pressure. J. Am. Soc. Echocardiogr. 1994, 7, 20–26. [Google Scholar] [CrossRef]
- Shimada, Y.J.; Shiota, M.; Siegel, R.J.; Shiota, T. Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: A meta-analysis study. J. Am. Soc. Echocardiogr. 2010, 23, 943–953. [Google Scholar] [CrossRef]
- Mele, D.; Pestelli, G.; Dini, F.; Dal Molin, D.; Smarazzo, V.; Trevisan, F.; Luisi, G.; Ferrari, R. Novel Echocardiographic Approach to Hemodynamic Phenotypes Predicts Outcome of Patients Hospitalized with Heart Failure. Circ. Cardiovasc. Imaging 2020, 13, e009939. [Google Scholar] [CrossRef]
- Medvedofsky, D.; Addetia, K.; Patel, A.R.; Sedlmeier, A.; Baumann, R.; Mor-Avi, V.; Lang, R.M. Novel Approach to Three-Dimensional Echocardiographic Quantification of Right Ventricular Volumes and Function from Focused Views. J. Am. Soc. Echocardiogr. 2015, 28, 1222–1231. [Google Scholar] [CrossRef]
- Sugeng, L.; Mor-Avi, V.; Weinert, L.; Niel, J.; Ebner, C.; Steringer-Mascherbauer, R.; Bartolles, R.; Baumann, R.; Schummers, G.; Lang, R.M.; et al. Multimodality comparison of quantitative volumetric analysis of the right ventricle. JACC Cardiovasc. Imaging 2010, 3, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Leibundgut, G.; Rohner, A.; Grize, L.; Bernheim, A.; Kessel-Schaefer, A.; Bremerich, J.; Zellweger, M.; Buser, P.; Handke, M. Dynamic assessment of right ventricular volumes and function by real-time three-dimensional echocardiography: A comparison study with magnetic resonance imaging in 100 adult patients. J. Am. Soc. Echocardiogr. 2010, 23, 116–126. [Google Scholar] [CrossRef]
- Tsang, W.; Salgo, I.S.; Medvedofsky, D.; Takeuchi, M.; Prater, D.; Weinert, L.; Yamat, M.; Mor-Avi, V.; Patel, A.R.; Lang, R.M. Transthoracic 3D Echocardiographic Left Heart Chamber Quantification Using an Automated Adaptive Analytics Algorithm. JACC Cardiovasc. Imaging 2016, 9, 769–782. [Google Scholar] [CrossRef]
- Medvedofsky, D.; Mor-Avi, V.; Amzulescu, M.; Fernández-Golfín, C.; Hinojar, R.; Monaghan, M.J.; Otani, K.; Reiken, J.; Takeuchi, M.; Tsang, W.; et al. Three-dimensional echocardiographic quantification of the left-heart chambers using an automated adaptive analytics algorithm: Multicentre validation study. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 47–58. [Google Scholar] [CrossRef]
- Narang, A.; Mor-Avi, V.; Prado, A.; Volpato, V.; Prater, D.; Tamborini, G.; Fusini, L.; Pepi, M.; Goyal, N.; Addetia, K.; et al. Machine learning based automated dynamic quantification of left heart chamber volumes. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 541–549. [Google Scholar] [CrossRef]
- Tamborini, G.; Piazzese, C.; Lang, R.M.; Muratori, M.; Chiorino, E.; Mapelli, M.; Fusini, L.; Ghulam Ali, S.; Gripari, P.; Pontone, G.; et al. Feasibility and Accuracy of Automated Software for Transthoracic Three-Dimensional Left Ventricular Volume and Function Analysis: Comparisons with Two-Dimensional Echocardiography, Three-Dimensional Transthoracic Manual Method, and Cardiac Magnetic Resonance Imaging. J. Am. Soc. Echocardiogr. 2017, 30, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Nakazono, A.; Salgo, I.S.; Lang, R.M.; Takeuchi, M. Three-Dimensional Echocardiographic Assessment of Left Heart Chamber Size and Function with Fully Automated Quantification Software in Patients with Atrial Fibrillation. J. Am. Soc. Echocardiogr. 2016, 29, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, G.; Marsan, N.A.; Gripari, P.; Maffessanti, F.; Brusoni, D.; Muratori, M.; Caiani, E.G.; Fiorentini, C.; Pepi, M. Reference values for right ventricular volumes and ejection fraction with real-time three-dimensional echocardiography: Evaluation in a large series of normal subjects. J. Am. Soc. Echocardiogr. 2010, 23, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Maffessanti, F.; Muraru, D.; Esposito, R.; Gripari, P.; Ermacora, D.; Santoro, C.; Tamborini, G.; Galderisi, M.; Pepi, M.; Badano, L.P. Age-, body size-, and sex-specific reference values for right ventricular volumes and ejection fraction by three-dimensional echocardiography: A multicenter echocardiographic study in 507 healthy volunteers. Circ. Cardiovasc. Imaging 2013, 6, 700–710. [Google Scholar] [CrossRef] [Green Version]
- Italiano, G.; Tamborini, G.; Fusini, L.; Mantegazza, V.; Doldi, M.; Celeste, F.; Gripari, P.; Muratori, M.; Lang, R.M.; Pepi, M. Feasibility and Accuracy of the Automated Software for Dynamic Quantification of Left Ventricular and Atrial Volumes and Function in a Large Unselected Population. J. Clin. Med. 2021, 10, 5030. [Google Scholar] [CrossRef]
- Namisaki, H.; Nabeshima, Y.; Kitano, T.; Otani, K.; Takeuchi, M. Prognostic Value of the Right Ventricular Ejection Fraction, Assessed by Fully Automated Three-Dimensional Echocardiography: A Direct Comparison of Analyses Using Right Ventricular-Focused Views versus Apical Four-Chamber Views. J. Am. Soc. Echocardiogr. 2021, 34, 117–126. [Google Scholar] [CrossRef]
- Otani, K.; Nabeshima, Y.; Kitano, T.; Takeuchi, M. Accuracy of fully automated right ventricular quantification software with 3D echocardiography: Direct comparison with cardiac magnetic resonance and semi-automated quantification software. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 787–795. [Google Scholar] [CrossRef]

| Total | Normal Subjects | Pathological Patients | |
|---|---|---|---|
| Patients (F/M) | 203 (86/117) | 122 (54/68) | 81 (31/50) |
| Age (years) | 57 ± 15 | 52 ± 14 | 65 ± 15 ** |
| Body surface area (m2) | 1.86 ± 0.22 | 1.87 ± 0.22 | 1.85 ± 0.21 |
| Left ventricular end diastolic volume (mL) | 105.3 ± 40.0 | 95.1 ± 24.5 | 120.8 ± 52.3 ** |
| Left ventricular end-systolic volume (mL) | 44.5 ± 33.2 | 36.7 ± 11.5 | 56.2 ± 48.4 ** |
| Left ventricular end diastolic volume index (mL/m2) | 56.3 ± 19.8 | 50.7 ± 10.8 | 64.8 ± 26.3 ** |
| Left ventricular end-systolic volume index (mL/m2) | 23.7 ± 17.0 | 19.5 ± 5.5 | 30.0 ± 24.9 ** |
| Left ventricular stroke volume (mL) | 61 ± 17 | 58 ± 16 | 64 ± 16 * |
| Left ventricular stroke volume index (mL/m2) | 33 ± 9 | 31 ± 8 | 35 ± 9 * |
| Left ventricular ejection fraction (%) | 60 ± 10 | 62 ± 7 | 58 ± 14 * |
| Left atrial diameter (mm) | 36.5 ± 5.9 | 35.7 ± 5.7 | 38.1 ± 6.0 * |
| Left ventricular volume (mL) | 56.1 ± 25.5 | 48.7 ± 16.8 | 67.2 ± 31.8 ** |
| Left ventricular volume index (mL/m2) | 30.3 ± 15.0 | 26.1 ± 8.8 | 36.7 ± 19.5 ** |
| Right ventricular end diastolic area (cm2) | 17.7 ± 4.6 | 17.2 ± 4.0 | 18.3 ± 5.3 |
| Right ventricular end-systolic area (cm2) | 9.2 ± 3.2 | 8.9 ± 2.5 | 9.8 ± 4.1 |
| Right ventricular fractional area change (%) | 47.6 ± 9.7 | 47.8 ± 8.6 | 47.2 ± 11.2 |
| Tricuspid annular plane systolic excursion (mm) | 23.7 ± 5.0 | 24.3 ± 4.2 | 22.8 ± 6.1 |
| Right atrial area (cm2) | 15.8 ± 7.4 | 14.2 ± 3.6 | 17.7 ± 10.1 * |
| Systolic pulmonary artery pressure (mmHg) | 30.6 ± 6.7 | 29.4 ± 3.8 | 32.4 ± 9.4 * |
| Automatic | Semi-Automatic | Bias | LOA | ICC | |
|---|---|---|---|---|---|
| Total | |||||
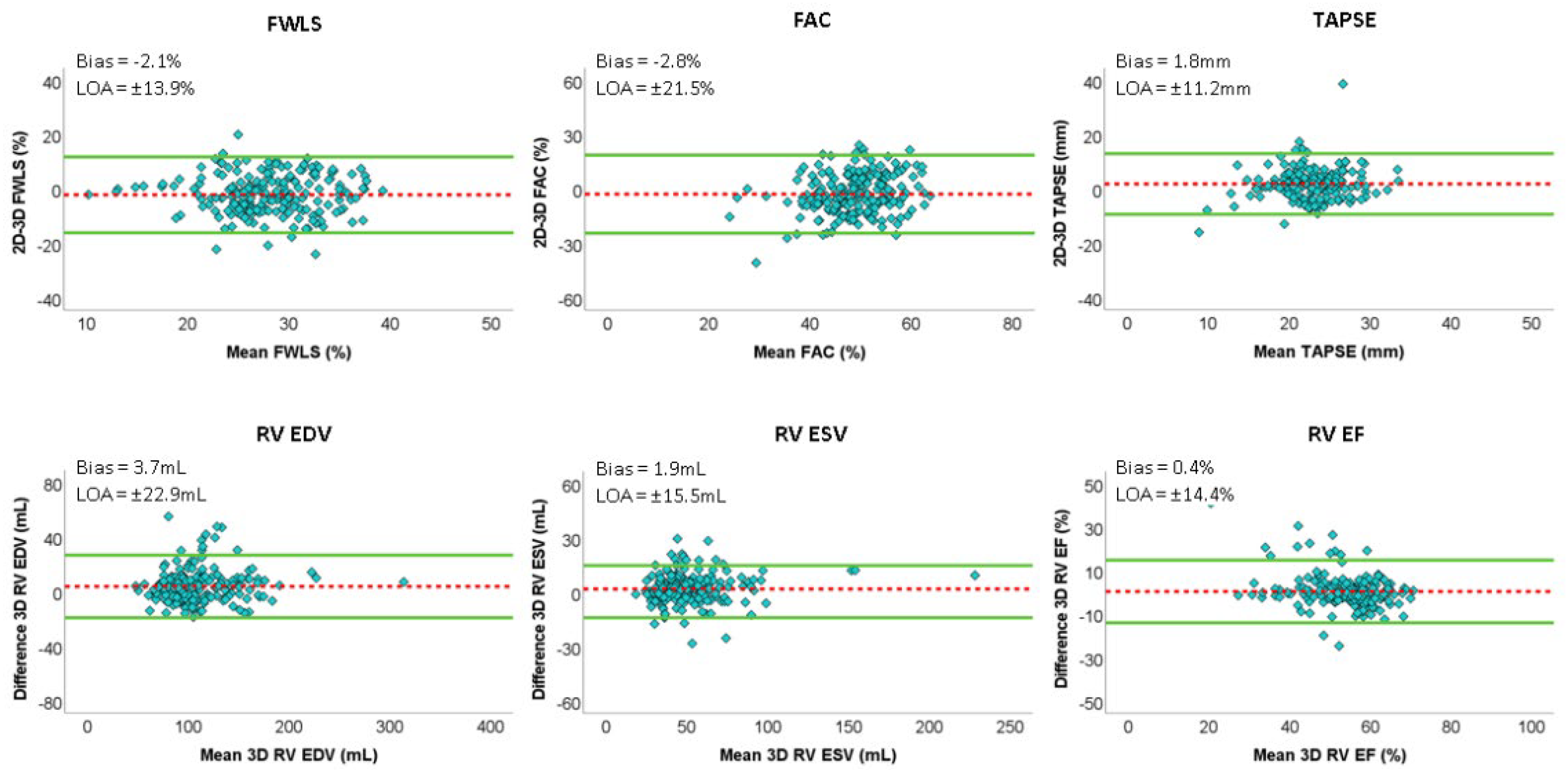
| Right ventricular end diastolic volume (mL) | 111.4 ± 34.4 | 107.6 ± 33.4 ** | 3.7 | 22.9 | 0.966 |
| Right ventricular end-systolic volume (mL) | 51.7 ± 23.6 | 50.0 ± 22.5 ** | 1.9 | 15.5 | 0.968 |
| Right ventricular ejection fraction (%) | 54 ± 8 | 54 ± 10 | 0.4 | 14.4 | 0.805 |
| Normal subjects | |||||
| Right ventricular end diastolic volume (mL) | 105.7 ± 28.5 | 104.3 ± 28.4 | 1.4 | 20.4 | 0.965 |
| Right ventricular end-systolic volume (mL) | 47.1 ± 15.9 | 45.8 ± 15.6 | 1.2 | 15.5 | 0.932 |
| Right ventricular ejection fraction (%) | 56 ± 7 | 56 ± 8 | −0.4 | 13.4 | 0.723 |
| Pathological patients | |||||
| Right ventricular end diastolic volume (mL) | 120.1 ± 40.6 | 112.8 ± 39.6 ** | 7.3 | 24.9 | 0.966 |
| Right ventricular end-systolic volume (mL) | 58.8 ± 30.7 | 56.0 ± 29.1 * | 2.8 | 15.6 | 0.98 |
| Right ventricular ejection fraction (%) | 52 ± 9 | 50 ± 12 | 1.6 | 15.6 | 0.832 |
| Automatic | 2D | Bias | LOA | ICC | |
|---|---|---|---|---|---|
| Total | |||||
| Right ventricular free wall longitudinal strain (%) | 29 ± 6 | 27 ± 6 ** | −2.1 | 13.9 | 0.479 |
| Right ventricular fractional area change (%) | 50.3 ± 7.8 | 47.6 ± 9.7 ** | −2.8 | 21.5 | 0.359 |
| Tricuspid annular plane systolic excursion (mm) | 21.9 ± 4.4 | 23.7 ± 5.1 ** | 1.8 | 11.2 | 0.424 |
| Normal subjects | |||||
| Right ventricular free wall longitudinal strain (%) | 29 ± 6 | 27 ± 6 ** | −2.2 | 14.2 | 0.288 |
| Right ventricular fractional area change (%) | 51.6 ± 6.8 | 47.8 ± 8.6 ** | −3.8 | 20.8 | 0.112 |
| Tricuspid annular plane systolic excursion (mm) | 22.2 ± 4.0 | 24.3 ± 4.2 ** | 2.1 | 11.2 | 0.062 |
| Pathological patients | |||||
| Right ventricular free wall longitudinal strain (%) | 28 ± 7 | 26 ± 7 * | −2 | 13.7 | 0.633 |
| Right ventricular fractional area change (%) | 48.4 ± 8.8 | 47.2 ± 11.3 | −1.2 | 22.2 | 0.546 |
| Tricuspid annular plane systolic excursion (mm) | 21.3 ± 5.0 | 22.8 ± 6.1 * | 1.4 | 11.1 | 0.637 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penso, M.; Ranalletta, R.A.; Pepi, M.; Garlaschè, A.; Ali, S.G.; Fusini, L.; Mantegazza, V.; Muratori, M.; Maragna, R.; Tamborini, G. Comparison between Automatic and Semiautomatic System for the 3D Echocardiographic Multiparametric Evaluation of RV Function and Dimension. J. Clin. Med. 2022, 11, 4528. https://doi.org/10.3390/jcm11154528
Penso M, Ranalletta RA, Pepi M, Garlaschè A, Ali SG, Fusini L, Mantegazza V, Muratori M, Maragna R, Tamborini G. Comparison between Automatic and Semiautomatic System for the 3D Echocardiographic Multiparametric Evaluation of RV Function and Dimension. Journal of Clinical Medicine. 2022; 11(15):4528. https://doi.org/10.3390/jcm11154528
Chicago/Turabian StylePenso, Marco, Remo Antonio Ranalletta, Mauro Pepi, Anna Garlaschè, Sarah Ghulam Ali, Laura Fusini, Valentina Mantegazza, Manuela Muratori, Riccardo Maragna, and Gloria Tamborini. 2022. "Comparison between Automatic and Semiautomatic System for the 3D Echocardiographic Multiparametric Evaluation of RV Function and Dimension" Journal of Clinical Medicine 11, no. 15: 4528. https://doi.org/10.3390/jcm11154528
APA StylePenso, M., Ranalletta, R. A., Pepi, M., Garlaschè, A., Ali, S. G., Fusini, L., Mantegazza, V., Muratori, M., Maragna, R., & Tamborini, G. (2022). Comparison between Automatic and Semiautomatic System for the 3D Echocardiographic Multiparametric Evaluation of RV Function and Dimension. Journal of Clinical Medicine, 11(15), 4528. https://doi.org/10.3390/jcm11154528








