Abstract
Dyslipidemia has been linked to breast cancer incidence. The aim of the present meta-analysis was to further investigate the relationship between the serum lipid profile and breast cancer risk. Databases such as PubMed, EMBASE, and Web of Sciences were searched up to the end of January 2021 using certain MeSH and non-MeSH keywords and combinations to extract related published articles. Twenty-six prospective studies involving 1,628,871 women, of whom 36,590 were diagnosed with breast cancer during the follow-up period met the inclusion criteria. A negative and significant association was found between the HDL-C level and the risk of breast cancer (relative risk (RR): 0.85, 95% CI: 0.72–0.99, I2: 67.6%, p = 0.04). In contrast, TG (RR: 1.02, 95% CI: 0.91–1.13, I2: 54.2%, p = 0.79), total cholesterol (TC) (RR: 0.98, 95% CI: 0.90–1.06, I2: 67.2%, p = 0.57), apolipoprotein A (ApoA) (RR: 0.96, 95% CI: 0.70–1.30, I2: 83.5%, p = 0.78) and LDL-C (RR: 0.93, 95% CI: 0.79–1.09, I2: 0%, p = 0.386) were not associated with breast cancer development. In studies adjusting for hormone use and physical activity, breast cancer risk was positively correlated with TC (RR: 1.05, 95% CI: 1.01–1.10). Similarly, TG was significantly related to breast cancer development after adjustment for baseline lipids (RR: 0.92, 95% CI: 0.85–0.99) and race (any races mentioned in each study) (RR: 1.80, 95% CI: 1.22–2.65). In the present meta-analysis, HDL-C was inversely related to breast cancer risk. Overall, data on the links between lipids and breast cancer are conflicting. However, there is increasing evidence that low HDL-C is related to an increased risk for this type of malignancy.
1. Introduction
Breast cancer is the most frequently diagnosed tumor in women according to the World Health Organization (WHO) [1]. According to the Centre for Disease Control (CDC), there are several predisposing factors to breast cancer, including older age, genetic mutations, early initiation of menstruation (at the age of <12 years), late menopause (at the age of >55 years), family history of breast or ovarian cancer, previous exposure to radiation, obesity, physical inactivity, alcohol use, and the use of hormone replacement therapy or oral contraceptives [2].
A link between dyslipidemia and breast cancer has been reported. For example, increased levels of triglycerides (TGs), low-density lipoprotein cholesterol (LDL-C) and very low-density lipoprotein cholesterol (VLDL) were observed in breast cancer patients compared with normal controls [3,4]. However, there are conflicting results [5,6].
The relationship between high-density lipoprotein cholesterol (HDL-C) and the risk for breast cancer remains unclear with some studies reporting an inverse association and others reporting the opposite or no association at all [7,8,9,10]. Furthermore, it has been proposed that HDL functionality may affect these links [7,8,9,10]. A Mendelian randomization study found that genetically elevated plasma LDL-C and HDL-C levels were associated with an increased breast cancer risk [11]. Of note, 27-hydroxycholesterol can facilitate metastasis via the induction of estrogen-receptor-positive breast cancer cells [12]. Furthermore, HDL glycation and oxidative modification of lipoproteins may activate certain inflammation-related pathways, leading to cell proliferation and migration, as well as inhibiting apoptosis [12]. Overall, the lipid profile may be a predictor of breast cancer occurrence and recurrence [13].
The aim of the present systematic review and meta-analysis was to further investigate the relationship between the serum lipid profile and breast cancer development.
2. Materials and Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to conduct the present systematic review and meta-analysis. This meta-analysis is registered in the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42021281278.
3. Search Strategy
We searched for papers published up to the end of January 2021 in databases such as PubMed, EMBASE, and Web of Sciences (ISI). The search strategy used MeSH and non-MeSH keywords and combinations to extract related published articles. The keywords used were “TC”, “HDL-C”, “LDL-C”, “TG”, “Apolipoprotein”, “lipoproteins”, “cholesterol”, “triglyceride”, “dyslipidemias”, “lipid profile”, “lipid component”, “blood lipid”, “plasma lipid”, “serum lipid”, “plasma lipoprotein, “dyslipoproteinemia”, “hypercholesterolemia”, “hypertriglyceridemia”, “hyperlipidemia”, “lipemia”, “ApoA”, “Apolipoproteins A”, “ApoB”, “Apolipoproteins B”, and “Metabolic syndrome”. Breast cancer was defined using the terms “breast neoplasm”, “breast cancer”, “breast tumor”, “breast tumour”, “breast malignancy”, and “breast carcinoma”. Furthermore, “Cohort”, “Prospective”, “Longitudinal”, “Follow-up”, “Nested”, and “Population-based” terms were used to limit the findings to cohort studies. No automatic filtering of databases was used during the database search. The references of papers were also checked. No time or language limitations were applied. The reference list of eligible articles was further searched, and authors were contacted by email for additional data, if needed.
4. Eligibility Criteria
Original articles that fulfilled the following criteria were included in the present systematic review and meta-analysis: (1) cohorts with a prospective design (the exposure takes place before the outcome), (2) participants free of cancer at baseline, and, (3) investigations of the relationship between the lipid profile and the risk of developing breast cancer.
After excluding duplicates and based on titles and abstracts, we excluded animal studies and those involving humans aged ≤ 18 years. In addition, supplementary hand searching of the reference lists of previous reviews or meta-analyses was conducted. Of 93 eligible full articles, 26 articles met the inclusion criteria (Figure 1).
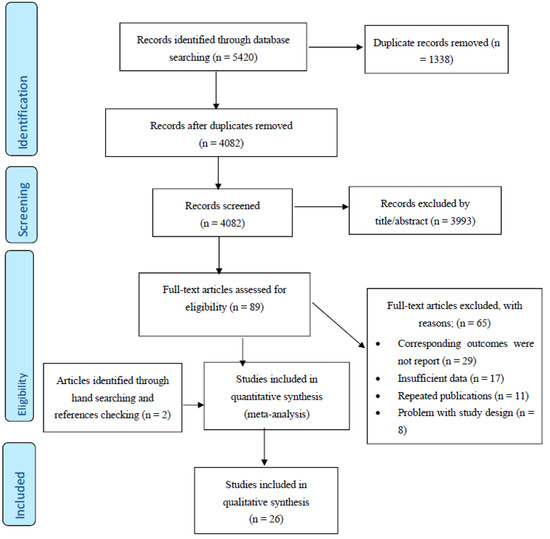
Figure 1.
PRISMA (The Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Flow Diagram.
5. Study Selection
Study selection started with the removal of duplicates, followed by the screening of titles and abstracts by two reviewers (M.N and M.A.M) blinded to the names, qualifications and institutional affiliations of the study authors. Agreement between the reviewers was excellent (Kappa index: 0.86; p < 0.001). Disagreements were resolved at a meeting between reviewers prior to selected articles being retrieved (a flow chart is available in Figure 1). We included studies if they met all of the following criteria: (1) the outcome of interest was the lipid profile; (2) the studies were population-based cohort studies and reported breast cancer data; (3) relative risk (RR), hazard ratio (HR) or odds ratio (OR) estimates with 95% confidence interval (CI) adjusted for multivariable factors were available or could be calculated; and (4) articles were original with full-text in English. Studies were excluded according to the following criteria: (1) reviews, letters, opinion papers, editorials, unpublished data or comments; (2) those published in languages other than English; (3) those that were not population-based cohort studies; or (4) RR, HR or OR estimates with 95% CI were not available or could not be calculated. Publications lacking primary data and/or explicit method descriptions were also excluded.
6. Data Extraction and Management
The full text versions of studies meeting the inclusion criteria were retrieved and screened to determine their eligibility by two reviewers (S.G and A.R.J). A study quality assessment was performed according to the Newcastle-Ottawa Scale (NOS, Table 1 [8,9,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]) as bias-assessment scores without an effect on selection [38]. By evaluating the selection, comparability and outcome of each study, the rating system scored studies from 0 (highest degree of bias) to 9 (lowest degree of bias). Furthermore, we investigated the funding sources of all eligible studies. Following an assessment of the methodological quality, two reviewers (S.G. and A.R.J.) extracted data using a purpose-designed data extraction form and wrote independent summaries on what they considered to be the most important results from each study. These summaries were compared, and any differences in opinion were resolved by discussion and consultation with a third reviewer (M.M). Any further calculations of the study data considered necessary were conducted by the first reviewer (S.G) and checked by the second (A.R.J). Information extracted from each eligible study included the following items: author, year and references, country, study name, men (%), mean age, follow-up time (years), number of cases, number of participants, parameters, outcomes and main confounders.
7. Data Synthesis and Statistical Analyses
We performed a random-effect meta-analysis as described by DerSimonian and Laird to estimate the summary effect size and 95% CI in the present highest vs. lowest meta-analysis. Comparisons between studies were ignored and no clear division was made [39]. We used the most fully adjusted hazard risk reported in the included studies. For studies with menopausal-specific effect sizes, we combined pre- and post-menopause estimates by employing a fixed-effects model and using the combined effect size for the analyses. Heterogeneity was tested using the Cochran Q and I2 statistics [40]. A series of subgroup analyses was also performed to identify potential sources of heterogeneity based on adjustments for the main confounders, including smoking, hormone, race, alcohol, body mass index, physical activity and lipid profile at baseline. The publication bias was evaluated through visual inspection of the funnel plot. A statistical assessment of the publication bias was conducted with Egger’s regression asymmetry [41] and Begg’s test [42]. All statistical analyses were performed using STATA version 16.0 (StataCorp, College Station, TX, USA), and two-sided p values of <0.05 were considered statistically significant.
8. Results
Of the 93 eligible full articles, 26 prospective studies met the inclusion criteria; their key characteristics are shown in Table 1. Among these studies, 21/26 were cohort studies [8,9,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] and 5/26 were nested case control studies [33,34,35,36,37]. Study sample sizes ranged from 594 to 288,057 persons aged >20 years. The mean follow up period was 12.41 years (range 7 to 26 years). Sixteen of the studies were conducted in European countries [8,9,14,15,16,18,24,25,26,28,29,30,32,34,35,36], 7 in the USA [17,19,20,27,31,33,37], 2 in Japan [22,23] and 1 in Korea [21]. Overall, 1,628,871 women were included in the present meta-analysis, of whom 36,590 were diagnosed with breast cancer during the follow-up period. The results of the NOS quality assessment are shown in the Table 1, with 18 studies scoring values ≥7 [8,9,17,18,19,20,23,24,25,26,27,28,29,30,31,32,35,36] and no studies scoring <5.

Table 1.
Summary of included studies.
Table 1.
Summary of included studies.
| Author’s Name, Country, Year | Sample Size | Age (Years) | Study Design | No. of Incident Breast Cancer/Case | Follow Up (Mean, Year) | Data Presented | Adjusted | Results | Study Score |
|---|---|---|---|---|---|---|---|---|---|
| Lars J. Vatten et al. Norway (1990) [14] | 24,329 | 31–51 | Cohort | 242 | 14 | TG, TC | Age, BMI | There were no statistically significant associations of lipid measures with breast cancer risk. | 5 |
| Annette Pernille Hoyer et al. Denmark (1992) [15] | 5207 | 30–80 | Cohort | 51 | 26 | HDL, LDL, TG, TC | Age, Smoking, Menopause Age, Alcohol Intake, BMI, Socioeconomic Status | There was a significant association of HDL with breast cancer risk. | 5 |
| Maria Gaard et al. Norway (1994) [16] | 30,666 | 20–54 | Cohort | 302 | 10.4 | HDL, LDL, TG, TC | Age, Smoking, Menopause Age, BMI, Lipid Baseline | There were no statistically significant associations of lipid measures with breast cancer risk. | 6 |
| Kyle Steenland et al. USA (1995) [17] | 14,407 | 25–74 | Cohort | 163 | 17 | TC | Age, Smoking, Menopause Age, Alcohol Intake, BMI, Socioeconomic Status, Physical Activity, Parity | There was no statistically significant association of TC with breast cancer risk. | 7 |
| Patricia. G Moorman et al. USA (1998) [33] | 400 (control), 196 (case) | 41.3 | Nested case-control | Case | _ | HDL | Age, Menopause Age, Education, BMI, Alcohol Intake, Family History Cancer, Hormone Use, History of Hysterectomy, Parity, Smoking | There was no statistically significant association of HDL with breast cancer risk. | 6 |
| J Manjer et al. Sweden (2001) [18] | 9738 | 49.6 ± 7.8 | Cohort | 269 | 13.1 | TG, TC | Age, Smoking, Alcohol Intake, BMI, Parity | There were no statistically significant associations of lipid measures with breast cancer risk. | 8 |
| Anne-Sofie Furberg et al. Norway (2004) [9] | 38,823 | 43 | cohort | 708 | 17.2 | HDL | Age, Menopause Age, Smoking Socioeconomic Status, BMI, Parity, Lipid Baseline | There was no statistically significant association of HDL with breast cancer risk. | 9 |
| A. Heather Eliassen et al. USA (2005) [19] | 71,921 | 66 (mean) | Cohort | 2468 | 10 | TC | Age, Menopause Age, Alcohol intake, BMI, Physical Activity, Parity, Family History Cancer, Hormone Use | There was no statistically significant association of TC with breast cancer risk. | 8 |
| Manami Inouea et al. Japan (2008) [22] | 18,176 | 55.5 ± 8.1 | Cohort | 120 | 10 | HDL, TG | Age, Smoking, Alcohol Intake, Lipid Baseline | There were no statistically significant associations of lipid measures with breast cancer risk. | 6 |
| Anna M. Kuchareska-Newton et al. USA (2008) [20] | 7575 | 53.7 ± 5.7 | Cohort | 359 | 13 | HDL | Age, Menopause Age, BMI, Race, Smoking Hormone Use | There was no statistically significant association of HDL with breast cancer risk. | 8 |
| Guy Fagherazzi et al. France (2009) [25] | 69,088 | 40–65 | Cohort | 2932 | 12 | TC | Menopause Age, Alcohol Intake, BMI, Family History Cancer, Hormone Use | There was no statistically significant association of TC with breast cancer risk. | 7 |
| Hiroyasu Iso et al. Japan (2009) [23] | 21,685 | 54.2 (mean) | Cohort | 178 | 12.4 | TC | Age, Smoking, Alcohol Intake, BMI | There was a significant association of TC with breast cancer risk. | 8 |
| H Ulmer et al. Austria (2009) [24] | 84,460 | 41.8 ± 15.1 | Cohort | 1204 | 10.6 | TG | Smoking, BMI, Socioeconomic status, Lipid Baseline | There was no statistically significant association of TG with breast cancer risk. | 9 |
| Mina Ha et al. Korean (2009) [21] | 170,374 | 40–64 | Cohort | 714 | 10 | TC | Age, Age at Menarche, Age at First Childbirth, Nulliparity, Hormone Replacement Therapy, Duration of Breast Feeding, Smoking Habit, Alcohol Consumption | There was a positive association between cholesterol level and breast cancer risk. | 6 |
| Immacolata Capasso et al. Italy (2010) [35] | 777 [control), 210 [case) | 57.5 | Nested case-control | Case | _ | HDL | Age, Menopause Age, BMI, Alcohol Intake, Family History cancer, Hormone Use, Parity, Smoking, Socioeconomic status | There was a significant association of HDL with breast cancer risk. | 7 |
| C. Agnoli et al. Italy (2010) [34] | 1089 (control), 163 (case) | 58 ± 5.6 | Nested case-control | Case | _ | HDL, TG | Age, Smoking, Menopause Age, Education, Alcohol Intake, Family History Cancer, Hormone Use | There were significant associations of HDL and TG with breast cancer risk. | 6 |
| Wegene Borena et al. Norway, Austria, and Swede (2011) [26] | 256,512 | 44.2 | Cohort | 5006 | 11.9 | TG | Age, Smoking, BMI | There was no statistically significant association of TG with breast cancer risk. | 8 |
| Jennifer C. Melvin et al. Sweden (2012) [28] | 234,494 | 25< | Cohort | 6105 | 8.25 | HDL, LDL, TG, TC, APO A, APO B, TC/HDL, LDL/HDL, TG/HD, APO B/APO A | Age, Socioeconomic Status, Lipid Baseline, Parity | There was a significant association of TG with breast cancer risk. | 7 |
| Jaclyn L. F. Bosco et al. USA (2012) [27] | 49,172 | 21–69 | Cohort | 1228 | 10.5 | TC | Age, Race, Education, BMI, Physical Activity | There was no statistically significant association of TC with breast cancer risk. | 7 |
| Susanne Strohmaier et al. Norway, Austria, and Sweden (2013) [29] | 288,057 | 33–48 | Cohort | 5228 | 11.7 | TC | Age, Smoking, BMI | There was a significant association of TC with breast cancer risk. | 8 |
| Signe Borgquist et al. Sweden (2016) [30] | 17,035 | 57.9 | Cohort | 1024 | 14.3 | APO A, APO B, APO B/APO A | Age, Menopause Age, Socioeconomic status, BMI, Hormone Use, Parity | There were significant associations of APO B/APO A with breast cancer risk. | 7 |
| Mathilde His et al. France (2017) [36] | 1043 (control), 583 [case) | 50–63 | Nested case-control | Case | _ | HDL, LDL, TG, TC, TC/HDL, LDL/HDL | Age, Menopause Age, Smoking, BMI, Family History Cancer, Education, Alcohol Intake, Hormone Use | There were no statistically significant associations of lipid measures with breast cancer risk. | 7 |
| Daniel T. Dibaba et al. USA (2018) [31] | 94,555 | 50–71 | Cohort | 5380 | 14 | TC | Age, Education, BMI, Physical Activity, Family History Cancer, Hormone Use, History of Hysterectomy, Parity, Smoking | There was a significant association of TC with breast cancer risk. | 8 |
| Kasper Mønsted Pedersen et al. Denmark (2020) [8] | 56,790 | 57.5 | Cohort | 1641 | 7.4 | HDL, APO A | Age, Smoking, BMI, Physical Activity, Education, Alcohol Intake, Socioeconomic status, Lipid Baseline | There was a significant association of Apo A with breast cancer risk. | 7 |
| Catherine Schairer et al. USA (2020) [37] | 2470 (control), 247 (case) | 60.7 | Nested case-control | Case | _ | HDL, TG, TG/HDL | Age, Race | There were significant associations of HDL and TG/HDL with breast cancer risk. | 6 |
| Rhonda S. Arthur et al. UK (2021) [32] | 58,629 | 60 (56–64) | Cohort | 1268 | 7 | HDL, TG | Age, BMI, Physical activity, Family History Cancer, Alcohol intake, Hormone Use, Smoking, Socioeconomic status | There were no statistically significant associations of lipid measures with breast cancer risk. | 7 |
Abbreviations: HDL–C: high-density lipoprotein cholesterol; LDL–C: low-density lipoprotein cholesterol; TG: triglyceride; TC: total cholesterol; APO: apolipoprotein; BMI: body mass index.
9. Associations of Lipid Profile with Risk of Breast Cancer
A negative and significant association was found between the HDL–C level and the risk of breast cancer (RR: 0.85, 95% CI: 0.72–0.99, n = 13 studies, Figure 2) with a moderate risk of heterogeneity (I2: 67.6, p = 0.04). In contrast, TG (RR: 1.02, 95% CI: 0.91–1.13, n = 12 studies, I2: 54.2%, p = 0.79, Figure 3), total cholesterol (TC) (RR: 0.98, 95% CI: 0.90–1.06, n = 14 studies, I2: 67.2%, p = 0.57, Figure 4), apolipoprotein A (ApoA1) (RR: 0.96, 95% CI: 0.70–1.30, n = 3 studies, I2: 83.5%, p = 0.78, Figure 5) and LDL–C (RR: 0.93, 95% CI: 0.79–1.09, n = 4 studies, I2: 0%, p = 0.38, Figure 6) were not associated with breast cancer development.
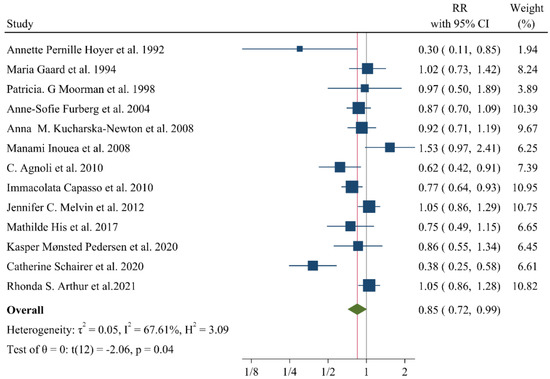
Figure 2.
Forest plot of the highest vs. lowest categories of serum HDL–C levels and breast cancer risk. HDL–C: high-density lipoprotein cholesterol [8,9,15,16,20,22,28,32,33,34,35,36,37].
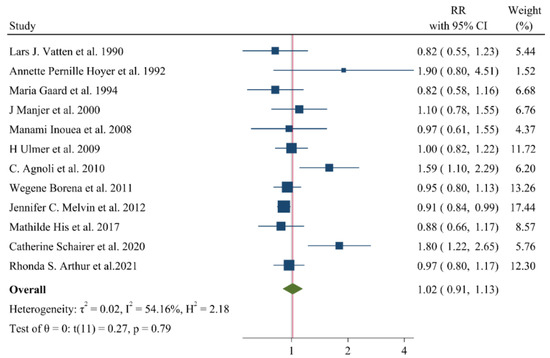
Figure 3.
Forest plot of the highest vs. lowest categories of serum triglyceride levels and breast cancer risk [14,15,16,18,22,24,26,28,32,34,36,37].
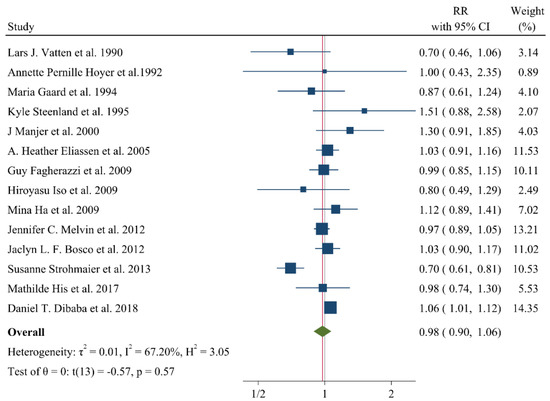
Figure 4.
Forest plot of the highest vs. lowest categories of serum total cholesterol levels and breast cancer risk [14,15,16,17,18,19,21,23,25,27,28,29,31,36].
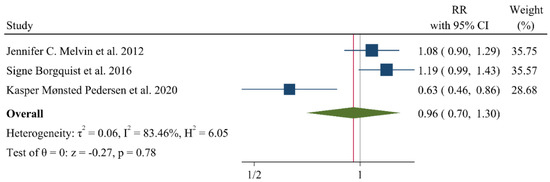
Figure 5.
Forest plot of the highest vs. lowest categories of serum Apolipoprotein A levels and breast cancer risk. Apoa: apolipoprotein A [8,28,30].
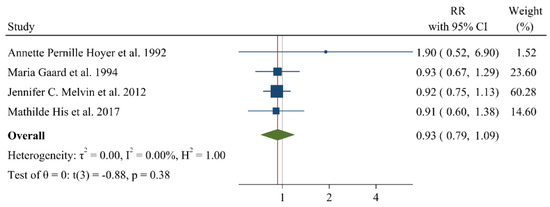
Figure 6.
Forest plot of the highest vs. lowest categories of serum LDL–C levels and breast cancer risk. LDL–C: low-density lipoprotein cholesterol [15,16,28,36].
Of note, a small but significant positive correlation was found between TC and breast cancer risk in studies adjusting for hormone use (RR: 1.05, 95% CI: 1.01–1.10) and physical activity (RR: 1.05, 95% CI: 1.01–1.10). Similarly, TG was significantly related to breast cancer development after adjustment for baseline lipids (RR: 0.92, 95% CI: 0.85–0.99) and race (any race mentioned in each study) (RR: 1.80, 95% CI: 1.22–2.65) (added in Supplementary File).
10. Publication Bias
Visual inspection of the funnel plot symmetry suggested no potential publication bias for the comparisons of HDL–C (Egger = 0.237), TG (Egger = 0.069), TC (Egger = 0.480) and LDL–C with the risk of breast cancer (Figure 7). Furthermore, Egger’s linear regression (intercept = −2.3, 95% CI: −6.91 to 6.00, two-tailed p = 0.542) and Begg’s rank correlation test (Kendall’s Tau with continuity correction =1.00, z = 0.342, two tailed p = 0.436) indicated the absence of a publication bias. After adjustment of the effect size for the potential publication bias using the ‘trim and fill’ correction, no potentially missing studies were added to the funnel plot for HDL–C, TG, TC or LDL–C.
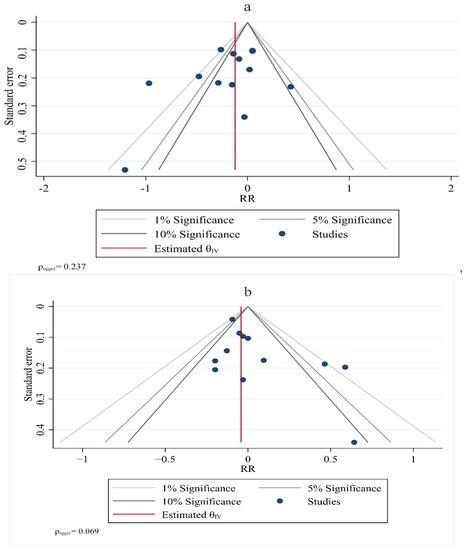
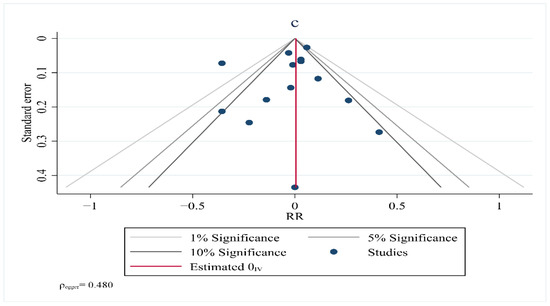
Figure 7.
Funnel plot showing lipid profiles (high–density lipoprotein (a), triglycerides (b), total cholesterol (c)) and risk of breast cancer.
11. Discussion
The present systematic review and meta-analysis evaluated the associations between lipid parameters and the risk of developing breast cancer in women. Only HDL-C was found to be significantly related to breast cancer development with an RR value of 0.85, thus highlighting the potential preventive effect of elevated HDL-C levels. Similarly, low HDL-C levels (<77 mg/dL) were correlated with an increased risk of breast cancer in the Copenhagen General Population Study [8]. Of note, the increased risks with lower HDL-C levels, i.e., HR, were 1.20 (95% CI: 1.01–1.41) and 1.36 (95% CI: 1.11–1.66) for HDL-C concentrations of 58–77 mg/dL and 39–58 mg/dL, respectively. A modest but non-significant, inverse association between HDL-C and breast cancer was reported in 2 previous meta-analyses produced in 2015; one by Touvier et al. which included 22 prospective cohort studies and 2 nested case-control studies (HR 0.90, 95% CI: 0.77–1.04, I2: 52%) [43], and the second by Ni et al., which included 15 prospective cohort studies with 1,189,635 participants and 23,369 breast cancer cases (RR 0.92, 95% CI: 0.73–1.16, I2: 65%) [44]. In the latter meta-analysis, the inverse association between HDL-C and breast cancer risk was significant among women who were postmenopausal at baseline (RR 0.77, 95% CI: 0.64–0.93), whereas the RR value was 0.84 (95% CI: 0.40–1.74) for premenopausal women [44]. Therefore, the potential involvement of HDL-C in breast cancer development seems more pronounced after menopause, highlighting the importance of implementing health policy strategies to increase HDL-C levels (and avoid their reduction) in postmenopausal women.
In the present meta-analysis, TC, TG and LDL-C were not found to be related to breast cancer risk. Similar results were reported in the abovementioned previous meta-analyses conducted by Touvier et al. [43] and Ni et al. [44]. Despite these findings, a high cholesterol intake has been positively related to an increased risk of breast cancer, and increased LDL receptor expression has been observed in breast cancer tissue to enhance LDL-C uptake from the circulation, since proliferating cancer cells require more cholesterol [12]. Furthermore, a recent Mendelian randomization study found that genetically elevated plasma LDL-C (OR 1.03, 95% CI: 1.01–1.07, p = 0.02) and HDL-C (OR 1.06, 95% CI: 1.03–1.10, p = 0.0049) correlated with an increased breast cancer risk [11].
As already mentioned, in the present meta-analysis, after adjustments for hormone use and physical activity, TC was found to be positively associated with breast cancer risk, as was TG when adjusted for baseline lipids and race. Overall, there are conflicting data regarding the links between lipids (especially TC, TG and LDL-C) and the risk for breast cancer [45]. For example, TGs have been considered a prognostic factor for breast cancer occurrence and recurrence, although not in all studies [45]. These contradictory findings may be attributed to the multifactorial nature of the disease and the presence of several confounding factors, such as age, race, menopausal status, obesity, genetic mutations, physical inactivity, and alcohol and hormone use. Large prospective and mechanistic studies (both in vitro and in vivo) are required to fully elucidate the role of lipids in breast cancer development in different populations. Furthermore, further investigations are warranted to investigate whether HDL-raising agents may protect against breast cancer development and progression. Of note, statins have been persistently reported to improve breast cancer outcomes and protect against the development and progression of this malignancy [46,47,48,49].
ApoA was also not significantly related to breast cancer risk in the present meta-analysis. Similarly, no association has previously been observed between ApoA1 and breast cancer incidence [30], whereas other studies have reported that ApoA1 is a risk factor for intraocular metastasis in patients with breast cancer [50,51]. Further research is needed in this field.
This study had some limitations. First, studies used several designs to define associations. Secondly, adjusted covariates differed between the included studies and this might have increased the risk of a confounding bias. Third, cutoff points for the first and last categories varied, and this might have led to study variation.
In conclusion, the present meta-analysis found a significant inverse association between HDL-C and the risk of breast cancer development. TC, TG, LDL-C and ApoA were not found to be significantly correlated with breast cancer development. Hormone use and physical activity affected the relationship of TC with this malignancy, as did race and baseline lipid values for TG. Data on the role of lipids on breast cancer risk are generally conflicting, with low HDL-C usually being related to an increased risk. Further basic and clinical research is required to elucidate the associations between specific cholesterol components and breast cancer risk in certain populations as well as the exact mechanisms underlying the lipid-related signaling pathway involved in the development of this malignancy. Such data will provide evidence on the potential clinical use of lipid-modifying drugs in relation to breast cancer prevention.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11154503/s1, Figure S1. Subgroup analysis for HDL-C. Figure S2. Subgroup analysis for triglyceride. Figure S3. Subgroup analysis for total cholesterol.
Author Contributions
M.N., S.F., N.K. and M.B. contributed to the research concept; M.N., M.A.M., S.G. and A.J. searched databases, screened articles, and extracted data; A.J. and M.M. performed statistical analysis. All authors contributed to the writing and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer 2019, 11, 151–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudet, M.M.; Gierach, G.L.; Carter, B.D.; Luo, J.; Milne, R.L.; Weiderpass, E.; Giles, G.G.; Tamimi, R.M.; Eliassen, A.H.; Rosner, B. Pooled analysis of nine cohorts reveals breast cancer risk factors by tumor molecular subtype. Cancer Res. 2018, 78, 6011–6021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laisupasin, P.; Thompat, W.; Sukarayodhin, S.; Sornprom, A.; Sudjaroen, Y. Comparison of serum lipid profiles between normal controls and breast cancer patients. J. Lab. Physicians 2013, 5, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Kapil, U.; Bhadoria, A.S.; Sareen, N.; Singh, P.; Dwivedi, S.N. Total cholesterol and triglyceride levels in patients with breast cancer. J. Breast Cancer 2013, 16, 129–130. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.-Q.; Cui, L.-H.; Li, C.-C.; Yu, Z.; Piao, J.-M. Effects of serum triglycerides on prostate cancer and breast cancer risk: A meta-analysis of prospective studies. Nutr. Cancer 2016, 68, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.-L.; Wu, Y.-T.; Wu, H.; Dai, W.; Arshad, B.; Xu, Z.; Li, H.; Wu, K.-N.; Kong, L.-Q. Status of lipid and lipoprotein in female breast cancer patients at initial diagnosis and during chemotherapy. Lipids Health Dis. 2018, 17, 91. [Google Scholar] [CrossRef] [Green Version]
- Owiredu, W.; Donkor, S.; Addai, B.W.; Amidu, N. Serum lipid profile of breast cancer patients. Pak. J. Biol. Sci. 2009, 12, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, K.M.; Çolak, Y.; Bojesen, S.E.; Nordestgaard, B.G. Low high-density lipoprotein and increased risk of several cancers: 2 population-based cohort studies including 116,728 individuals. J. Hematol. Oncol. 2020, 13, 129. [Google Scholar] [CrossRef]
- Furberg, A.-S.; Veierød, M.B.; Wilsgaard, T.; Bernstein, L.; Thune, I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J. Natl. Cancer Inst. 2004, 96, 1152–1160. [Google Scholar] [CrossRef]
- Samadi, S.; Ghayour-Mobarhan, M.; Mohammadpour, A.; Farjami, Z.; Tabadkani, M.; Hosseinnia, M.; Miri, M.; Heydari-Majd, M.; Mehramiz, M.; Rezayi, M. High-density lipoprotein functionality and breast cancer: A potential therapeutic target. J. Cell. Biochem. 2019, 120, 5756–5765. [Google Scholar] [CrossRef]
- Johnson, K.E.; Siewert, K.M.; Klarin, D.; Damrauer, S.M.; Program, V.M.V.; Chang, K.-M.; Tsao, P.S.; Assimes, T.L.; Maxwell, K.N.; Voight, B.F. The relationship between circulating lipids and breast cancer risk: A Mendelian randomization study. PLoS Med. 2020, 17, e1003302. [Google Scholar] [CrossRef] [PubMed]
- Cedó, L.; Reddy, S.T.; Mato, E.; Blanco-Vaca, F. HDL and LDL: Potential new players in breast cancer development. J. Clin. Med. 2019, 8, 853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fichtali, K.; Bititi, A.; Elghanmi, A.; Ghazi, B. Serum Lipidomic Profiling in Breast Cancer to Identify Screening, Diagnostic, and Prognostic Biomarkers. BioResearch Open Access 2020, 9, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatten, L.J.; Foss, O.P. Total serum cholesterol and triglycerides and risk of breast cancer: A prospective study of 24,329 Norwegian women. Cancer Res. 1990, 50, 2341–2346. [Google Scholar] [PubMed]
- Høyer, A.P.; Engholm, G. Serum lipids and breast cancer risk: A cohort study of 5,207 Danish women. Cancer Causes Control 1992, 3, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Gaard, M.; Tretli, S.; Urdal, P. Risk of breast cancer in relation to blood lipids: A prospective study of 31,209 Norwegian women. Cancer Causes Control 1994, 5, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Nowlin, S.; Palu, S. Cancer incidence in the National Health and Nutrition Survey, I. Follow-up data: Diabetes, cholesterol, pulse and physical activity. Cancer Epidemiol. Prev. Biomark. 1995, 4, 807–811. [Google Scholar]
- Manjer, J.; Kaaks, R.; Riboli, E.; Berglund, G. Risk of breast cancer in relation to anthropometry, blood pressure, blood lipids and glucose metabolism: A prospective study within the Malmö Preventive Project. Eur. J. Cancer Prev. 2001, 10, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Eliassen, A.H.; Colditz, G.A.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Serum lipids, lipid-lowering drugs, and the risk of breast cancer. Arch. Intern. Med. 2005, 165, 2264–2271. [Google Scholar] [CrossRef]
- Kucharska-Newton, A.M.; Rosamond, W.D.; Mink, P.J.; Alberg, A.J.; Shahar, E.; Folsom, A.R. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann. Epidemiol. 2008, 18, 671–677. [Google Scholar] [CrossRef]
- Ha, M.; Sung, J.; Song, Y.-M. Serum total cholesterol and the risk of breast cancer in postmenopausal Korean women. Cancer Causes Control 2009, 20, 1055–1060. [Google Scholar] [CrossRef]
- Inoue, M.; Noda, M.; Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Iso, H.; Tsugane, S. Impact of metabolic factors on subsequent cancer risk: Results from a large-scale population-based cohort study in Japan. Eur. J. Cancer Prev. 2009, 18, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Ikeda, A.; Inoue, M.; Sato, S.; Tsugane, S. Serum cholesterol levels in relation to the incidence of cancer: The JPHC study cohorts. Int. J. Cancer 2009, 125, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, H.; Borena, W.; Rapp, K.; Klenk, J.; Strasak, A.; Diem, G.; Concin, H.; Nagel, G. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br. J. Cancer 2009, 101, 1202–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagherazzi, G.; Fabre, A.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F. Serum cholesterol level, use of a cholesterol-lowering drug, and breast cancer: Results from the prospective E3N cohort. Eur. J. Cancer Prev. 2010, 19, 120–125. [Google Scholar] [CrossRef]
- Borena, W.; Stocks, T.; Jonsson, H.; Strohmaier, S.; Nagel, G.; Bjørge, T.; Manjer, J.; Hallmans, G.; Selmer, R.; Almquist, M. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. Cancer Causes Control 2011, 22, 291–299. [Google Scholar] [CrossRef]
- Bosco, J.L.; Palmer, J.R.; Boggs, D.A.; Hatch, E.E.; Rosenberg, L. Cardiometabolic factors and breast cancer risk in US black women. Breast Cancer Res. Treat. 2012, 134, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Melvin, J.C.; Seth, D.; Holmberg, L.; Garmo, H.; Hammar, N.; Jungner, I.; Walldius, G.; Lambe, M.; Wigertz, A.; Van Hemelrijck, M. Lipid profiles and risk of breast and ovarian cancer in the Swedish AMORIS study. Cancer Epidemiol. Prev. Biomark. 2012, 21, 1381–1384. [Google Scholar] [CrossRef] [Green Version]
- Strohmaier, S.; Edlinger, M.; Manjer, J.; Stocks, T.; Bjørge, T.; Borena, W.; Häggström, C.; Engeland, A.; Nagel, G.; Almquist, M. Total serum cholesterol and cancer incidence in the Metabolic syndrome and Cancer Project (Me-Can). PLoS ONE 2013, 8, e54242. [Google Scholar] [CrossRef] [Green Version]
- Borgquist, S.; Butt, T.; Almgren, P.; Shiffman, D.; Stocks, T.; Orho-Melander, M.; Manjer, J.; Melander, O. Apolipoproteins, lipids and risk of cancer. Int. J. Cancer 2016, 138, 2648–2656. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Braithwaite, D.; Akinyemiju, T. Metabolic syndrome and the risk of breast cancer and subtypes by race, menopause and BMI. Cancers 2018, 10, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, R.S.; Dannenberg, A.J.; Rohan, T.E. The association of prediagnostic circulating levels of cardiometabolic markers, testosterone and sex hormone-binding globulin with risk of breast cancer among normal weight postmenopausal women in the UK Biobank. Int. J. Cancer 2021, 149, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Moorman, P.G.; Hulka, B.S.; Hiatt, R.A.; Krieger, N.; Newman, B.; Vogelman, J.H.; Orentreich, N. Association between high-density lipoprotein cholesterol and breast cancer varies by menopausal status. Cancer Epidemiol. Prev. Biomark. 1998, 7, 483–488. [Google Scholar]
- Agnoli, C.; Berrino, F.; Abagnato, C.A.; Muti, P.; Panico, S.; Crosignani, P.; Krogh, V. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: A nested case–control study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capasso, I.; Esposito, E.; Pentimalli, F.; Crispo, A.; Montella, M.; Grimaldi, M.; De Marco, M.R.; Cavalcanti, E.; D’Aiuto, M.; Fucito, A. Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer Biol. Ther. 2010, 10, 1240–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- His, M.; Dartois, L.; Fagherazzi, G.; Boutten, A.; Dupré, T.; Mesrine, S.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; Dossus, L. Associations between serum lipids and breast cancer incidence and survival in the E3N prospective cohort study. Cancer Causes Control 2017, 28, 77–88. [Google Scholar] [CrossRef]
- Schairer, C.; Laurent, C.A.; Moy, L.M.; Gierach, G.L.; Caporaso, N.E.; Pfeiffer, R.M.; Kushi, L.H. Obesity and related conditions and risk of inflammatory breast cancer: A nested case–control study. Breast Cancer Res. Treat. 2020, 183, 467–478. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Touvier, M.; Fassier, P.; His, M.; Norat, T.; Chan, D.S.; Blacher, J.; Hercberg, S.; Galan, P.; Druesne-Pecollo, N.; Latino-Martel, P. Cholesterol and breast cancer risk: A systematic review and meta-analysis of prospective studies. Br. J. Nutr. 2015, 114, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Liu, H.; Gao, R. Serum Lipids and Breast Cancer Risk: A Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2015, 10, e0142669. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Huang, Y.; Yang, W.; Huang, Q.; Chen, Y.; Zeng, K.; Chen, J.; Chen, J. The significances and clinical implications of cholesterol components in human breast cancer. Sci. Prog. 2021, 104, 368504211028395. [Google Scholar] [CrossRef] [PubMed]
- Van Wyhe, R.D.; Rahal, O.M.; Woodward, W.A. Effect of statins on breast cancer recurrence and mortality: A review. Breast Cancer 2017, 9, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Borgquist, S.; Broberg, P.; Tojjar, J.; Olsson, H. Statin use and breast cancer survival—A Swedish nationwide study. BMC Cancer 2019, 19, 54. [Google Scholar] [CrossRef] [Green Version]
- Beckwitt, C.H.; Brufsky, A.; Oltvai, Z.N.; Wells, A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. 2018, 20, 144. [Google Scholar] [CrossRef]
- Lv, H.; Shi, D.; Fei, M.; Chen, Y.; Xie, F.; Wang, Z.; Wang, Y.; Hu, P. Association Between Statin Use and Prognosis of Breast Cancer: A Meta-Analysis of Cohort Studies. Front. Oncol. 2020, 10, 556243. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Yuan, Q.; Lin, Q.; Shi, W.Q.; Zhu, P.W.; Min, Y.L.; Ge, Q.M.; Shao, Y. Apolipoprotein A1 and Low-Density Lipoprotein as Risk Factors for Intraocular Metastases in Postmenopausal Breast Cancer. Technol. Cancer Res. Treat. 2021, 20, 1533033820984180. [Google Scholar] [CrossRef]
- Liu, J.X.; Yuan, Q.; Min, Y.L.; He, Y.; Xu, Q.H.; Li, B.; Shi, W.Q.; Lin, Q.; Li, Q.H.; Zhu, P.W.; et al. Apolipoprotein A1 and B as risk factors for development of intraocular metastasis in patients with breast cancer. Cancer Manag. Res. 2019, 11, 2881–2888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).