High Tg/HDL-Cholesterol Ratio Highlights a Higher Risk of Metabolic Syndrome in Children and Adolescents with Severe Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometric Data
2.3. Blood Pressure Measurements
2.4. Laboratory Analyses
2.5. Definitions
2.6. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, A.; Buoncristiano, M.; Kovacs, V.A.; Yngve, A.; Spiroski, I.; Obreja, G.; Starc, G.; Pérez, N.; Rito, A.I.; Kunešová, M.; et al. Prevalence of Severe Obesity among Primary School Children in 21 European Countries. Obes. Facts 2019, 12, 244–258. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation (WHO). Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 January 2022).
- Browne, N.T.; Snethen, J.A.; Greenberg, C.S.; Frenn, M.; Kilanowski, J.F.; Gance-Cleveland, B.; Burke, P.J.; Lewandowski, L. When Pandemics Collide: The Impact of COVID-19 on Childhood Obesity. J. Pediatr. Nurs. 2021, 56, 90–98. [Google Scholar] [CrossRef]
- Chung, Y.L.; Rhie, Y.J. Severe Obesity in Children and Adolescents: Metabolic Effects, Assessment, and Treatment. J. Obes. Metab. Syndr. 2021, 30, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Kinge, J.M.; Morris, S. The Impact of Childhood Obesity on Health and Health Service Use. Health Serv. Res. 2018, 53, 1621–1643. [Google Scholar] [CrossRef]
- World Health Organization. Report of the Commission on Ending Childhood Obesity; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Kim, S.H.; Després, J.P.; Koh, K.K. Obesity and cardiovascular disease: Friend or foe? Eur. Heart J. 2016, 37, 3560–3568. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Jin, C.; Guan, Q. Causal Effects of Overall and Abdominal Obesity on Insulin Resistance and the Risk of Type 2 Diabetes Mellitus: A Two-Sample Mendelian Randomization Study. Front. Genet. 2020, 11, 603. [Google Scholar] [CrossRef]
- Allison, D.B.; Zhu, S.K.; Plankey, M.; Faith, M.S.; Heo, M. Differential associations of body mass index and adiposity with all cause mortality among men in the first and second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) follow-up studies. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C.M.; Su, H.; Thomas, D.M.; Heo, M.; Golnabi, A.H.; Pietrobelli, A.; Heymsfield, S.B. Tri-ponderal mass index vs body mass index in estimating body fat during adolescence. JAMA Pediatr. 2017, 171, 629–636. [Google Scholar] [CrossRef]
- Rankinen, T.; Kim, S.Y.; Perusse, L.; Despres, J.P.; Bouchard, C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study Group. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010, 33, 920–922. [Google Scholar] [CrossRef] [Green Version]
- Maffeis, C.; Banzato, C.; Talamini, G. Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology. Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J. Pediatr. 2008, 152, 207–213. [Google Scholar] [CrossRef]
- Radetti, G.; Fanolla, A.; Grugni, G.; Lupi, F.; Sartorio, A. Indexes of adiposity and body composition in the prediction of metabolic syndrome in obese children and adolescents: Which is the best? Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Datta Banik, S.; Pacheco-Pantoja, E.; Lugo, R.; Gómez-de-Regil, L.; Chim Aké, R.; Méndez González, R.M.; Gutiérrez Solis, A.L. Evaluation of Anthropometric Indexes and Lipid Parameters to Predict Metabolic Syndrome Among Adults in Mexico. Diabetes Metab. Syndr. Obes. 2021, 14, 691–701. [Google Scholar] [CrossRef]
- Krawczyk, M.; Rumińska, M.; Witkowska-Sędek, E.; Majcher, A.; Pyrżak, B. Usefulness of the Triglycerides to High-Density Lipoprotein Cholesterol ratio (TG/HDL-C) in prediction of metabolic syndrome in Polish obese children and adolescents. Acta Biochim. Pol. 2018, 65, 605–611. [Google Scholar] [CrossRef]
- Aslan Çin, N.N.; Yardımcı, H.; Koç, N.; Uçaktürk, S.A.; Akçil Ok, M. Triglycerides/high-density lipoprotein cholesterol is a predictor similar to the triglyceride-glucose index for the diagnosis of metabolic syndrome using International Diabetes Federation criteria of insulin resistance in obese adolescents: A cross-sectional study. J. Pediatr. Endocrinol. Metab. 2020, 33, 777–784. [Google Scholar]
- Radetti, G.; Fanolla, A.; Grugni, G.; Lupi, F.; Tamini, S.; Cicolini, S.; Sartorio, A. The Role of Different Indexes of Adiposity and Body Composition for the Identification of Metabolic Syndrome in Women with Obesity. J. Clin. Med. 2021, 10, 1975. [Google Scholar] [CrossRef]
- Vizzuso, S.; Del Torto, A.; Dilillo, D.; Calcaterra, V.; Di Profio, E.; Leone, A.; Gilardini, L.; Bertoli, S.; Battezzati, A.; Zuccotti, G.V.; et al. Visceral Adiposity Index (VAI) in Children and Adolescents with Obesity: No Association with Daily Energy Intake but Promising Tool to Identify Metabolic Syndrome (MetS). Nutrients 2021, 13, 413. [Google Scholar] [CrossRef]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity and stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- Barlow, S.E.; Expert Committee. Recommendations regarding the prevention, assessment and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120, S164–S192. [Google Scholar] [CrossRef] [Green Version]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, treatment and prevention of pediatric obesity: Consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The WHO Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards/standards (accessed on 15 February 2022).
- Zimmet, P.; Alberti, K.G.; Kaufmann, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennet, P.; Shaw, J.; Caprio, S. IDF consensus group. The metabolic syndrome in children and adolescents-An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- McCharty, H.D.; Jarret, K.V.; Crawley, H.F. The development of waist circumference percentiles in British children aged 5.0-16.9 y. Eur. J. Clin. Nutr. 2001, 55, 902–907. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Obesity and Overweight. WHO Fact Sheet No. 311. 2014. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/# (accessed on 17 December 2021).
- Quijada, Z.; Paoli, M.; Zerpa, Y.; Camacho, N.; Cichetti, R.; Villarroel, V.; Arata-Bellabarba, G.; Lanes, R. The triglyceride/HDL-cholesterol ratio as a marker of cardiovascular risk in obese children; association with traditional and emergent risk factors. Pediatric Diabetes 2008, 9, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, I.; Daimon, T. The “cardiometabolic index” as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin. Chim. Acta 2015, 438, 274–278. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Srinivasan, S.R.; Chen, W.; Malina, R.M.; Bouchard, C.; Berenson, G.S. Body mass index, waist circumference, and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics 2004, 114, e198–e205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Chen, C. Growth Charts of Body Mass Index (BMI) with Quantile Regression. 2005. Available online: https://www.researchgate.net/profile/Colin-Chen-4/publication/220979218_Growth_Charts_of_Body_Mass_Index_BMI_With_Quantile_Regression/links/02bfe50ef9479c0a2f000000/Growth-Charts-of-Body-Mass-Index-BMI-With-Quantile-Regression.pdf (accessed on 7 February 2022).
- Cole, T.J.; Freeman, J.V.; Preece, M.A. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998, 17, 407–429. [Google Scholar] [CrossRef]
- Pinhas-Hamiel, O.; Hamiel, U.; Bendor, C.D.; Bardugo, A.; Twig, G.; Cukierman-Yaffe, T. The Global Spread of Severe Obesity in Toddlers, Children, and Adolescents: A Systematic Review and Meta-Analysis. Obes. Facts 2022, 15, 118–134. [Google Scholar]
- Center for Disease Control and Prevention. Prevalence of Overweight, Obesity, and Severe Obesity Among Children and Adolescents Aged 2–19 Years: United States, 1963–1965 through 2017–2018. Available online: https://www.cdc.gov/nchs/data/hestat/obesity-child-17-18/obesity-child.htm (accessed on 9 April 2022).
- Lauria, L.; Spinelli, A.; Buoncristiano, M.; Nardone, P. Decline of childhood overweight and obesity in Italy from 2008 to 2016: Results from 5 rounds of the population-based surveillance system. BMC Public Health 2019, 19, 618. [Google Scholar]
- Lombardo, F.L.; Spinelli, A.; Lazzeri, G.; Lamberti, A.; Mazzarella, G.; Nardone, P.; Pilato, V.; Buoncristiano, M.; Caroli, M.; OKkio alla SALUTE Group 2010. Severe obesity prevalence in 8- to 9-year-old Italian children: A large population-based study. Eur. J. Clin. Nutr. 2015, 69, 603–608. [Google Scholar] [CrossRef]
- Nur Zati Iwani, A.K.; Jalaludin, M.Y.; Yahya, A.; Mansor, F.; Md Zain, F.; Hong, J.Y.H.; Wan Mohd Zin, R.M.; Mokhtar, A.H. TG: HDL-C Ratio as Insulin Resistance Marker for Metabolic Syndrome in Children with Obesity. Front. Endocrinol. 2022, 13, 852290. [Google Scholar] [CrossRef]
- Chu, S.Y.; Jung, J.H.; Park, M.J.; Kim, S.H. Risk assessment of metabolic syndrome in adolescents using the triglyceride/high-density lipoprotein cholesterol ratio and the total cholesterol/high-density lipoprotein cholesterol ratio. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Nie, G.; Hou, S.; Zhang, M.; Peng, W. High TG/HDL ratio suggests a higher risk of metabolic syndrome among an elderly Chinese population: A cross-sectional study. BMJ Open 2021, 11, e041519. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Moio, N.; Scilla, C.; Cavuto, L.; Sibilio, G.; Sanguigno, E.; Forziato, C.; Saitta, F.; Iardino, M.R.; Di Carluccio, C.; et al. Usefulness of the High Triglyceride-to- HDL Cholesterol Ratio to Identify Cardiometabolic Risk Factors and Preclinical Signs of Organ Damage in Outpatient Children. Diabetes Care 2012, 35, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Di Bonito, P.; Valerio, G.; Grugni, G.; Licenziati, M.R.; Maffeis, C.; Manco, M.; Miraglia del Giudice, E.; Pacifico, L.; Pellegrin, M.C.; Tomat, M.; et al. CARdiometabolic risk factors in overweight and obese children in ITALY (CARITALY) Study Group. Comparison of non-HDL-cholesterol versus triglycerides-to-HDL-cholesterol ratio in relation to cardiometabolic risk factors and preclinical organ damage in overweight/obese children: The CARITALY study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 489–494. [Google Scholar]
- Grugni, G.; Licenziati, M.R.; Valerio, G.; Crinò, A.; Maffeis, C.; Tanas, R.; Morino, G.S.; Childhood Obesity Study Group of the Italian Society for Pediatric Endocrinology and Diabetology (ISPED). The rehabilitation of children and adolescents with severe or medically complicated obesity: An ISPED expert opinion document. Eat Weight Disord. 2017, 22, 3–12. [Google Scholar] [CrossRef]
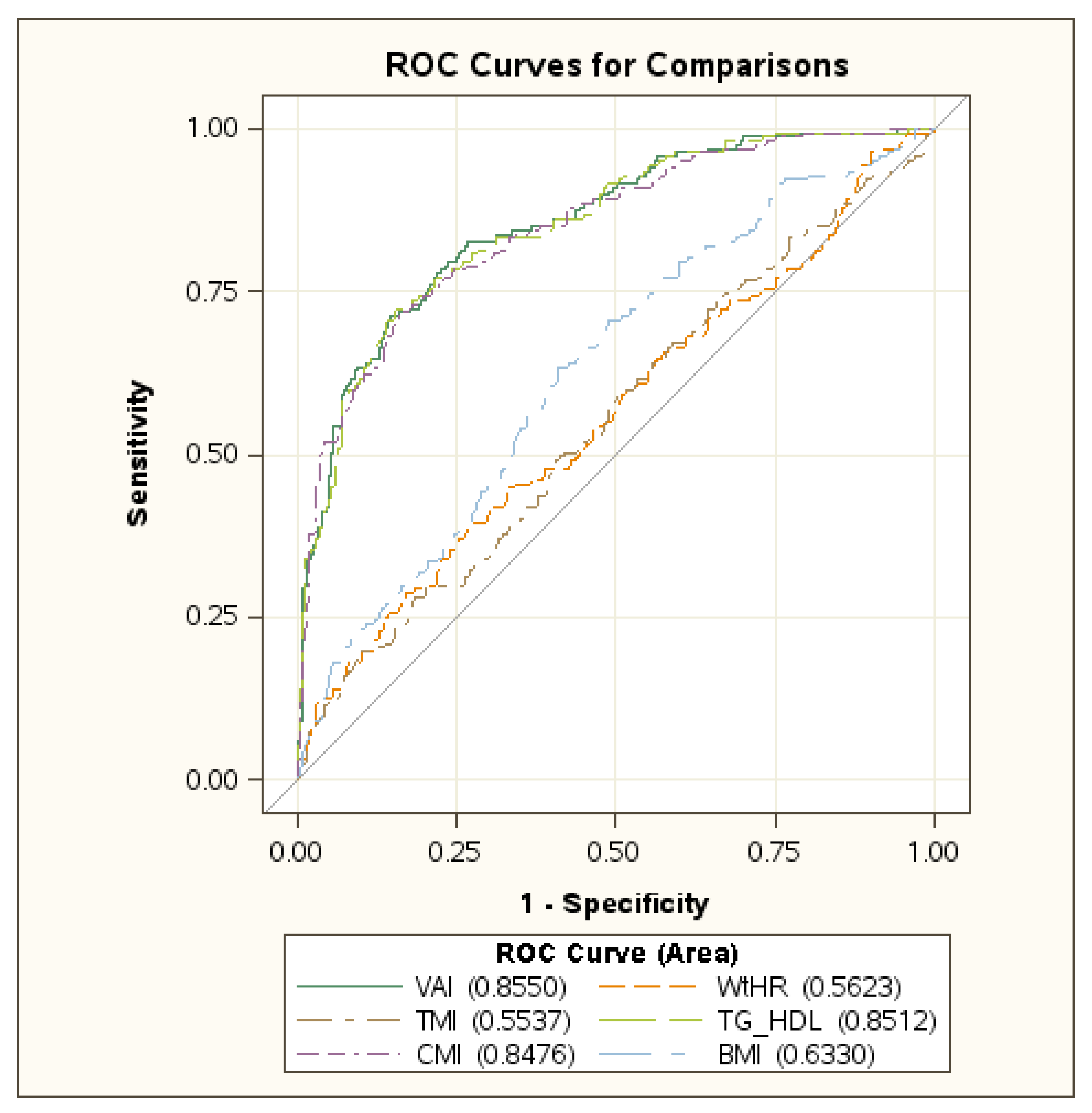
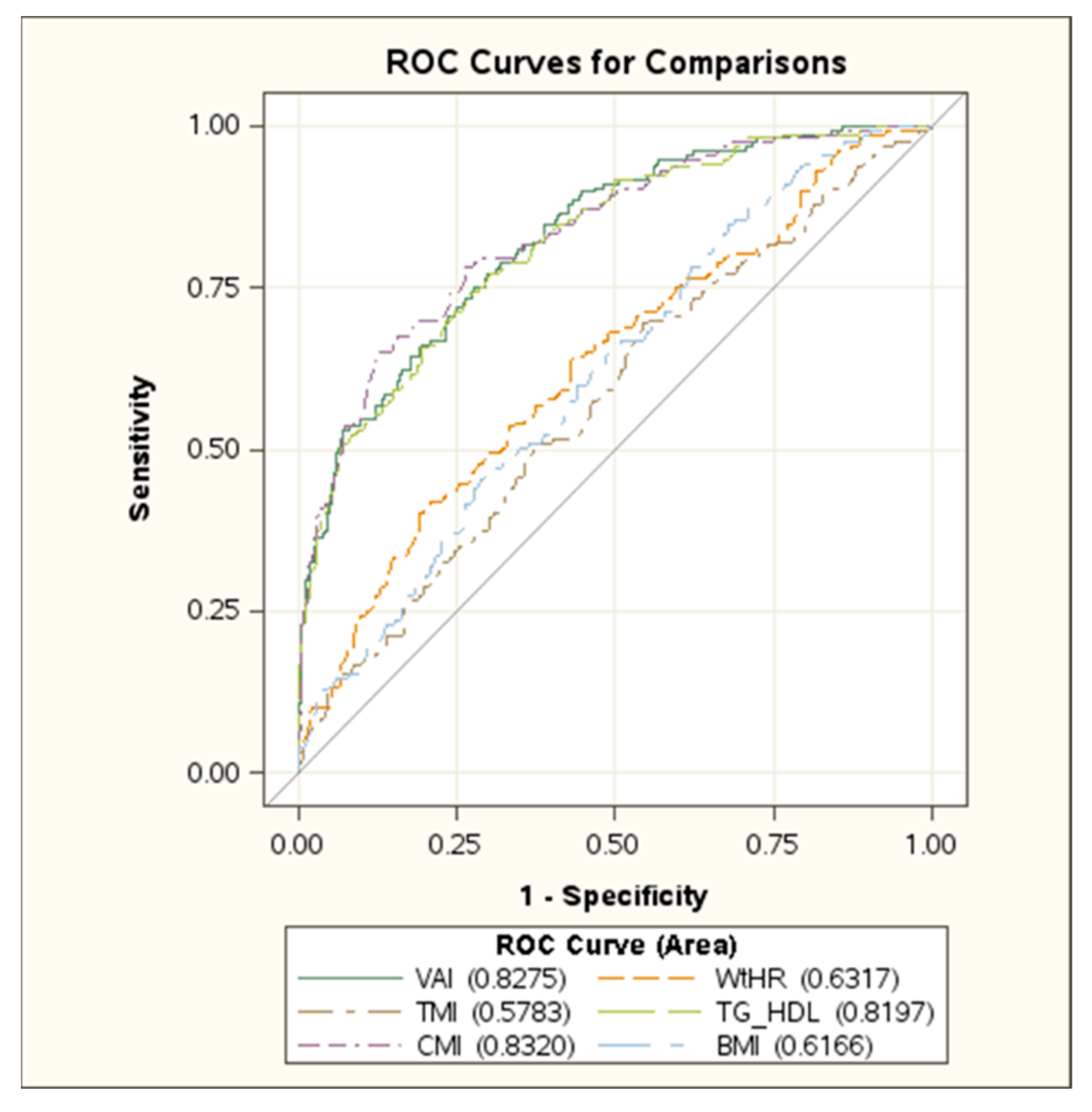
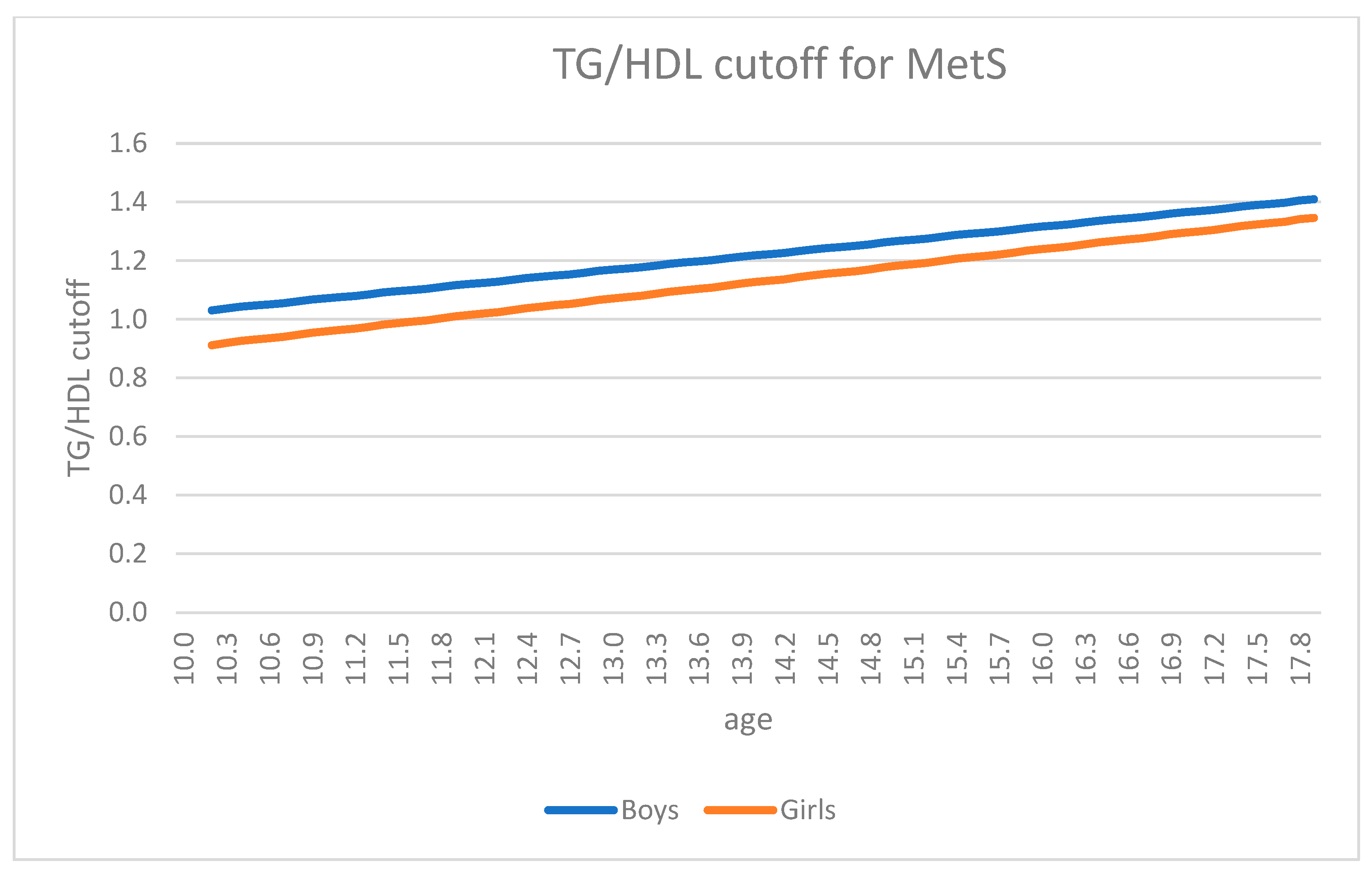
| Total (n = 1065) | Boys (n = 502) | Girls (n = 563) | |
|---|---|---|---|
| Age (yr) | 14.9 (13–16.4) | 14.7 (12.7–16.2) & | 15 (13.2–16.5) |
| Weight (kg) | 105.2 (93.1–119.4) | 110.5 (93.1–126.9) * | 101.8 (93–114) |
| Height (cm) | 1.6 (1.6–1.7) | 1.7 (1.6–1.8) | 1.6 (1.6–1.7) |
| BMI (kg/m2) | 38.8 (36–42.6) | 38.3 (35.3–41.8) * | 39.3 (36.7–42.8) |
| BMI SDS | 3.5 (3.2–3.7) | 3.5 (3.3–3.7) | 3.4 (3.2–3.7) |
| Waist Circumference (cm) | 117 (107–126) | 120 (110–129) * | 115 (106–124) |
| Glucose (mmol/L) | 79 (74–84) | 80 (76–84) $ | 79 (73–83) |
| Insulin (µU/mL) | 14.8 (10–19.9) | 15.3 (10–20.1) | 14.5 (10–19.7) |
| HOMA | 2.9 (1.9–3.9) | 3 (2–4.1) | 2.8 (1.9–3.8) |
| HDL Cholesterol (mg/dL) | 42 (36–49) | 41 (35–47) * | 44 (37–50) |
| LDL Cholesterol (mg/dL) | 104 (86–123) | 106.5 (87–126) | 102 (84–122) |
| Triglycerides (mg/dL) | 90 (69–119) | 93 (71–125) & | 88 (68–113) |
| SBP (mm/Hg) | 125 (120–130) | 130 (120–140) * | 120 (120–130) |
| DBP (mm/Hg) | 80 (70–80) | 80 (70–80) & | 80 (70–80) |
| VAI | 1.5 (1.1–2.2) | 1.4 (0.9–1.9) * | 1.7 (1.2–2.4) |
| WtHR (cm/cm) | 0.72 (0.67–0.76) | 0.71 (0.67–0.76) | 0.72 (0.66–0.77) |
| TMI (kg/m3) | 23.8 (22–25.9) | 22.7 (21.1–25) * | 24.5 (22.8–26.7) |
| TG/HDL | 0.9 (0.7–1.3) | 1 (0.7–1.4) * | 0.9 (0.6–1.2) |
| CMI | 0.7 (0.5–1) | 0.7 (0.5–1) * | 0.6 (0.4–0.9) |
| MetS | No MetS | |
|---|---|---|
| Number of subjects | 741 | 324 |
| Age (yr) | 15.8 (14.2–16.8) * | 14.5 (12.6–16) |
| Weight (kg) | 114.4 (100.8–130.6) * | 101.2 (90.1–115.8) |
| Height (cm) | 1.7 (1.6–1.7) * | 1.6 (1.6–1.7) |
| BMI (kg/m2) | 40 (37.3–43.9) * | 38.3 (35.7–41.7) |
| BMI SDS | 3.5 (3.3–3.8) | 3.4 (3.2–3.7) |
| WC (cm) | 123 (113–133) * | 115 (106–124) |
| Glucose (mmol/L) | 79 (75–84) | 79 (74–84) |
| Insulin (µU/mL) | 17.1 (13.1–22.8) * | 13.4 (9.3–18.7) |
| HOMA | 3.3 (2.5–4.6) * | 2.6 (1.8–3.7) |
| HDL Cholesterol (mg/dL) | 35 (32–38.5) * | 46 (41–51) |
| LDL Cholesterol (mg/dL) | 108 (86.5–127) & | 102 (86–123) |
| Triglycerides (mg/dL) | 117 (88–157) * | 83 (66–105) |
| SBP (mm/Hg) | 130 (130–140) * | 120 (120–130) |
| DBP (mm/Hg) | 80 (80–90) * | 80 (70–80) |
| VAI | 2.3 (1.7–3.3) * | 1.3 (0.9–1.7) |
| WtHR (cm/cm) | 0.73 (0.68–0.79) * | 0.71 (0.67–0.75) |
| TMI (kg/m3) | 24.1 (22.2–26.7) $ | 23.6 (21.9–25.8) |
| TG/HDL | 1.5 (1.1–2.1) * | 0.8 (0.6–1.1) |
| CMI | 1.1 (0.8–1.5) * | 0.6 (0.4–0.8) |
| (a) | ||
|---|---|---|
| Boys | Girls | |
| Sensitivity | 0.713 (0.673–0.752) | 0.752 (0.716–0.787) |
| Specificity | 0.854 (0.823–0.885) | 0.724 (0.687–0.761) |
| PPV | 0.708 (0.669–0.748) | 0.513 (0.472–0.554) |
| NPV | 0.856 (0.826–0.887) | 0.883 (0.856–0.909) |
| PLR | 4.9 | 2.7 |
| NLR | 0.3 | 0.3 |
| (b) | ||
| Boys | Girls | |
| Sensitivity | 0.719 (0.679–0.758) | 0.65 (0.61–0.689) * |
| Specifity | 0.836 (0.803–0.868) | 0.872 (0.844–0.9) * |
| PPV | 0.686 (0.645–0.726) | 0.662 (0.623–0.701) * |
| NPV | 0.856 (0.826–0.887) | 0.866 (0.837–0.894) |
| PLR | 4.4 | 5.1 * |
| NLR | 0.3 | 0.4 |
| (c) | ||
| Boys | Girls | |
| Sensitivity | 0.725 (0.685–0.764) | 0.745 (0.709–0.781) |
| Specifity | 0.845 (0.813–0.876) | 0.724 (0.687–0.761) |
| PPV | 0.699 (0.659–0.74) | 0.511 (0.47–0.552) |
| NPV | 0.86 (0.83–0.891) | 0.88 (0.853–0.907) |
| PLR | 4.7 | 2.7 |
| NLR | 0.3 | 0.4 |
| Total | Boys | Girls | |
|---|---|---|---|
| Weight (kg) | 0.25 * | 0.25 * | 0.08 |
| Height (cm) | 0.19 * | 0.19 * | 0.04 |
| BMI (kg/m2) | 0.22 * | 0.22 * | 0.08 |
| WC (cm) | 0.23 * | 0.23 * | 0.17 * |
| Glucose (mmol/L) | −0.01 | 0.00 | 0.04 |
| HDL Cholesterol (mg/dL) | −0.68 * | −0.67 * | −0.70 * |
| LDL Cholesterol (mg/dL) | 0.22 * | 0.22 * | 0.42 * |
| Triglycerides (mg/dL) | 0.89 * | 0.89 * | 0.90 * |
| SBP (mm/Hg) | 0.11 & | 0.11 & | 0.10 & |
| DBP (mm/Hg) | 0.08 | 0.08 | 0.05 |
| BMI SDS | 0.16 $ | 0.16 $ | 0.08 |
| Boys | Girls | |||
|---|---|---|---|---|
| OR (95% CI) | Pseudo R Square | OR (95% CI) | Pseudo R Square | |
| Large WC (IDF) | n.c. * | n.c. * | 267.1 (0.1 to infinite) | 0.138 |
| High Glucose (IDF) | 1.7 (0.5–6.1) | 0.025 | 1.8 (0.6–5.2) | 0.034 |
| Low HDL cholesterol (IDF) | 17.1 (9.8–29.9) & | 0.43 | 22.9 (12.4–42.2) & | 0.456 |
| High Triglycerides (IDF) | 121.2 (39.6–370.6) & | 0.681 | 167.3 (50.3–556.9) & | 0.759 |
| High Blood Pressure (IDF) | 0.9 (0.6–1.2) | 0.19 | 1.3 (1–1.7) | 0.056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radetti, G.; Grugni, G.; Lupi, F.; Fanolla, A.; Caroli, D.; Bondesan, A.; Sartorio, A. High Tg/HDL-Cholesterol Ratio Highlights a Higher Risk of Metabolic Syndrome in Children and Adolescents with Severe Obesity. J. Clin. Med. 2022, 11, 4488. https://doi.org/10.3390/jcm11154488
Radetti G, Grugni G, Lupi F, Fanolla A, Caroli D, Bondesan A, Sartorio A. High Tg/HDL-Cholesterol Ratio Highlights a Higher Risk of Metabolic Syndrome in Children and Adolescents with Severe Obesity. Journal of Clinical Medicine. 2022; 11(15):4488. https://doi.org/10.3390/jcm11154488
Chicago/Turabian StyleRadetti, Giorgio, Graziano Grugni, Fiorenzo Lupi, Antonio Fanolla, Diana Caroli, Adele Bondesan, and Alessandro Sartorio. 2022. "High Tg/HDL-Cholesterol Ratio Highlights a Higher Risk of Metabolic Syndrome in Children and Adolescents with Severe Obesity" Journal of Clinical Medicine 11, no. 15: 4488. https://doi.org/10.3390/jcm11154488
APA StyleRadetti, G., Grugni, G., Lupi, F., Fanolla, A., Caroli, D., Bondesan, A., & Sartorio, A. (2022). High Tg/HDL-Cholesterol Ratio Highlights a Higher Risk of Metabolic Syndrome in Children and Adolescents with Severe Obesity. Journal of Clinical Medicine, 11(15), 4488. https://doi.org/10.3390/jcm11154488







