Pachychoroid Spectrum Diseases in Patients with Cushing’s Syndrome: A Systematic Review with Meta-Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Eligibility Criteria
2.2. Information Sources, Search Strategy, and Study Selection
2.3. Data Extraction and Risk of Bias within Studies
2.4. Synthesis of the Results and Risk of Bias across Studies
3. Results
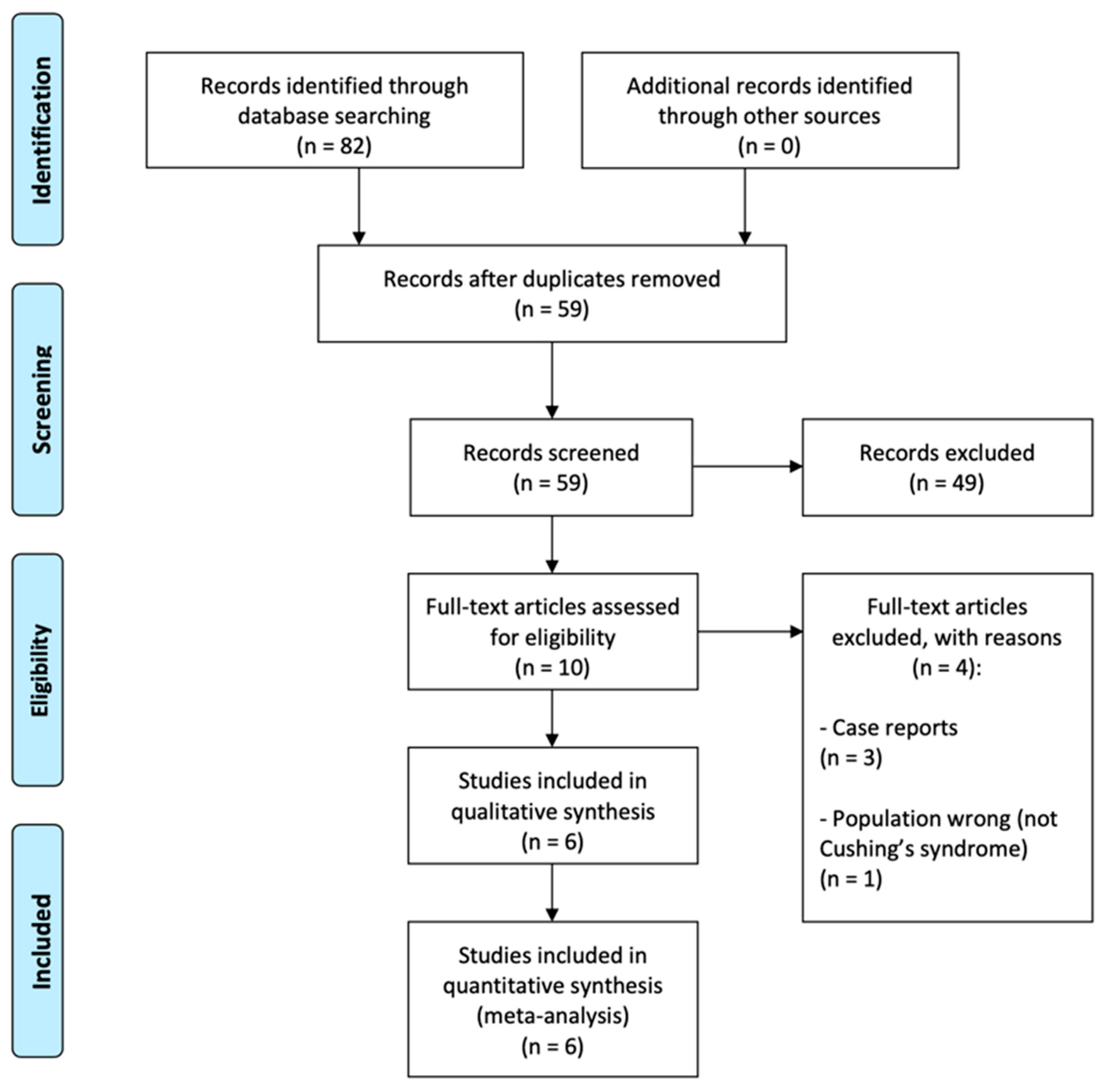
3.1. Literature Search and Study Selection
3.2. Study Characteristics
3.3. Results of Individual Studies
3.4. Risk of Bias of Individual Studies
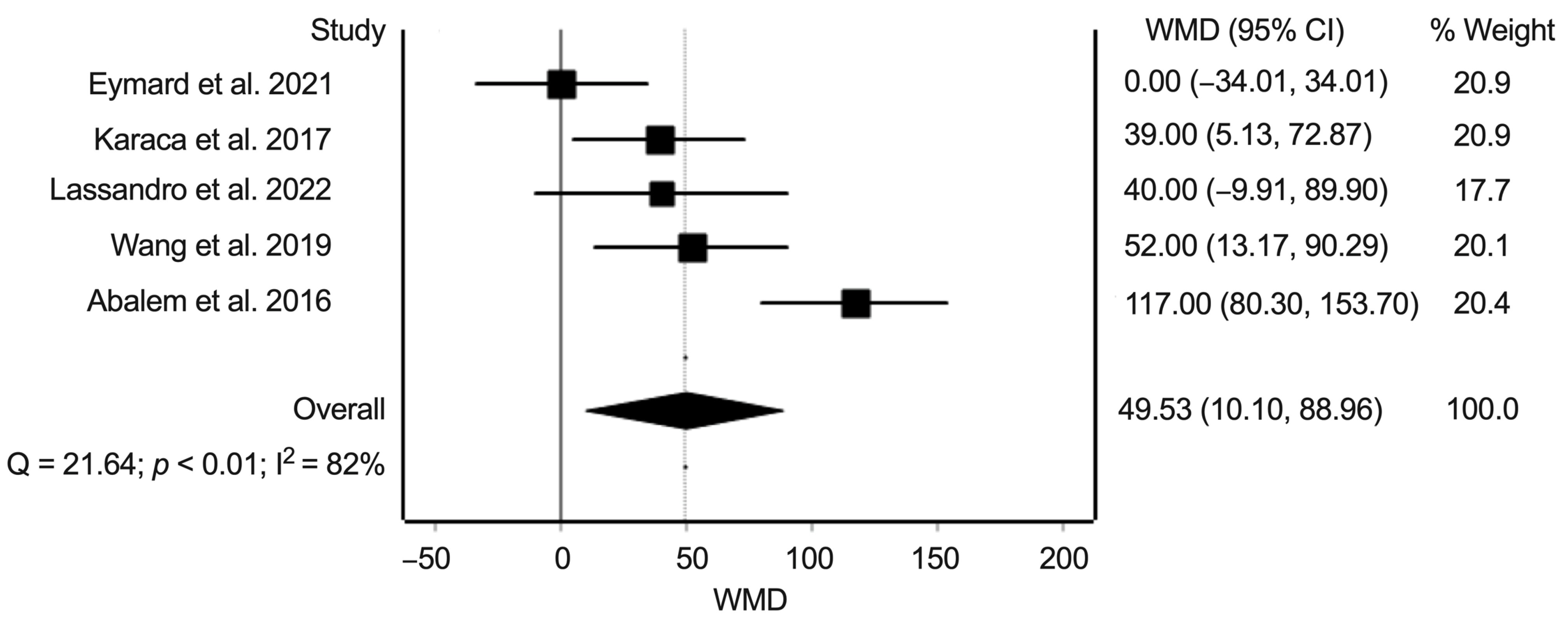
3.5. Synthesis of Results: Difference in Subfoveal Choroidal Thickness between Eyes from Patients with Cushing’s Syndrome and Healthy Controls
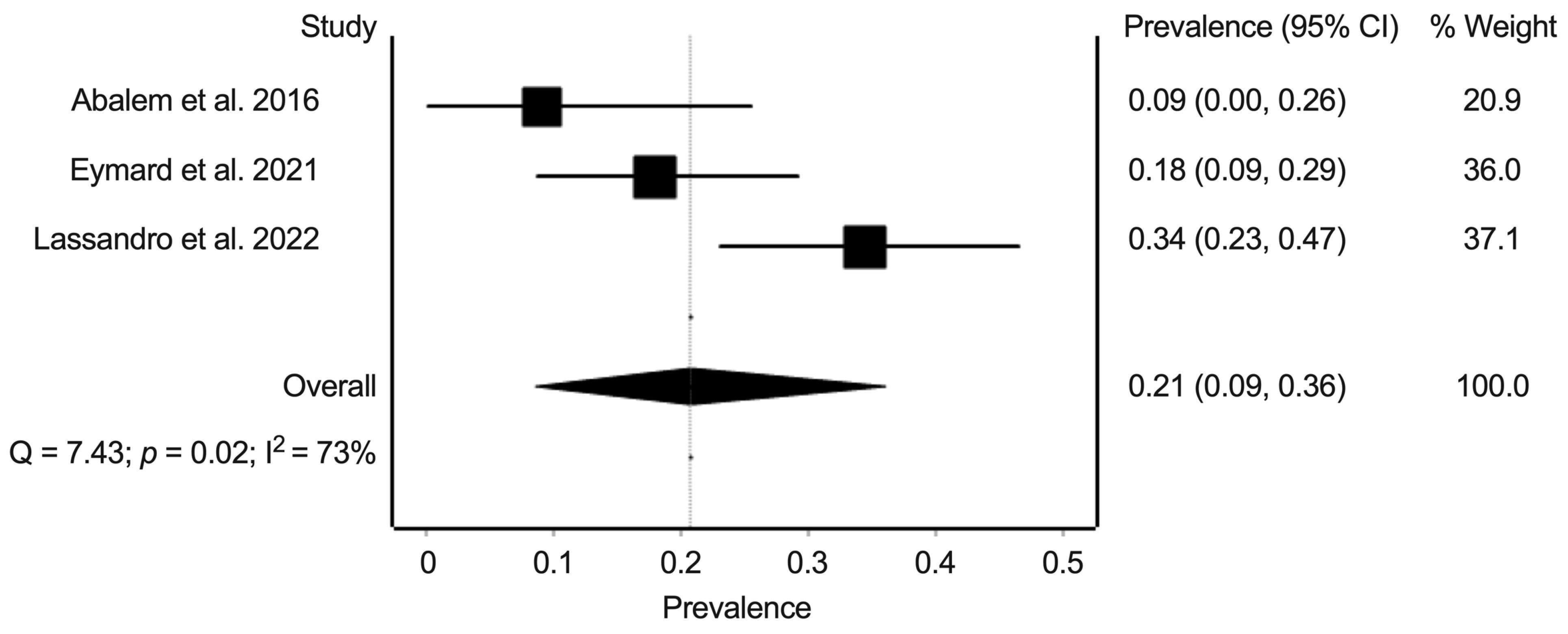
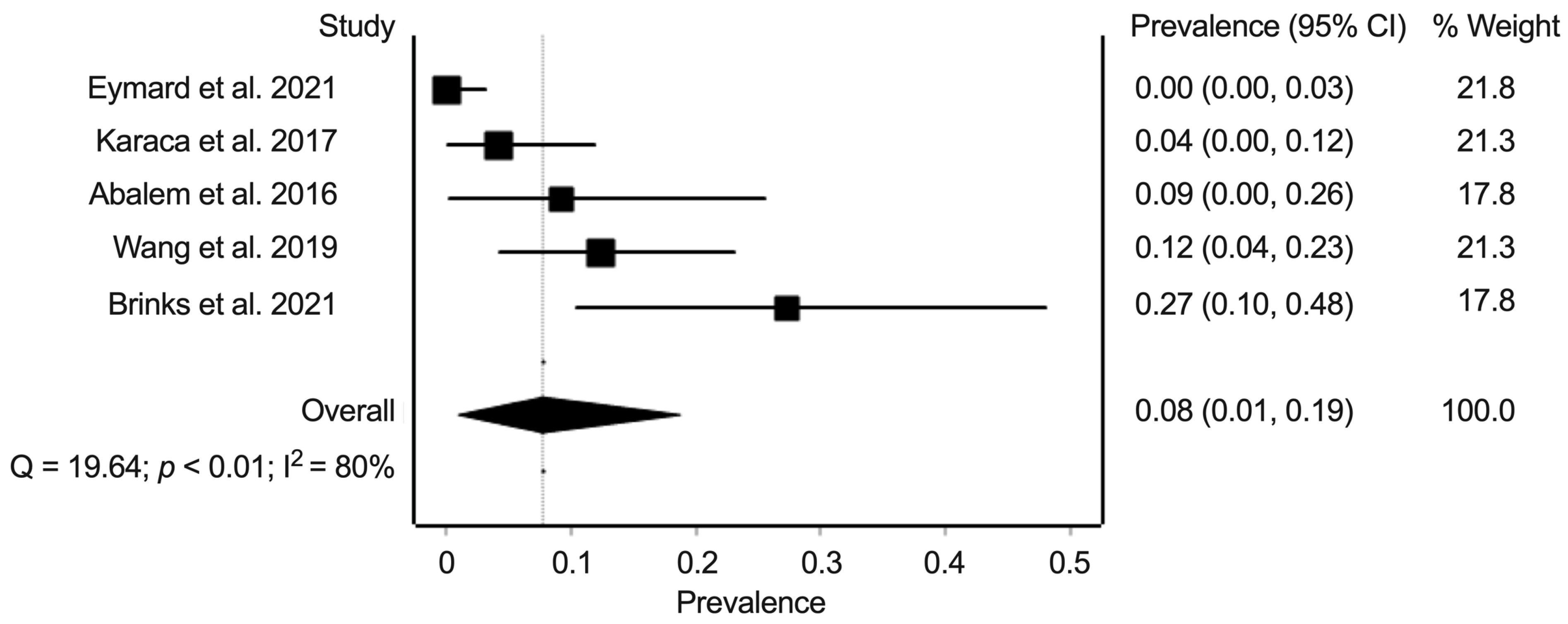
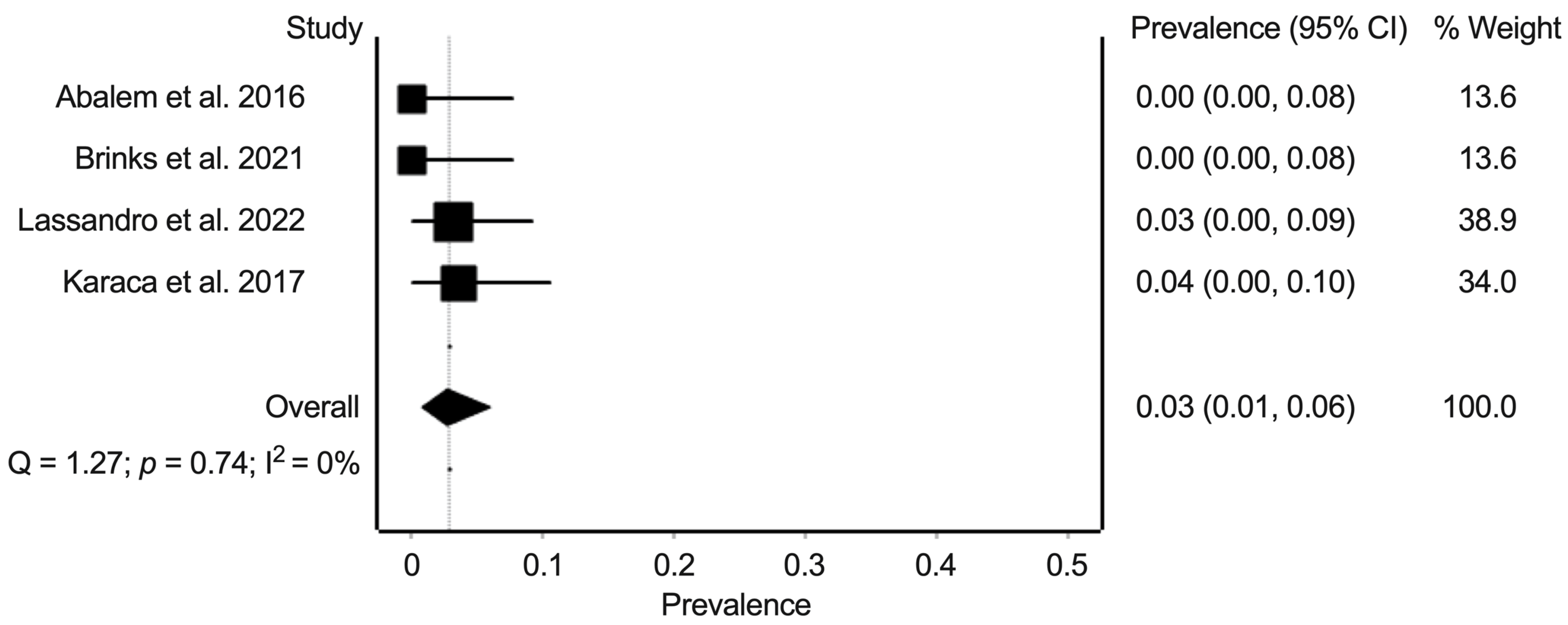
3.6. Synthesis of Results: Prevalence of Pachychoroid Pigment Epitheliopathy, Central Serous Chorioretinopathy, and Polypoidal Choroidal Vasculopathy in Eyes from Patients with Cushing’s Syndrome and Healthy Controls
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Haalen, F.M.; Broersen, L.H.A.; Jorgensen, J.O.; Pereira, A.M.; Dekkers, O.M. Management of endocrine disease: Mortality remains increased in Cushing’s disease despite biochemical remission: A systematic review and meta-analysis. Eur. J. Endocrinol. 2015, 172, R143–R149. [Google Scholar] [CrossRef] [Green Version]
- Limumpornpetch, P.; Morgan, A.W.; Tiganescu, A.; Baxter, P.D.; Nyaga, V.N.; Pujades-Rodriguez, M.; Stewart, P.M. The effect of endogenous Cushing’s syndrome on all-cause and cause-specific mortality: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2022, 107, 2377–2388. [Google Scholar] [CrossRef]
- Nieman, L.K. Cushing’s syndrome: Update on signs, symptoms and biochemical screening. Eur. J. Endocrinol. 2015, 173, M33–M38. [Google Scholar] [CrossRef] [Green Version]
- Hakami, O.A.; Ahmed, S.; Karavitaki, N. Epidemiology and mortality of Cushing’s syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101521. [Google Scholar] [CrossRef]
- Zhou, X.; Komuku, Y.; Araki, T.; Hozumi, K.; Terasaki, H.; Miki, A.; Kuwayama, S.; Niki, M.; Matsubara, H.; Kinoshita, T.; et al. A multicentre study of the risk factors associated with recurrence of central serous chorioretinopathy. Acta Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Azad, A.D.; Zhou, M.; Afshar, A.R.; Bakri, S.J.; Pershing, S. Systemic Corticosteroid Use after Central Serous Chorioretinopathy Diagnosis. Ophthalmology 2021, 128, 121–129. [Google Scholar] [CrossRef]
- Spaide, R.F.; Cheung, C.M.G.; Matsumoto, H.; Kishi, S.; Boon, C.J.F.; van Dijk, E.H.C.; Mauget-Faysse, M.; Behar-Cohen, F.; Hartnett, M.E.; Sivaprasad, S.; et al. Venous overload choroidopathy: A hypothetical framework for central serous chorioretinopathy and allied disorders. Prog. Retin. Eye Res. 2022, 86, 100973. [Google Scholar] [CrossRef]
- Brinks, J.; van Dijk, E.H.C.; Meijer, O.C.; Schlingemann, R.O.; Boon, C.J.F. Choroidal arteriovenous anastomoses: A hypothesis for the pathogenesis of central serous chorioretinopathy and other pachychoroid disease spectrum abnormalities. Acta Ophthalmol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Borooah, S.; Sim, P.Y.; Phatak, S.; Moraes, G.; Wu, C.Y.; Cheung, C.M.G.; Pal, B.; Bujarborua, D. Pachychoroid spectrum disease. Acta Ophthalmol. 2021, 99, e806–e822. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid disease. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 2015, 8, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Abalem, M.F.; Machado, M.C.; Santos, H.N.V.D.; Garcia, R.; Helal, J., Jr.; Corricondo, P.C.; Pimentel, S.L.G.; Monteiro, M.L.R.; Qian, C.X.; Bronstein, M.D.; et al. Choroidal and Retinal Abnormalities by Optical Coherence Tomography in Endogenous Cushing’s Syndrome. Front. Endocrinol. 2016, 7, 154. [Google Scholar] [CrossRef] [Green Version]
- Brinks, J.; van Haalen, F.M.; van Rijssen, T.J.; Biermasz, N.R.; Meijer, O.C.; Pereira, A.M.; Boon, C.J.F.; van Dijk, E.H.C. Central serous chorioretinopathy in active endogenous Cushing’s syndrome. Sci. Rep. 2021, 11, 2748. [Google Scholar] [CrossRef]
- Eymard, P.; Gerardy, M.; Bouys, L.; Mehanna, C.; Bertherat, J.; Behar-Cohen, F.; Bousquet, E. Choroidal imaging in patients with Cushing syndrome. Acta Ophthalmol. 2021, 99, 533–537. [Google Scholar] [CrossRef]
- Karaca, C.; Karaca, Z.; Kahraman, N.; Sirakaya, E.; Oner, A.; Mirza, G.E. Is there a role of ACTH in increased choroidal thickness in Cushing syndrome? Retina 2017, 37, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Lassandro, N.V.; Nicolai, M.; Arnaldi, G.; Franceschi, A.; Pelliccioni, P.; Cantini, L.; Gesuita, R.; Faragalli, A.; Mariotti, C. Pachychoroid spectrum disease and choriocapillary flow analysis in patients with Cushing disease: An optical coherence tomography angiography study. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 1535–1542. [Google Scholar] [CrossRef]
- Wang, E.; Chen, S.; Yang, H.; Yang, J.; Li, Y.; Chen, Y. Choroidal Thickening and pachychoroid in Cushing syndrome: Correlation with endogenous cortisol level. Retina 2019, 39, 408–414. [Google Scholar] [CrossRef]
- Li, Y.; You, Q.S.; Wei, W.B.; Xu, J.; Chen, C.X.; Wang, Y.X.; Xu, L.; Jonas, J.B. Prevalence and associations of central serous chorioretinopathy in elderly Chinese. The Beijing Eye Study 2011. Acta Ophthalmol. 2016, 94, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, E.H.C.; Holtz, J.K.; Sirks, M.J.; Larsson, J.M.E.; Diederen, R.M.H.; Schlingemann, R.O.; Boon, C.J.F.; Subhi, Y. European Prevalence of Polypoidal Choroidal Vasculopathy: A Systematic Review, Meta-Analysis, and Forecasting Study. 2022; submitted, in review. [Google Scholar]
- Brinks, J.; van Dijk, E.H.C.; Klaassen, I.; Schlingemann, R.O.; Kielbasa, S.M.; Emri, E.; Quax, P.H.A.; Bergen, A.A.; Meijer, O.C.; Boon, C.J.F. Exploring the choroidal vascular labyrinth and its molecular and structural roles in health and disease. Prog. Retin. Eye Res. 2022, 87, 100994. [Google Scholar] [CrossRef] [PubMed]
- Subhi, Y.; Forshaw, T.; Sørensen, T.L. Macular thickness and volume in the elderly: A systematic review. Ageing Res. Rev. 2016, 29, 42–49. [Google Scholar] [CrossRef]
- Borgersen, N.J.; Møller-Lorentzen, T.; Sørensen, T.L.; Subhi, Y. Association between C-reactive protein and polypoidal choroidal vasculopathy: A systematic review and meta-analysis. Acta Ophthalmol. 2021, 99, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Subhi, Y.; Nielsen, M.K.; Molbech, C.R.; Oishi, A.; Singh, A.; Nissen, M.H.; Sørensen, T.L. Polypoidal Choroidal Vasculopathy Associate With Diminished Regulatory T Cells That Are Polarized Into a T Helper 2-Like Phenotype. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2583–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinks, J.; van Dijk, E.H.C.; Kielbasa, S.M.; Mei, H.; van der Veen, I.; Peters, H.A.B.; Sips, H.C.M.; Notenboom, R.G.E.; Quax, P.H.A.; Boon, C.J.F.; et al. The Cortisol Response of Male and Female Choroidal Endothelial Cells: Implications for Central Serous Chorioretinopathy. J. Clin. Endocrinol. Metab. 2022, 107, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.; Yang, E.; Lee, W.K.; Lee, G.K.; Mathur, R.; Cheng, J.; Wong, D.; Wong, T.Y.; Lai, T.Y. The natural history of polypoidal choroidal vasculopathy: A multi-center series of untreated Asian patients. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 2075–2085. [Google Scholar] [CrossRef]
- Subhi, Y.; Sørensen, T.L. Valsalva-Related Subretinal Hemorrhage as a Presenting Symptom of Polypoidal Choroidal Vasculopathy. Case Rep. Ophthalmol. Med. 2017, 2017, 9650287. [Google Scholar] [CrossRef] [Green Version]
| Reference | Country | Study Design | Study Population | Definition of Cushing’s Syndrome |
|---|---|---|---|---|
| Abalem et al., 2016 [15] | Brazil | Cross-sectional |
| ≥2 abnormal screening tests (insufficient suppression of cortisol after low dose dexamethasone suppression test, increased salivary cortisol, or increased 24-h urinary free cortisol). |
| Brinks et al., 2021 [16] | Netherlands | Cross-sectional |
| ≥2 abnormal screening tests (insufficient suppression of cortisol after low dose dexamethasone suppression test, increased salivary cortisol, or increased 24-h urinary free cortisol). Etiology of the disease was assessed by MRI or CT. |
| Eymard et al., 2021 [17] | France | Cross-sectional |
| Hormone testing (not specified in detail). Etiology of the disease was assessed by imaging. |
| Karaca et al., 2017 [18] | Turkey | Cross-sectional |
| Low-dose dexamethasone suppression test, midnight serum cortisol test, adrenocorticotropic hormone test, and dehydroepiandrosterone sulfate test. Etiology of the disease was assessed by imaging. |
| Lassandro et al., 2022 [19] | Italy | Cross-sectional |
| ≥2 abnormal screening tests (insufficient suppression of cortisol after low-dose dexamethasone suppression test, increased salivary cortisol, or increased 24-h urinary free cortisol). |
| Wang et al., 2019 [20] | China | Cross-sectional |
| Presence of cushingoid appearance, failure to achieve midnight nadir in cortisol diurnal rhythm, lack of negative feedback in low-dose dexamethasone suppression test, increased excretion of urine-free cortisol, and imaging of the pituitary and the adrenal gland. All patients with Cushing’s syndrome had plasma-free cortisol, 24-h urinary free cortisol, and plasma adrenocorticotropic hormone test. |
| Reference | General Ophthalmic Examination | OCT Protocol | Outcomes of Interest for This Review |
|---|---|---|---|
| Abalem et al., 2016 [15] | Slit-lamp biomicroscopy, indirect fundoscopy, axial length measurement, and retinal OCT. Pupillary dilation was not reported. | SD-OCT (Spectralis, Heidelberg Engineering) with an EDI protocol (horizontal and vertical scans, seven sections, high resolution mode, 25 frames). All OCTs were obtained at the same time (without further specification). | SFCT was measured. Patients with any PPE, CSC, and PCV were reported. |
| Brinks et al., 2021 [16] | Indirect ophthalmoscopy, fundus photography, fundus autofluorescence, fluorescein angiography, and retinal OCT. Pupils were dilated. | SD-OCT (Spectralis HRA + OCT, Heidelberg Engineering) with an EDI protocol. | SFCT was measured. Presence of any CSC was reported. |
| Eymard et al., 2021 [17] | Slit-lamp biomicroscopy, fundus photography, fundus photography, fundus autofluorescence, and retinal OCT. Pupillary dilation was not reported. | SD-OCT (Spectralis, Heidelberg Engineering) with an EDI protocol and OCT-angiography (OCT-A, RTVue XR Avanti, Optovue Inc.). EDI scans were all performed between 2 pm and 5 pm. | SFCT was measured. Presence of any PPE, CSC, and PCV were reported. |
| Karaca et al., 2017 [18] | Slit-lamp biomicroscopy, indirect fundoscopy, axial length measurement, and retinal OCT. Pupillary dilation was not reported. | SD-OCT (Spectralis, Heidelberg Engineering) with an EDI protocol (horizontal scans, seven sections, 100 averaged images). | SFCT was measured. Presence of any CSC was reported. |
| Lassandro et al., 2022 [19] | Slit-lamp biomicroscopy, retinal OCT, and in select cases also fluorescein and indocyanine green angiography. Pupillary dilation was not reported. | SD-OCT (RS-3000 Advance 2, Nidek Co., Ltd.) with an EDI protocol. OCT scans were all performed between 1 pm and 5 pm. | SFCT was measured. Presence of any PPE, CSC, and PCV were reported. |
| Wang et al., 2019 [20] | Slit-lamp biomicroscopy, indirect ophthalmoscopy, axial length measurement, and retinal OCT. Pupillary dilation was not reported. | SS-OCT (DRI OCT Triton plus, Topcon Corp.). All scans were performed in the afternoon. | SFCT was measured. Presence of any CSC was reported. |
| Reference | Patients with Cushing’s Syndrome | Healthy Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Age | Females | Etiology | Duration of Disease | N | Age | Females | |
| Abalem et al., 2016 [15] | 11 | 38 ± 16 | 100% | 8 pituitary adenoma, 1 adrenocortical adenoma, 1 adrenocortical carcinoma, 1 primary macronodular adrenal hyperplasia. | 5–360 months | 12 | 51 ± 17 | 100% |
| Brinks et al., 2021 [16] | 11 | 53 ± 16 | 64% | 7 pituitary adenoma, 3 adrenal adenoma, 1 undetermined. | 1–25 weeks | — | ||
| Eymard et al., 2021 [17] | 28 | 47 ± 15 | 82% | 19 pituitary adenoma, 4 primary macronodular adrenal hyperplasia, 2 adrenocortical carcinoma, 2 ectopic ACTH secretion, 1 undetermined. | — | 28 | 46 ± 12 | 82% |
| Karaca et al., 2017 [18] | 28 | 43 ± 13 | 75% | 16 ACTH secreting pituitary adenoma, 10 unilateral cortisol-producing adenoma, 2 bilateral macronodular adrenal hyperplasia | — | 38 | 44 ± 12 | 76% |
| Lassandro et al., 2022 [19] | 32 | Median 48 | 84% | — | Mean 95 months | 32 | Median 48 | 86% |
| Wang et al., 2019 [20] | 49 | 41 ± 12 | 88% | 44 pituitary adenoma, 5 adrenal gland adenoma | — | 49 | 41 ± 12 | 88% |
| Reference | Defines Source | Eligibility Criteria | Time Period | Consecutive Recruitment | Quality Assurance | Explains Exclusions |
|---|---|---|---|---|---|---|
| Abalem et al., 2016 [15] | Yes | Yes | Yes | Unclear | Yes | Not relevant |
| Brinks et al., 2021 [16] | Yes | Yes | Yes | Yes | Yes | Not relevant |
| Eymard et al., 2021 [17] | Yes | Yes | Yes | Yes | Yes | Not relevant |
| Karaca et al., 2017 [18] | Yes | Yes | Yes | Unclear | No | Yes |
| Lassandro et al., 2022 [19] | Yes | Yes | Yes | Yes | Yes | Yes |
| Wang et al., 2019 [20] | Yes | Yes | Yes | Yes | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holtz, J.K.; Larsson, J.M.E.; Hansen, M.S.; van Dijk, E.H.C.; Subhi, Y. Pachychoroid Spectrum Diseases in Patients with Cushing’s Syndrome: A Systematic Review with Meta-Analyses. J. Clin. Med. 2022, 11, 4437. https://doi.org/10.3390/jcm11154437
Holtz JK, Larsson JME, Hansen MS, van Dijk EHC, Subhi Y. Pachychoroid Spectrum Diseases in Patients with Cushing’s Syndrome: A Systematic Review with Meta-Analyses. Journal of Clinical Medicine. 2022; 11(15):4437. https://doi.org/10.3390/jcm11154437
Chicago/Turabian StyleHoltz, Jeppe K., Janni M. E. Larsson, Michael S. Hansen, Elon H. C. van Dijk, and Yousif Subhi. 2022. "Pachychoroid Spectrum Diseases in Patients with Cushing’s Syndrome: A Systematic Review with Meta-Analyses" Journal of Clinical Medicine 11, no. 15: 4437. https://doi.org/10.3390/jcm11154437
APA StyleHoltz, J. K., Larsson, J. M. E., Hansen, M. S., van Dijk, E. H. C., & Subhi, Y. (2022). Pachychoroid Spectrum Diseases in Patients with Cushing’s Syndrome: A Systematic Review with Meta-Analyses. Journal of Clinical Medicine, 11(15), 4437. https://doi.org/10.3390/jcm11154437







