Time to Surgery Does Not Affect Overall or Disease-Free Survival of Patients with Primary Resectable PDAC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Procedures and Postoperative Course
2.2. Statistical Analysis
3. Results
3.1. Dataset of Patients
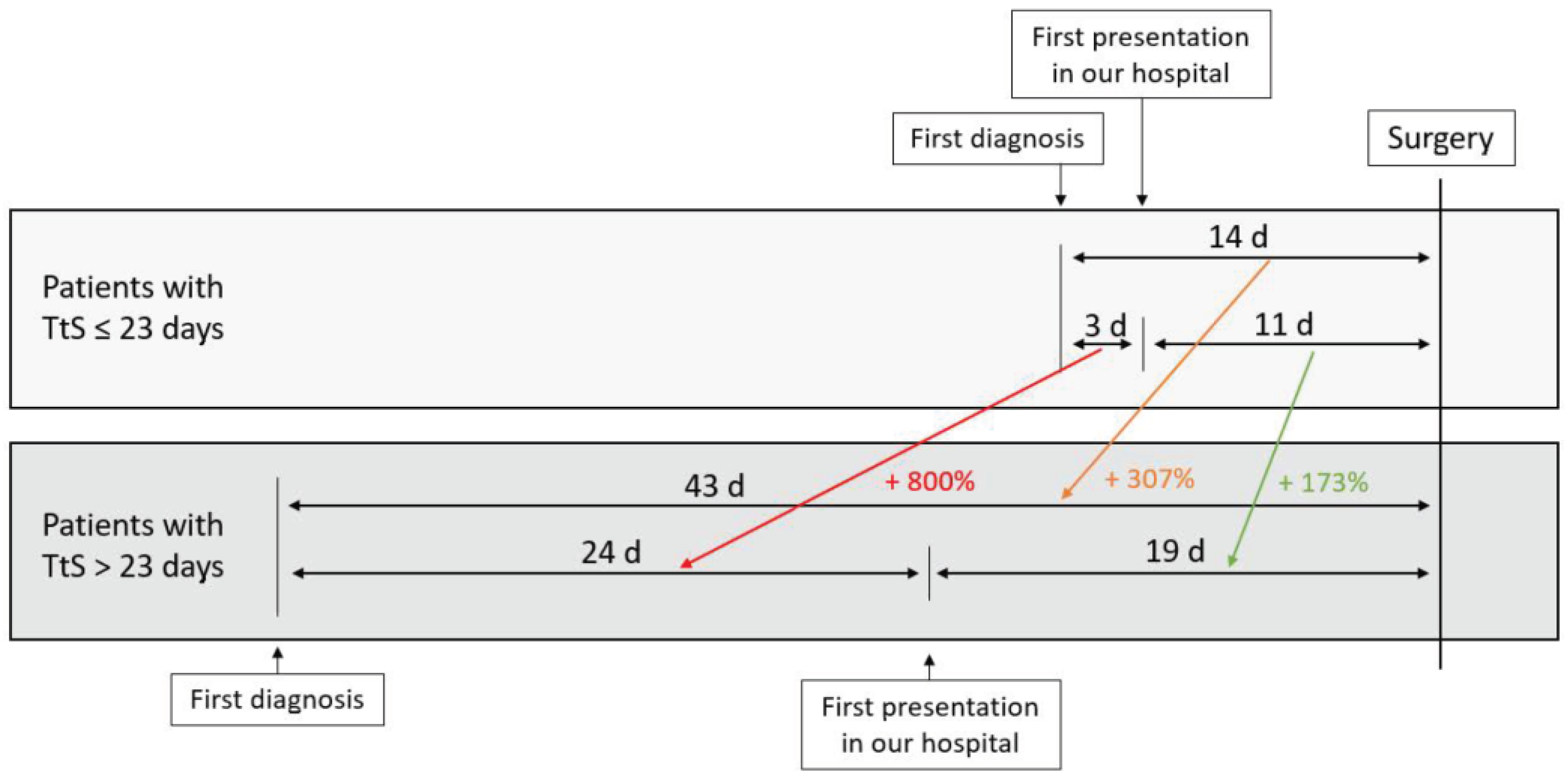
3.2. Time to Surgery
3.3. Patient Characteristics
3.4. Surgical and Histopathological Details
3.5. Short-Term Postoperative Outcome Parameters
3.6. Overall and Disease-Free Survival
3.7. Prognostic Factors for Overall and Disease-Free Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J. Clin. Oncol. 2022, 40, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Yu, J.; Blackford, A.L.; Dal Molin, M.; Wolfgang, C.L.; Goggins, M. Time to progression of pancreatic ductal adenocarcinoma from low-to-high tumour stages. Gut 2015, 64, 1783–1789. [Google Scholar] [CrossRef] [Green Version]
- Jooste, V.; Dejardin, O.; Bouvier, V.; Arveux, P.; Maynadie, M.; Launoy, G.; Bouvier, A.M. Pancreatic cancer: Wait times from presentation to treatment and survival in a population-based study. Int. J. Cancer 2016, 139, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Eshuis, W.J.; van der Gaag, N.A.; Rauws, E.A.; van Eijck, C.H.; Bruno, M.J.; Kuipers, E.J.; Coene, P.P.; Kubben, F.J.; Gerritsen, J.J.; Greve, J.W.; et al. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann. Surg. 2010, 252, 840–849. [Google Scholar] [CrossRef] [Green Version]
- Steen, M.W.; van Rijssen, L.B.; Festen, S.; Busch, O.R.; Groot Koerkamp, B.; van der Geest, L.G.; de Hingh, I.H.; van Santvoort, H.C.; Besselink, M.G.; Gerhards, M.F. Impact of time interval between multidisciplinary team meeting and intended pancreatoduodenectomy on oncological outcomes. BJS Open 2020, 4, 884–892. [Google Scholar] [CrossRef]
- Joliat, G.R.; Labgaa, I.; Gilgien, J.; Demartines, N. Prognostic Impact of Time to Surgery in Patients with Resectable Pancreatic Ductal Adenocarcinoma. Pancreas 2021, 50, 104–110. [Google Scholar] [CrossRef]
- Marchegiani, G.; Andrianello, S.; Perri, G.; Secchettin, E.; Maggino, L.; Malleo, G.; Bassi, C.; Salvia, R. Does the surgical waiting list affect pathological and survival outcome in resectable pancreatic ductal adenocarcinoma? HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2018, 20, 411–417. [Google Scholar] [CrossRef]
- Sanjeevi, S.; Ivanics, T.; Lundell, L.; Kartalis, N.; Andrén-Sandberg, Å.; Blomberg, J.; Del Chiaro, M.; Ansorge, C. Impact of delay between imaging and treatment in patients with potentially curable pancreatic cancer. Br. J. Surg. 2016, 103, 267–275. [Google Scholar] [CrossRef]
- UICC (Interntional Union of Cancer Control). TNM Classification of Malignant Tumours, 8th ed.; Wiley Blackwell: Oxford, UK, 2017. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Bassi, C.; Dervenis, C.; Butturini, G.; Fingerhut, A.; Yeo, C.; Izbicki, J.; Neoptolemos, J.; Sarr, M.; Traverso, W.; Buchler, M.; et al. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery 2005, 138, 8–13. [Google Scholar] [CrossRef]
- Panwar, R.; Pal, S. The International Study Group of Pancreatic Surgery definition of delayed gastric emptying and the effects of various surgical modifications on the occurrence of delayed gastric emptying after pancreatoduodenectomy. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 353–363. [Google Scholar] [CrossRef]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy hemorrhage (PPH): An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef]
- Raptis, D.A.; Fessas, C.; Belasyse-Smith, P.; Kurzawinski, T.R. Clinical presentation and waiting time targets do not affect prognosis in patients with pancreatic cancer. Surg. J. R. Coll. Surg. Edinb. Irel. 2010, 8, 239–246. [Google Scholar] [CrossRef]
- McLean, S.R.; Karsanji, D.; Wilson, J.; Dixon, E.; Sutherland, F.R.; Pasieka, J.; Ball, C.; Bathe, O.F. The effect of wait times on oncological outcomes from periampullary adenocarcinomas. J. Surg. Oncol. 2013, 107, 853–858. [Google Scholar] [CrossRef]
- Mirkin, K.A.; Hollenbeak, C.S.; Wong, J. Time to Surgery: A Misguided Quality Metric in Early Stage Pancreatic Cancer. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2018, 22, 1365–1375. [Google Scholar] [CrossRef]
- Glant, J.A.; Waters, J.A.; House, M.G.; Zyromski, N.J.; Nakeeb, A.; Pitt, H.A.; Lillemoe, K.D.; Schmidt, C.M. Does the interval from imaging to operation affect the rate of unanticipated metastasis encountered during operation for pancreatic adenocarcinoma? Surgery 2011, 150, 607–616. [Google Scholar] [CrossRef]
- Roberts, K.J.; Prasad, P.; Steele, Y.; Marcon, F.; Faulkner, T.; Cilliers, H.; Dasari, B.; Abradelo, M.; Marudanayagam, R.; Sutcliffe, R.P.; et al. A reduced time to surgery within a ‘fast track’ pathway for periampullary malignancy is associated with an increased rate of pancreatoduodenectomy. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2017, 19, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassen, K.; Coolsen, M.M.; Slim, K.; Carli, F.; de Aguilar-Nascimento, J.E.; Schafer, M.; Parks, R.W.; Fearon, K.C.; Lobo, D.N.; Demartines, N.; et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin. Nutr. 2012, 31, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Gurusamy, K.S.; Wang, Q.; Davidson, B.R.; Lin, H.; Xie, X.; Wang, C. Meta-analysis of randomized clinical trials on safety and efficacy of biliary drainage before surgery for obstructive jaundice. Br. J. Surg. 2013, 100, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- van der Gaag, N.A.; Rauws, E.A.; van Eijck, C.H.; Bruno, M.J.; van der Harst, E.; Kubben, F.J.; Gerritsen, J.J.; Greve, J.W.; Gerhards, M.F.; de Hingh, I.H.; et al. Preoperative biliary drainage for cancer of the head of the pancreas. N. Engl. J. Med. 2010, 362, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.R.; van Lanschot, J.J.; van der Velden, J.; Kloek, J.J.; Gouma, D.J.; Sprangers, M.A. Quality of life in newly diagnosed cancer patients waiting for surgery is seriously impaired. J. Surg. Oncol. 2006, 93, 571–577. [Google Scholar] [CrossRef]
| Number (n) | % of All Patients | |
|---|---|---|
| TtS ≤ 10 days | 26 | 13.5 |
| TtS > 10 and ≤ 20 days | 51 | 26.6 |
| TtS > 20 and ≤ 30 days | 49 | 25.5 |
| TtS > 30 and ≤ 40 days | 29 | 15.1 |
| TtS > 40 and ≤ 50 days | 17 | 8.9 |
| TtS > 50 and ≤ 60 days | 4 | 2.1 |
| TtS > 60 and ≤ 90 days | 11 | 5.7 |
| TtS > 90 and ≤ 120 days | 5 | 2.6 |
| TtS ≤ 23 Days | TtS > 23 Days | p-Value | |
|---|---|---|---|
| Number | 90 | 102 | |
| Age (years), median (IQR) | 68 (15) | 69 (14) | 0.433 |
| Gender, n (%) | 1.000 | ||
| Female | 44 (49) | 50 (49) | |
| Male | 46 (51) | 52 (51) | |
| ASA (n = 180) *, n (%) | 0.769 | ||
| I | 2 (2) | 1 (1) | |
| II | 51 (61) | 61 (64) | |
| III | 31 (37) | 34 (35) | |
| BMI (kg/m2), median (IQR) | 25.5 (4.4) | 25.6 (6.4) | 0.965 |
| Alcohol abuse (n = 169) *, n (%) | 44 (56) | 43 (47) | 0.280 |
| Nicotine abuse (n = 189) *, n (%) | 19 (22) | 23 (23) | 0.863 |
| Comorbidity, n (%) | |||
| Hypertension | 48 (53) | 62 (61) | 0.310 |
| Diabetes | 20 (22) | 34 (33) | 0.108 |
| Cardiovascular | 10 (11) | 14 (14) | 0.665 |
| Pulmonary | 8 (9) | 9 (9) | 1.000 |
| Cerebrovascular | 4 (4) | 7 (7) | 0.546 |
| Liver disease | 7 (8) | 9 (9) | 1.000 |
| Preoperative biliary stenting, n (%) | 45 (50) | 56 (55) | 0.561 |
| Preoperative hemoglobin (g/dL), median (IQR) | 12.9 (2.4) | 12.7 (2.3) | 0.587 |
| Preoperative WBC (109/L), median (IQR) | 6.8 (3.2) | 7.2 (3.6) | 0.402 |
| Preoperative albumin (g/L), median (IQR) | 40.4 (6.6) | 40.1 (7.8) | 0.954 |
| Preoperative CRP (mg/L), median (IQR) | 8 (20) | 5 (15) | 0.140 |
| Preoperative CA19-9 (U/mL) (n = 174) *, median (IQR) | 118 (619) | 88 (213) | 0.265 |
| Preoperative CEA (ng/mL) (n = 137) *, median (IQR) | 3.0 (3.9) | 2.3 (3.3) | 0.197 |
| TtS ≤ 23 Days (n = 90) | TtS > 23 Days (n = 102) | p-Value | |
|---|---|---|---|
| Kind of surgery | 0.743 | ||
| Pancreatic head resection | 69 (77) | 76 (74) | |
| Distal pancreatectomy | 17 (19) | 23 (23) | |
| Total pancreatectomy | 4 (4) | 3 (3) | |
| Portal vein resection, n (%) | 26 (29) | 30 (29) | 1.000 |
| Arterial resection, n (%) | 3 (3) | 1 (1) | 0.342 |
| Multi-visceral resection, n (%) | 19 (21) | 15 (15) | 0.262 |
| Operative time (min), median (IQR) | 280 (101) | 278 (108) | 0.494 |
| Intraoperative blood loss (mL), median (IQR) | 600 (575) | 500 (700) | 0.809 |
| Intraoperative blood transfusion, n (%) | 23 (26) | 31 (30) | 0.521 |
| T category | 0.664 | ||
| pT1 | 4 (4) | 8 (8) | |
| pT2 | 16 (18) | 20 (20) | |
| pT3 | 68 (76) | 73 (72) | |
| pT4 | 2 (2) | 1 (1) | |
| n category | 0.662 | ||
| pN0 | 35 (39) | 43 (42) | |
| pN+ | 55 (61) | 59 (58) | |
| M category | 0.621 | ||
| pM0 | 83 (92) | 91 (89) | |
| pM1 | 7 (8) | 11 (11) | |
| R-status | 0.687 | ||
| R0 | 78 (87) | 90 (88) | |
| R1 | 8 (9) | 10 (10) | |
| R2 | 4 (4) | 2 (2) | |
| Differentiation | 0.550 | ||
| G1 | 3 (3) | 1 (1) | |
| G2 | 30 (33) | 35 (34) | |
| G3 | 57 (63) | 66 (65) |
| TtS ≤ 23 Days (n = 90) | TtS > 23 Days (n = 102) | p-Value | |
|---|---|---|---|
| Morbidity, n (%) | 56 (62) | 62 (61) | 0.882 |
| Major morbidity, n (%) | 22 (24) | 32 (31) | 0.336 |
| Mortality, n (%) | 1 (1) | 6 (6) | 0.123 |
| Re-operation, n (%) | 8 (9) | 10 (10) | 1.000 |
| POPF, n (%) | 13 (14) | 21 (21) | 0.344 |
| DGE, n (%) | 32 (36) | 29 (28) | 0.352 |
| PPH, n (%) | 1 (1) | 0 (0) | 0.469 |
| Surgical site infection, n (%) | 8 (9) | 4 (4) | 0.232 |
| Length of postoperative stay (days), median (IQR) | 18 (15) | 18 (12) | 0.366 |
| Adjuvant chemotherapy, n (%) | 47 (52) | 58 (57) | 0.534 |
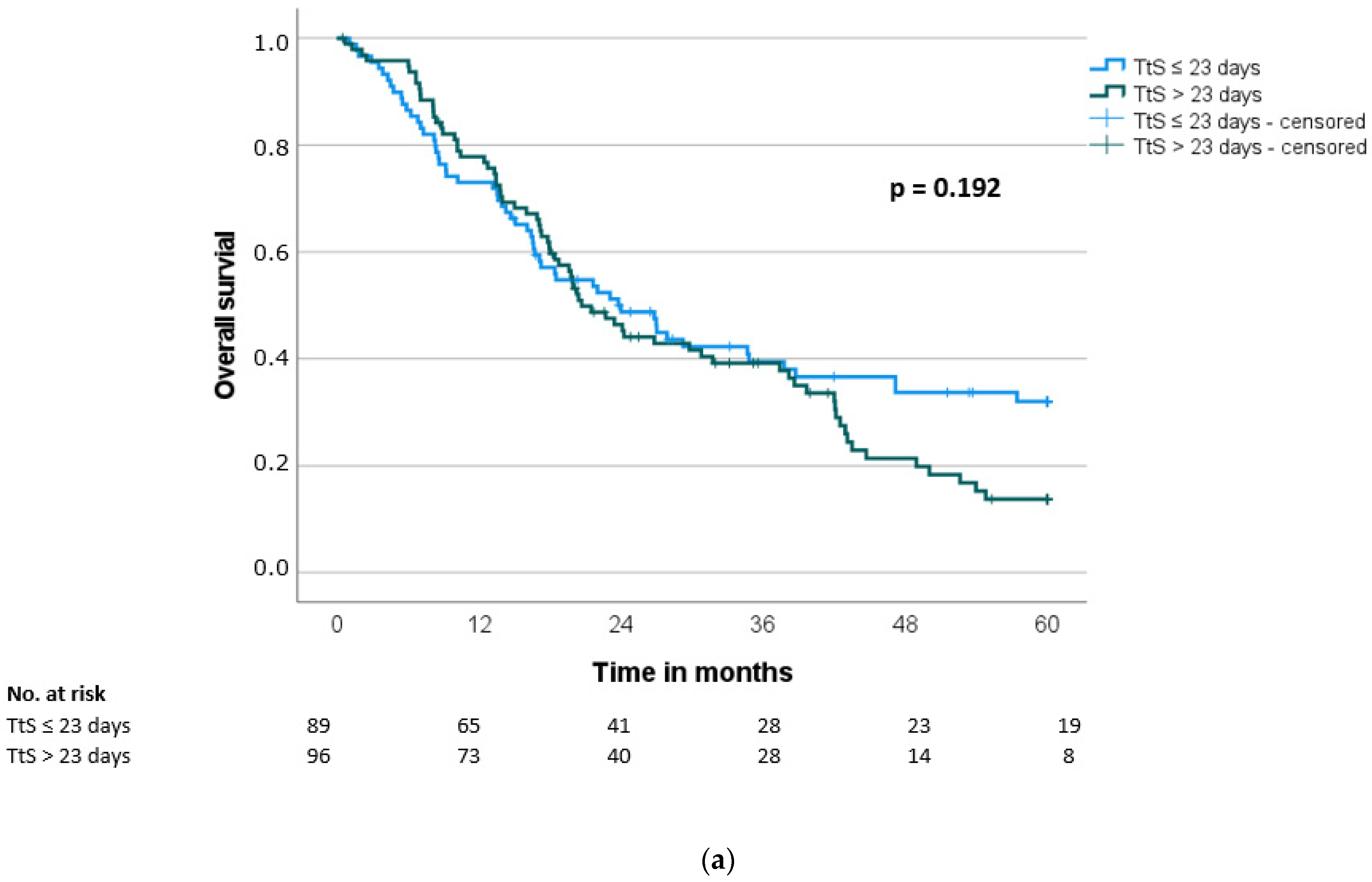
| Overall survival (months) *, median (SD) | 24.0 (4.2) | 20.7 (2.1) | 0.192 |
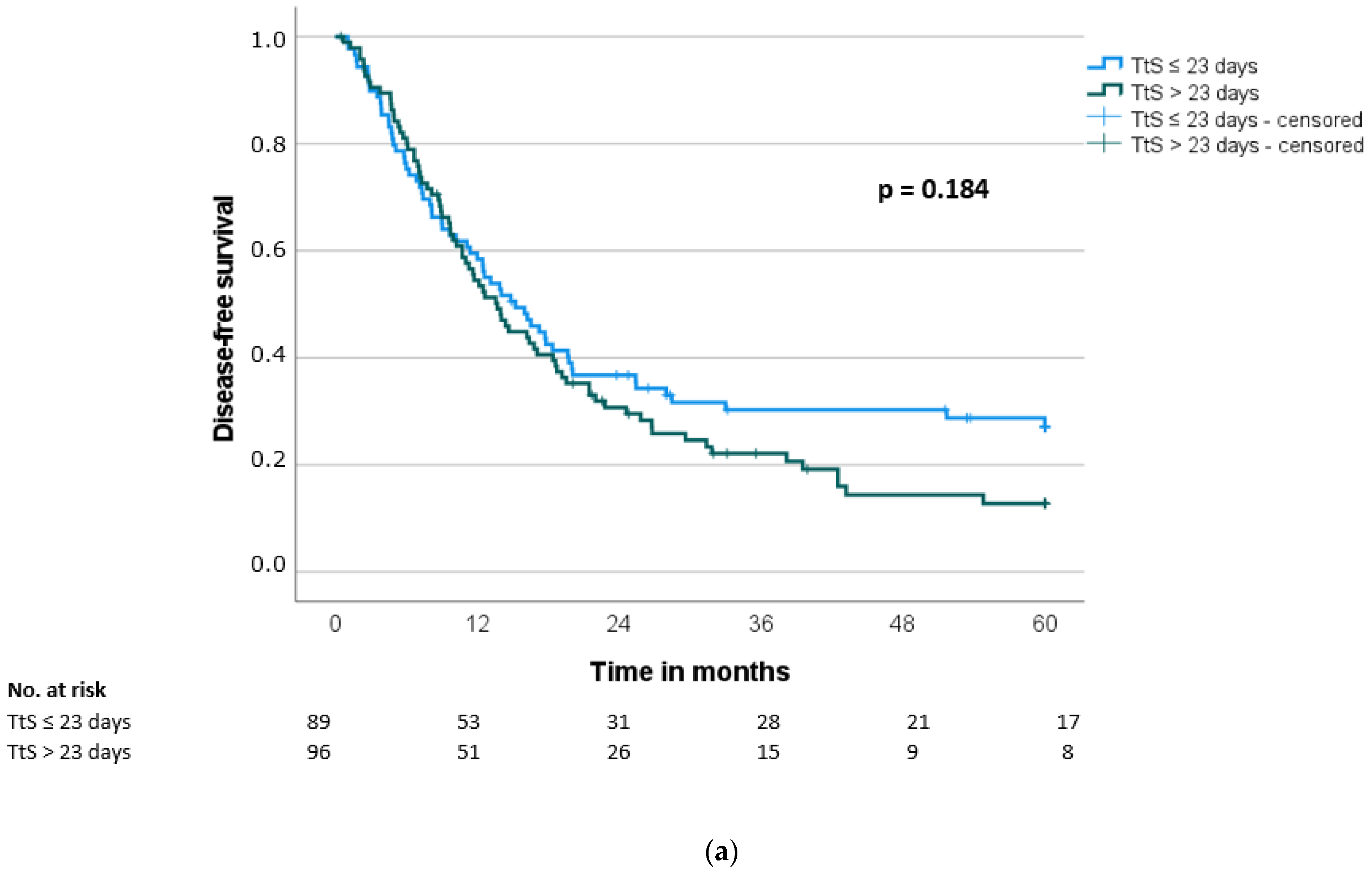
| Disease-free survival (months) *, median (SD) | 15.2 (2.4) | 13.6 (1.5) | 0.187 |
| Overall Survival (OS) * | Disease-Free Survival (DFS) * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||||
| n | Median OS | p | HR | 95% CI | p-Value | Median DFS | p | HR | 95% CI | p-Value | |
| Age | 0.002 | 2.39 | 1.63–3.49 | <0.001 | 0.054 | 1.51 | 1.04–2.19 | 0.032 | |||
| ≤70 years | 108 | 37.4 | 16.5 | ||||||||
| >70 years | 77 | 17.9 | 11.0 | ||||||||
| Gender | 0.505 | 0.764 | |||||||||
| Female | 92 | 26.8 | 13.7 | ||||||||
| Male | 93 | 21.5 | 14.4 | ||||||||
| ASA (n = 174) ** | 0.035 | 1.27 | 0.87–1.86 | 0.210 | 0.053 | 1.16 | 0.79–1.70 | 0.444 | |||
| I/II | 112 | 26.8 | 15.2 | ||||||||
| III | 62 | 16.9 | 11.1 | ||||||||
| Ca19-9 (n = 170) ** | 0.197 | 0.070 | 1.01 | 0.69–1.50 | 0.945 | ||||||
| <50 U/mL | 64 | 24.2 | 16.8 | ||||||||
| ≥50 U/mL | 106 | 22.7 | 12.5 | ||||||||
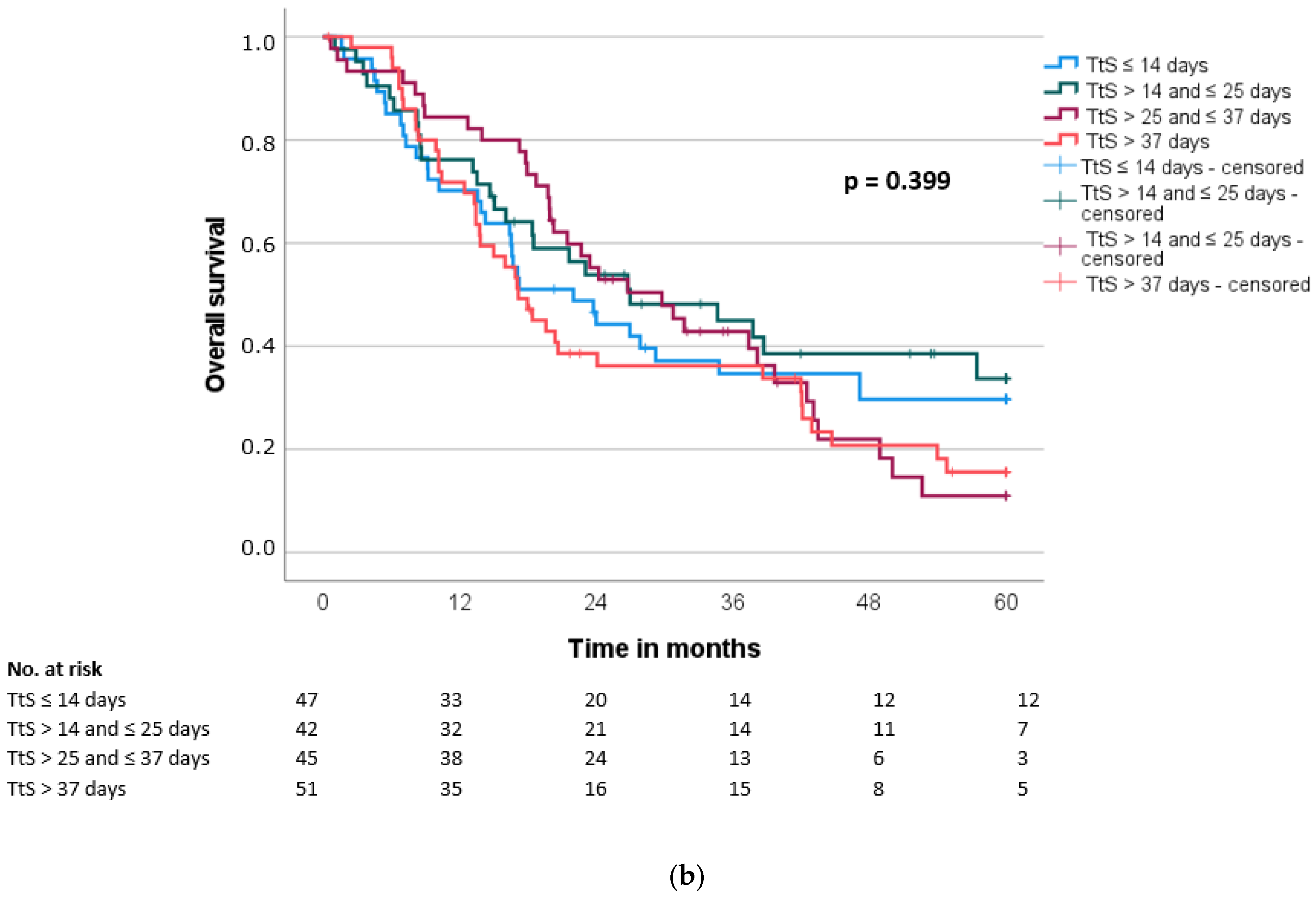
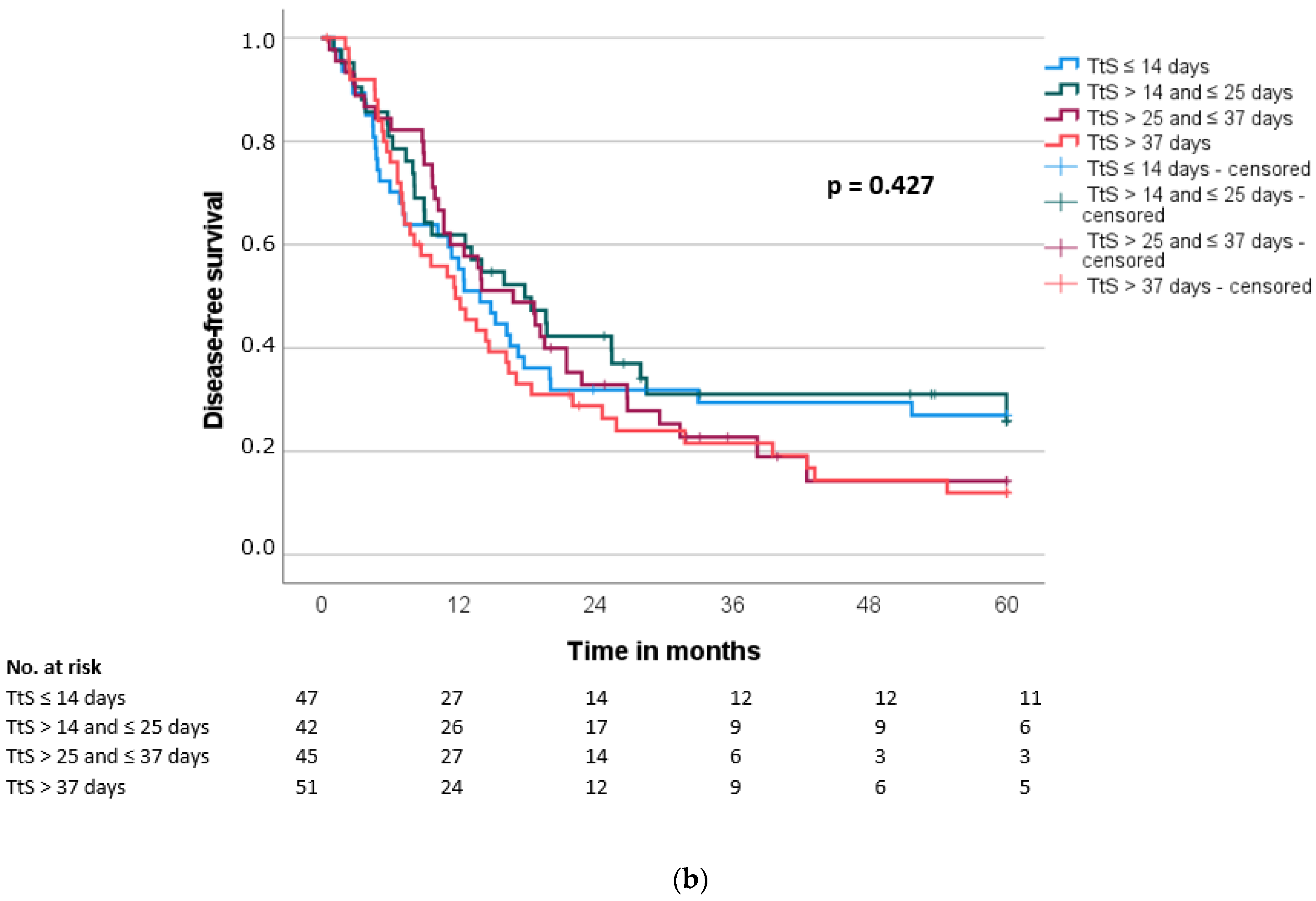
| Interval to surgery | 0.192 | 0.184 | |||||||||
| ≤23 days | 89 | 24.0 | 15.2 | ||||||||
| >23 days | 96 | 20.7 | 13.7 | ||||||||
| Kind of surgery | 0.333 | 0.395 | |||||||||
| Pancreatic head resection | 141 | 24.1 | 16.2 | ||||||||
| Distal pancreatectomy | 38 | 18.0 | 10.7 | ||||||||
| Total pancreatectomy | 6 | 6.7 | 6.7 | ||||||||
| Vascular resection | 0.190 | 0.260 | |||||||||
| Yes | 54 | 17.2 | 9.7 | ||||||||
| No | 131 | 23.7 | 15.2 | ||||||||
| Multi-visceral resection | 0.329 | 0.316 | |||||||||
| Yes | 30 | 18.0 | 9.7 | ||||||||
| No | 155 | 24.0 | 14.7 | ||||||||
| T category | 0.011 | 1.36 | 0.83–2.15 | 0.234 | 0.004 | 1.32 | 0.83–2.09 | 0.243 | |||
| pT1/pT2 | 47 | 37.8 | 22.8 | ||||||||
| pT3/pT4 | 138 | 19.9 | 12.5 | ||||||||
| N category | <0.001 | 2.00 | 1.34–3.00 | <0.001 | <0.001 | 1.78 | 1.19–2.67 | 0.005 | |||
| pN0 | 77 | 39.7 | 19.2 | ||||||||
| pN+ | 108 | 18.4 | 12.5 | ||||||||
| M category | 0.032 | 0.74 | 0.37–1.50 | 0.405 | 0.158 | ||||||
| M0 | 168 | 24.0 | 14.8 | ||||||||
| pM1 | 17 | 12.7 | 9.6 | ||||||||
| R-status | <0.001 | 2.69 | 1.48–4.89 | 0.001 | 0.013 | 1.74 | 1.00–3.02 | 0.050 | |||
| R0 | 162 | 24.2 | 16.0 | ||||||||
| R1/R2 | 23 | 10.2 | 8.8 | ||||||||
| Differentiation | <0.001 | 1.84 | 1.23–2.76 | 0.003 | < 0.001 | 1.62 | 1.09–2.40 | 0.017 | |||
| G1/G2 | 68 | 38.2 | 20.0 | ||||||||
| G3 | 117 | 17.2 | 12.2 | ||||||||
| Morbidity | 0.323 | 0.737 | |||||||||
| Yes | 111 | 20.4 | 14.0 | ||||||||
| No | 74 | 31.7 | 14.0 | ||||||||
| Re-operation | 0.334 | 0.683 | |||||||||
| Yes | 16 | 17.2 | 14.0 | ||||||||
| No | 167 | 23.4 | 14.0 | ||||||||
| Adjuvant chemotherapy | 0.721 | 0.981 | |||||||||
| Yes | 105 | 23.8 | 14.8 | ||||||||
| No | 80 | 18.5 | 12.2 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobsen, A.; Hobbs, M.; Merkel, S.; Mittelstädt, A.; Czubayko, F.; Krautz, C.; Weber, G.F.; Grützmann, R.; Brunner, M. Time to Surgery Does Not Affect Overall or Disease-Free Survival of Patients with Primary Resectable PDAC. J. Clin. Med. 2022, 11, 4433. https://doi.org/10.3390/jcm11154433
Jacobsen A, Hobbs M, Merkel S, Mittelstädt A, Czubayko F, Krautz C, Weber GF, Grützmann R, Brunner M. Time to Surgery Does Not Affect Overall or Disease-Free Survival of Patients with Primary Resectable PDAC. Journal of Clinical Medicine. 2022; 11(15):4433. https://doi.org/10.3390/jcm11154433
Chicago/Turabian StyleJacobsen, Anne, Mirianna Hobbs, Susanne Merkel, Anke Mittelstädt, Franziska Czubayko, Christian Krautz, Georg F. Weber, Robert Grützmann, and Maximilian Brunner. 2022. "Time to Surgery Does Not Affect Overall or Disease-Free Survival of Patients with Primary Resectable PDAC" Journal of Clinical Medicine 11, no. 15: 4433. https://doi.org/10.3390/jcm11154433
APA StyleJacobsen, A., Hobbs, M., Merkel, S., Mittelstädt, A., Czubayko, F., Krautz, C., Weber, G. F., Grützmann, R., & Brunner, M. (2022). Time to Surgery Does Not Affect Overall or Disease-Free Survival of Patients with Primary Resectable PDAC. Journal of Clinical Medicine, 11(15), 4433. https://doi.org/10.3390/jcm11154433







