Abstract
(1) Background: The clinical burden of aortic stenosis (AS) remains high in Western countries. Yet, there are no screening algorithms for this condition. We developed a risk prediction model to guide targeted screening for patients with AS. (2) Methods: We performed a cross-sectional analysis of all echocardiographic studies performed between 2013 and 2018 at a tertiary academic care center. We included reports of unique patients aged from 40 to 95 years. A logistic regression model was fitted for the risk of moderate and severe AS, with readily available demographics and comorbidity variables. Model performance was assessed by the C-index, and its calibration was judged by a calibration plot. (3) Results: Among the 38,788 reports yielded by inclusion criteria, there were 4200 (10.8%) patients with ≥moderate AS. The multivariable model demonstrated multiple variables to be associated with AS, including age, male gender, Caucasian race, Body Mass Index ≥ 30, and cardiovascular comorbidities and medications. C-statistics of the model was 0.77 and was well calibrated according to the calibration plot. An integer point system was developed to calculate the predicted risk of ≥moderate AS, which ranged from 0.0002 to 0.7711. The lower 20% of risk was approximately 0.15 (corresponds to a score of 252), while the upper 20% of risk was about 0.60 (corresponds to a score of 332 points). (4) Conclusions: We developed a risk prediction model to predict patients’ risk of having ≥moderate AS based on demographic and clinical variables from a large population cohort. This tool may guide targeted screening for patients with advanced AS in the general population.
1. Introduction
Calcific aortic stenosis (AS) is the most common valvular heart disease in Western countries [1] and the third most common cardiovascular disease, next only to hypertension and coronary artery disease (CAD) [2]. Prevalence of moderate or severe AS increases with age, affecting 12.4% of the population aged ≥ 75 years [3]. The prevalence of the disease is also projected to increase due to the aging population and the high burden of atherosclerotic risk factors. By 2025, 0.8 million people in North America will be suffering from symptomatic severe AS, and by 2050 the number is expected to approach 1.4 million [3]. The 2-year mortality of symptomatic severe AS without intervention is >50% [4]. In addition, patients with AS have a high incidence of heart failure (HF), repeated hospitalizations, and poor quality of life [5]. Yet, there are no screening programs for such a common and morbid disease.
AS can be readily diagnosed using Doppler echocardiography with accuracy, reasonable cost, and at no risk to the patient. Once reliably diagnosed, it can be treated effectively with interventions that improve survival and quality of life [6,7]. In the absence of a screening program, many patients are diagnosed late in the course of the disease, mainly because of the reliance on symptom appearance to prompt testing [8]. The disease typically runs an asymptomatic course. Even patients with severe AS are frequently asymptomatic or endorse non-specific symptoms [9,10]. These challenging aspects of AS have led to under-diagnosis and consequently under-treatment of the disease with devastating consequences for the lethality and morbidity of the disease. Hence, there is a need for tools that guide screening programs for early diagnosis in various settings (cardiology offices, primary care offices, online health clinics, etc.) and timely treatment of AS patients.
Previous efforts have not been sufficient to reliably characterize AS risk factors and successfully use them to generate a risk score for general screening recommendations to identify patients with moderate or severe AS. In particular, this was due to the small size of the available cohorts and/or the lack of control groups. Towards this end, we leveraged a large echocardiography database to develop a prediction model to identify patients with AS based on readily available demographic and comorbidity information.
2. Materials and Methods
2.1. Patient Population and Data Source
This cross-sectional study was conducted at Yale-New Haven Hospital, a tertiary care center in the United States, serving the community of the greater New Haven area and a large fraction of the population of the state of Connecticut (with multiple ethnic backgrounds, age groups, and comorbidity profiles). Echocardiography data and electronic health records (EHR) were queried for all patients ≥ 18 years old who had at least one study during the calendar years 2013–2018. These criteria yielded 146,876 studies obtained on 48,524 unique patients. The Institutional Review Board at Yale University approved this study, and individual consent was waived.
2.2. Analytic Cohort Building
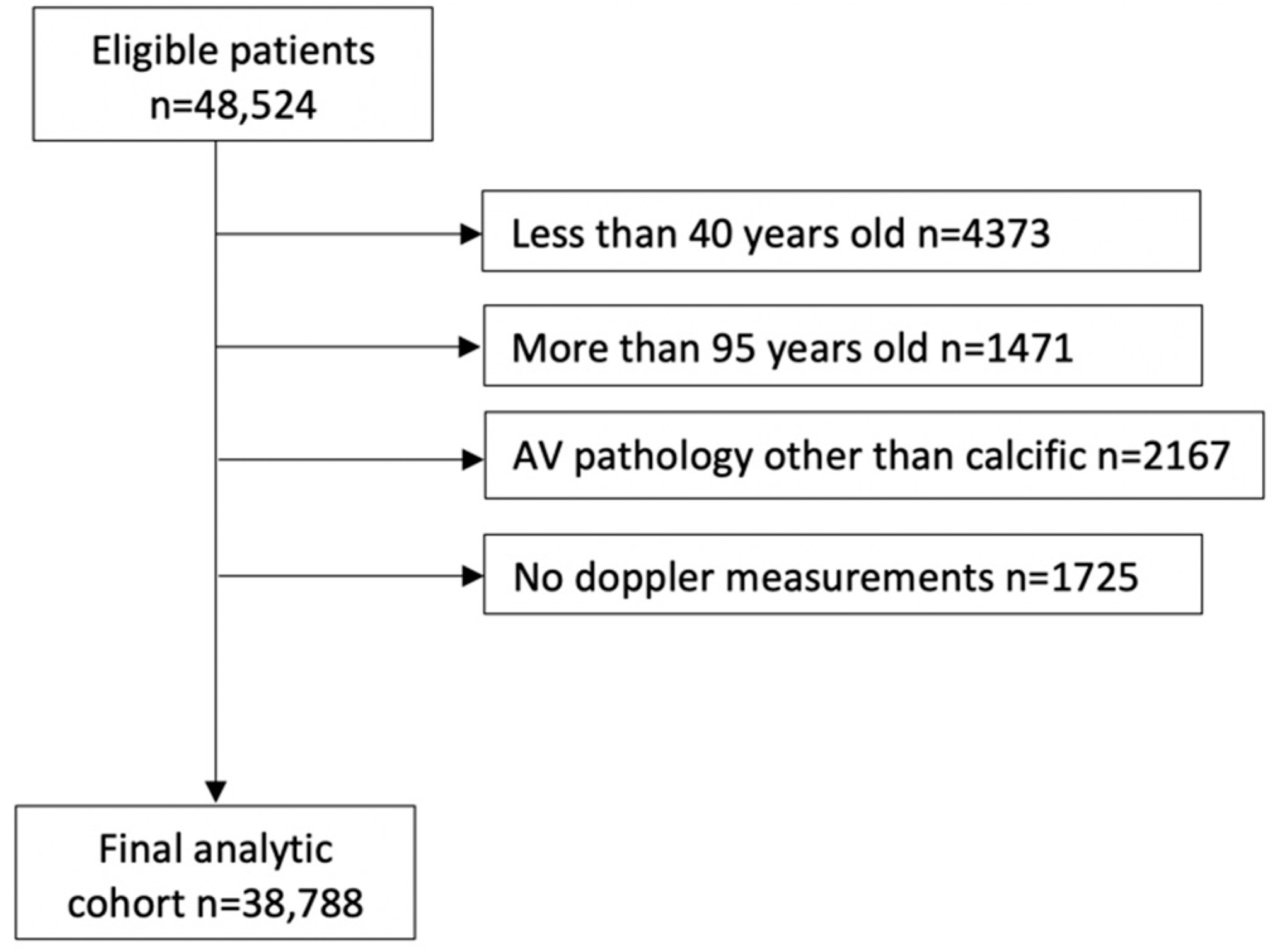
A series of exclusions led to the final analytic cohort of 38,788 patients, as shown in Figure 1. Given that AS is rare before age 40, patients less than 40 years old at the time of their initial study were excluded. Patients more than 95 years old at the time of their initial study were also excluded because intervention adds minimal benefit to longevity at this old age. We also excluded patients with prosthetic aortic valves (AV) on their initial echocardiography during the study period, patients with AV pathology other than calcific AS (rheumatic AS, endocarditis, HOCM, moderate and severe AI, and aortic valve tumor), patients who had aortic valve replacement (AVR) as part of aortic aneurysm or dissection repair, and patients who received heart transplantation or ventricular assist device treatment. Studies missing all AV Doppler parameters were also excluded. We intentionally excluded patients with mild AS from the models because the focus of this analysis was to develop a screening algorithm to help identify patients with ≥moderate AS.
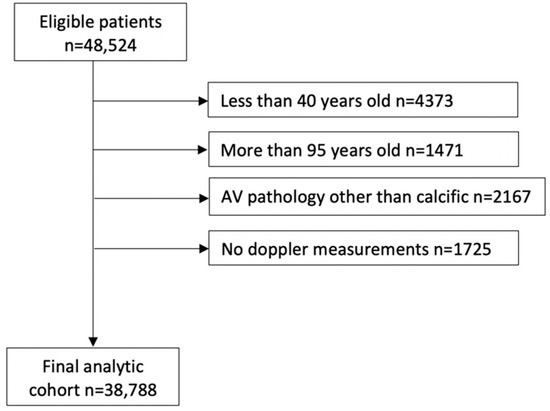
Figure 1.
Analytic cohort building. Consort diagram describing the inclusion and exclusion steps that led to the final analytic cohort.
2.3. Moderate and Severe AS
Patients with ≥ moderate AS were identified using the Doppler echocardiography parameters clinically used to define AS. The most severe value of the following parameters was considered [11]:
Aortic valve area (AVA) ≤ 1.5 cm2 and or dimensionless valve index (DVI) ≤ 0.5 and or maximum flow velocity (V-max) ≥ 3 m/s, and or mean pressure gradient (PG-mean) ≥ 20 mmHg.
2.4. Predictor Variables
Candidate variables included demographics (age, sex, and race), clinical characteristics (body mass index, smoking history, and other comorbidities that are common in this age group), medications (beta blocking agents, calcium channel blocking agents, diuretics, angiotensin-converting enzyme pathway inhibitors/blockers, aspirin, and statins), and previous interventions including coronary artery bypass graft (CABG), percutaneous coronary intervention (PCI), defibrillator and/or pacemaker implantation. Demographics, BMI, history of smoking, and medications were directly abstracted from the EHR. Comorbidities and interventions were queried in the form of ICD-10 codes (Appendix A).
2.5. Variable Selection and Model Development
Distribution of patient demographics, comorbidity, and medications between patients with ≥moderate AS and those with no AS were compared by Student’s t-test for continuous variables and by chi-squared test for categorical variables, respectively. Association between the risk of moderate/severe AS and patient demographics, comorbidity, and medications were evaluated using the multivariable logistic regression. The variables included in the regression model were chosen based on stepwise variable selection, optimizing the Akaike Information Criterion (AIC) value.
2.6. Performance Metrics
We evaluated the model discrimination ability using the area under the receiver-operator characteristics curve (AUROC), which characterizes model discrimination and ranges between 0 and 1, with a higher value corresponding to better discrimination. AUROC is the proportion of the times patients with an event were accurately classified to have a higher probability of event within all possible pairs of patients with and without an event. Calibration was characterized using continuous calibration plot.
2.7. Clinical Interpretability
To enhance clinical implementation of the models in assessing patients’ risk of having the disease and, accordingly, the need for echocardiographic testing, we further developed a simple integer scoring system based on the β coefficients for each predictor variable in the logistic regression models. A two-tailed p < 0.05 was considered statistically significant. All analyses were carried out using SAS 9.4 (SAS Institute, Cary, NC, USA).
3. Results
A series of exclusions led to the final analytic cohort of 38,788 patients (Figure 1). Among them, 4200 (10.8%) patients met the criteria for ≥moderate AS. On unadjusted comparisons, patients with ≥moderate AS, compared to patients with no AS, were older (mean age: 76.6 ± 11.3 vs. 66.4 ± 13.3 years, p < 0.0001), more likely to be males, Caucasian race, with higher prevalence of hypertension, dyslipidemia, coronary artery disease (CAD), pulmonary hypertension, heart block (HB), atrial fibrillation (AF), cerebral infarction, peripheral vascular disease (PVD), chronic kidney disease (CKD), diabetes mellitus (DM), dementia, inability to walk, heart failure (HF), dilated cardiomyopathy, pacemaker, defibrillator, PCI, and CABG and were more likely to be on beta-blockers, CCBs, ACEIs, and diuretics (Table 1).

Table 1.
Distribution of the potential predictors between patients with advanced AS and those without AS.
In the multivariable model, the risk of having moderate and severe AS, in reference to no AS, was associated with increasing age (OR = 1.055 CI = 1.051–1.059 for every one-year increment from 40), use of aspirin (OR = 1.21, CI = 1.11–1.31), use of CCBs (OR = 1.2, CI = 1.12–1.29), use of diuretics (OR = 1.58, CI = 1.46–1.7), CABG (OR = 1.37, CI = 1.22–1.53), CAD (OR = 1.14, CI = 1.05–1.24), dyslipidemia (OR = 1.23, CI = 1.13–1.32), pulmonary hypertension (OR = 1.69, CI = 1.39–2.04), cardiomyopathy (OR = 1.74, CI = 1.12–2.71), HB (OR = 1.31, CI = 1.14–1.5), AF (OR = 1.15, CI = 1.06–1.24), CVD (OR = 1.24, CI = 1.02–1.5), PVD (OR = 1.29, CI = 1.14–1.46), CKD (OR = 1.2, CI = 1.09–1.33), DM (OR = 1.08, CI = 1.00–1.16), chronic liver disease (OR = 1.64, CI = 1.38–1.97), history of obesity (OR = 1.21, CI = 1.06–1.39), pacemaker (OR = 1.2, CI = 1.01–1.42), and heart failure (OR = 1.39, CI = 1.26–1.52). The risk of having moderate or severe AS decreased with African American race (AA) (OR = 0.5, CI = 0.44–0.56), races other than Caucasian and AA (OR = 0.83, CI = 0.72–0.95) and history of dementia (OR = 0.7, CI = 0.6–0.81) (Table 2).

Table 2.
Logistic regression coefficients and ORs for advanced AS.
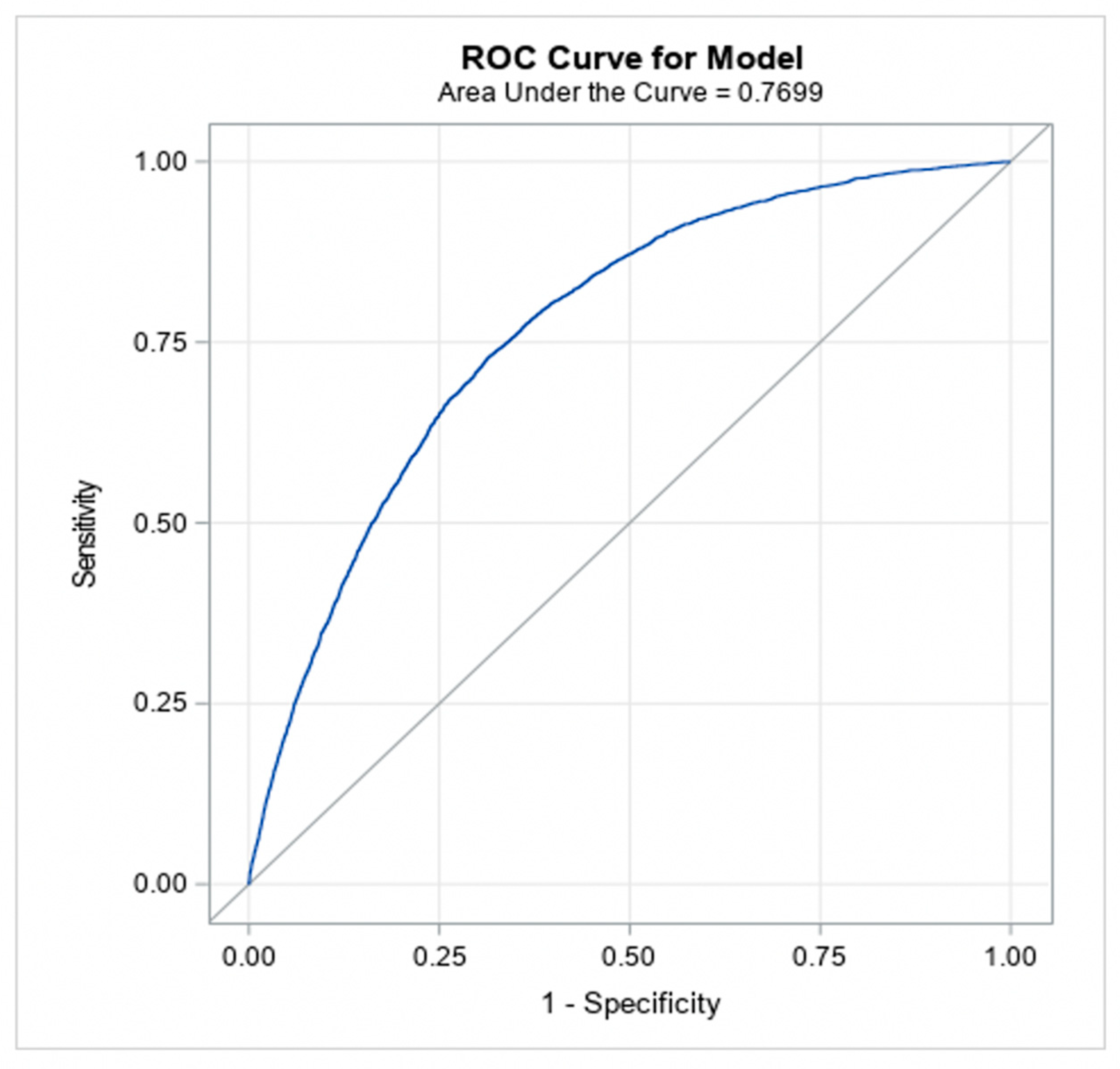
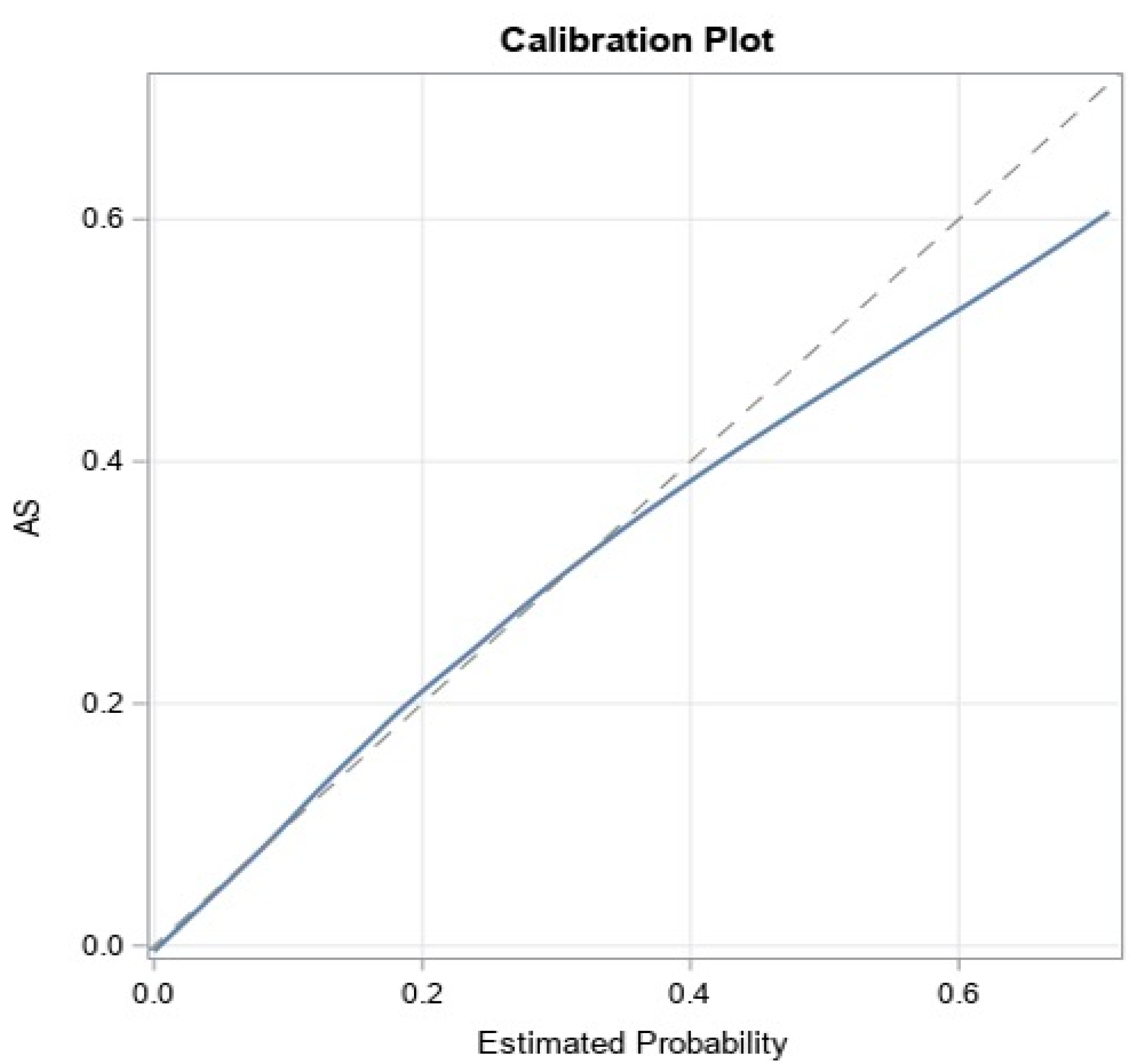
The models achieved good predictive discrimination with an AUC of 0.77 (Figure 2) and were well calibrated by visual examination of the calibration plots (Figure 3). The coefficients were transformed to risk scores that can be translated into the probability of individual patient having ≥moderate AS (Table 3). The risk scores assigned to each risk factor are summarized in a nomogram, which also shows translation between the overall points and the predicted probability of ≥moderate AS (Table 4).
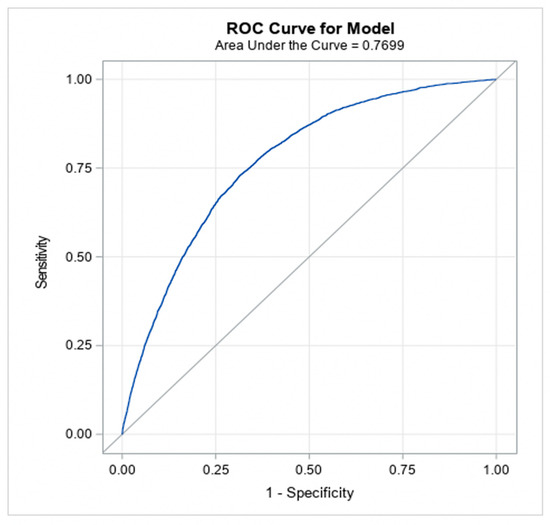
Figure 2.
Discrimination ability of the model (to classify patients into moderate/severe AS versus no AS).
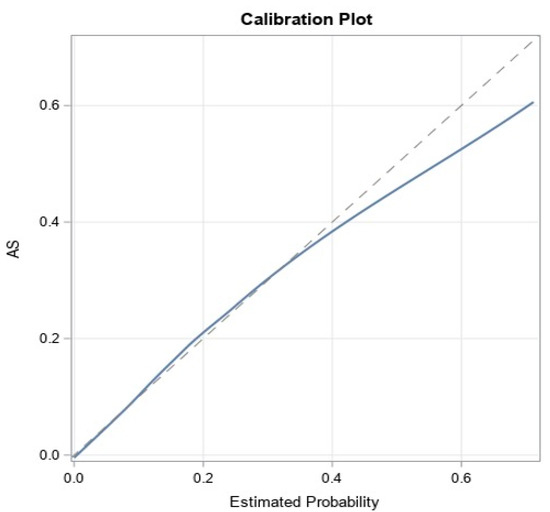
Figure 3.
Calibration of the model.

Table 3.
Score prediction for ≥ moderate AS.

Table 4.
Linear project to the risk probability and total score.
4. Discussion
The current lack of clinical tools to facilitate targeted screening of the population for patients with increased risk of ≥moderate AS is concerning given the increasing burden of the disease and its association with poor outcomes when diagnosed late or left untreated. Furthermore, timely intervention in this subpopulation can lead to significantly improved quality of life and survival, and therefore, a reliable screening program for AS would have an impact on patient outcomes. This contrasts with other conditions such as abdominal aortic aneurysm and lung cancer, for which successful implementation of nationwide screening programs led to the mitigation of their consequences [12,13,14]. Given that the prevalence of AS is much higher than these conditions for which there are screening programs, the necessity for the development of a screening tool for AS is warranted. To facilitate systematic screening of AS, we developed a model to predict a patient’s risk of having moderate/severe AS based on clinical parameters. The model is based on readily available demographic and comorbidity information that was further integrated into an integer scoring system that can be used to predict patients’ risk of having AS via the simple addition of points.
Many of the risk factors that showed the importance in the model are shared by other atherosclerotic diseases such as CAD and PAD. According to the model, the risk of advanced AS incrementally increased after age 40, although fast progression and earlier disease onset have been shown in certain groups, including patients with bicuspid aortic valve and patients with chronic kidney disease [15,16]. Advanced AS (i.e., ≥moderate) was also associated with male gender and Caucasian race, and the association with gender was less prominent compared to the association with race as shown by the ß-estimates. This agrees with the report by Patel et al., where AA patients were less likely to have severe AS (OR = 0.41) [17]; and the report by Owens et al. from the Multi-Ethnic Study of Atherosclerosis that showed that the risk of progression of AV calcification was associated with male sex [18] The integer scoring system that we developed in this model collectively calculates the risk of AS based on multiple factors rather than considering one specific risk factor. For example, an AA woman (theoretically least likely to develop moderate/severe AS) may score up to 332 points, which corresponds to a risk of 0.6.
The model has the potential to be a valuable tool in clinical practice, given its simplification to an integer (points) system and reliance on variables that are readily available in most EHR systems. Yet, implementation of the model in a clinical setting should start with caution as the model has not been validated in prospective studies. The results of the model do not show a certain threshold at which screening should be recommended. A score of 207–235 (predicted risk of 5–10%) can be used as a threshold in the beginning, and further validating studies can help fine-tune it. Starting with a low threshold is reasonable considering the high prevalence of AS and the safety profile of echocardiography without contrast exposure. Additionally, echocardiography as a screening test for AS can be valuable as a screening test for other diseases that are prevalent in this population, such as thoracic aortic aneurysms, by providing information on aortic size.
Limitations
This study has some limitations. It is a single-center study, which might limit the generalizability of the results, even though the cohort is very large and included patients from nearly all backgrounds. However, the model has not been externally validated, and its implication in clinical settings requires caution until its validity is proven by other studies. The study included all comers with ≥moderate AS and the history of previously diagnosed versus incidental finding of AS is unknown, and the effect of such information on the analysis is uncertain. Finally, the study relied on claims data (ICD-10 codes) to assess the presence or absence of comorbidities, and such data are reported to be subject to over- or under-reporting.
5. Conclusions
We developed a risk model to identify patients aged between 40 and 95 years who are at increased risk of having ≥moderate AS based on demographic and clinical characteristics. Discrimination and calibration of the model were good. This prediction rule may guide the targeted screening of patients at increased risk of having advanced AS.
Author Contributions
Conceptualization: S.Y. and P.V.; Methodology: S.Y., A.A., C.R., H.H., V.K., S.S., R.A. (Ritu Agarwal), R.A. (Roland Assi), R.K.M., Y.Z., P.A.P., M.K., A.G. and P.V.; Software: H.H.; Validation: S.Y., A.A., C.R., H.H., V.K., S.S., R.A. (Roland Assi), R.K.M., Y.Z., P.A.P., M.K., A.G. and P.V.; Formal analysis: S.Y. and H.H.; Investigation: S.Y., A.A., H.H., V.K., S.S., R.A. (Roland Assi), R.K.M., Y.Z., P.A.P., M.K., A.G. and P.V.; Resources: S.Y., A.A., H.H., V.K., S.S., Ritu Agarwal, R.K.M., Y.Z., P.A.P., M.K., A.G. and P.V.; Data curation: S.Y. and H.H.; Writing—original draft preparation: S.Y.; Writing—review and editing: S.Y., A.A., H.H., V.K., S.S., R.A. (Roland Assi), R.K.M., Y.Z., P.A.P., M.K., A.G. and P.V.; Visualization: S.Y., A.A., H.H., V.K., S.S., R.A. (Roland Assi), R.K.M., Y.Z., P.A.P., M.K., A.G. and P.V.; Supervision: R.K.M., R.A. (Roland Assi), Y.Z., M.K., A.G. and P.V.; Project administration: S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at Yale University (protocol code 2000028791, date of approval 9 February 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Geirsson receives a consulting fee for being a member of the Medtronic Strategic Surgical Advisory Board. Medtronic produces valve products for valve replacement and repairs. Krane is a physician proctor and a member of the medical advisory board for JOMDD, a physician proctor for Peter Duschek, and has received speakers’ honoraria from Medtronic and Terumo. The remaining authors have nothing to disclose.
Appendix A

Table A1.
ICD-10 code used to define comorbidities.
Table A1.
ICD-10 code used to define comorbidities.
| Hypertension | I10, I11, I11.0, I11.9, I12, I12.0, I12.9, I13, I13.0, I13.1, I13.10, I13.11, I13.2, I15, I15.0, I15.1, I15.2, I15.8, I15.9 |
| Coronary Heart Disease | I20, I20.0, I20.1, I20.8, I20.9, I21, I21.0, I21.01, I21.02, I21.09, I21.1, I21.11, I21.19, I21.2, I21.21, I21.29, I21.3, I21.4, I21.9, I24.8, I24.9, I25, I25.1, I25.10, I25.11, I25.11, I25.110, I25.111, I25.118, I25.119, I25.2, I25.3, I25.5, I25.6, I25.8, I25.81, I25.82, I25.83, I25.84, I25.89 |
| Dyslipidemia | E78, E78.0, E78.00, E78.01, E78.1, E78.2, E78.3, E78.4, E78.41, E78.49, E78.5, E78.6 |
| Pulmonary Hypertension | I27.0, I27.2, I27.20, I27.21, I27.22, I27.23, I27.24, I27.29, I27.81 |
| Endocarditis | I33.0, I33.9, I38, I39 |
| Mitral Regurgitation | I34.0, I34.1, I34.8, I34.9 |
| Aortic Stenosis | I35.0, I35.2 |
| Tricuspid Regurgitation | I36.1, I36.8, I36.9 |
| Cardiomyopathy | I42.0, I42.1, I42.2 |
| Heart Block | I44.0, I44.1, I44.2, I44.30, I44.39, I44.4, I44.5, I44.60, I44.69, I44.7, I45.0, I45.10, I45.19, I45.2, I45.3, I45.4, I45.6, I45.81, I45.89, I45.9 |
| Cardiac Arrest | I46.2, I46.8, I46.9 |
| Atrial Fibrillation | I48.0, I48.1, I48.11, I48.19, I48.2, I48.20, I48.21, I48.91, I48.3, I48.4, I48.92, I49.5 |
| Heart Failure | I11.0, I13.0, I13.1, I13.10, I13.11, I13.2, I50.1, I50.2, I50.20, I50.21, I50.22, I50.23, I50.3, I50.30, I50.31, I50.32, I50.33, I50.4, I50.40, I50.41, I50.42, I50.43, I50.8, I50.81, I50.810, I50.811, I50.812, I50.813, I50.814, I50.82, I50.84, I50.9 |
| Stroke | I60, I60.0, I60.00, I60.01, I60.02, I60.1, I60.10, I60.11, I60.12, I60.2, I60.3, I60.30, I60.31, I60.32, I60.4, I60.5, I60.50, I60.51, I60.52, I60.6, I60.6, I60.7, I60.8, I60.9, I61, I61.0, I61.1, I61.2, I61.3, I61.4, I61.5, I61.6, I61.8, I61.9, I62, I62.0, I62.00, I62.01, I62.02, I62.03, I62.1, I62.9, I63, I63.0, I63.00, I63.01, I63.011, I63.012, I63.013, I63.019, I63.03, I63.031, I63.032, I63.033, I63.039, I63.09, I63.1, I63.10, I63.11, I63.111, I63.112, I63.113, I63.119, I63.12, I63.13, I63.131, I63.132, I63.133, I63.139, I63.19, I63.2, I63.20, I63.21, I63.211, I63.212, I63.213, I63.219, I63.22, I63.23, I63.231, I63.232, I63.233, I63.239, I63.29, I63.3, I63.30, I63.31, I63.311, I63.312, I63.313, I63.319, I63.32, I63.321, I63.322, I63.323, I63.329, I63.33, I63.331, I63.332, I63.333, I63.339, I63.34, I63.341, I63.342, I63.343, I63.349, I63.39, I63.4, I63.40, I63.41, I63.411, I63.412, I63.413, I63.419, I63.42, I63.421, I63.422, I63.423, I63.429, I63.43, I63.431, I63.432, I63.433, I63.439, I63.44, I63.441, I63.442, I63.443, I63.449, I63.49, I63.5, I63.50, I63.51, I63.511, I63.512, I63.513, I63.519, I63.52, I63.521, I63.522, I63.523, I63.529, I63.53, I63.531, I63.532, I63.533, I63.539, I63.54, I63.541, I63.542, I63.543, I63.549, I63.59, I63.6, I63.8, I63.81,I63.89, I63.9 |
| Cerebrovascular Disease | I65.0, I65.01, I65.02, I65.03, I65.09, I65.1, I65.2, I65.21, I65.22, I65.23, I65.29, I65.8, I66, I66.0, I66.01, I66.02, I66.03, I66.09, I66.1, I66.11, I66.12, I66.13, I66.19, I66.2, I66.21, I66.22, I66.23, I66.29, I66.3, I66.8, I66.9, I67, I67.0, I67.1, I67.2, I67.3, I67.4, I67.5, I67.6, I67.7, I67.8, I67.81, I67.82, I67.83, I67.84, I67.9 |
| Peripheral Vascular Disease | I73.9, I73.89, E08.5, E09.5, E10.5, E11.5, E13.5, I70.20, I70.21, I70.22, I70.26, I70.29, I70.23, I70.24, I70.25, I70.211, I70.212, I70.213, I70.218, I70.311, I70.312, I70.313, I70.318, I70.611 |
| Chronic Lung Disease | J40, J41, J41.0, J41.1, J41.8, J42, J43, J43.9, J44, J44.0, J44.1, J44.9, J45, J45.2, J45.20, J45.21, J45.22, J45.3, J45.30, J45.31, J45.32, J45.4, J45.40, J45.41, J45.42, J45.5, J45.50, J45.51, J45.52, J45.9, J45.90, J45.901, J45.902, J45.909, J45.99, J45.990, J45.991, J45.998, J47, J47.0, J47.1, J47.9 |
| Chronic Kidney Disease | N18, N18.1, N18.2, N18.3, N18.4, N18.5, N18.6, N18.9, N19 |
| Diabetes Mellitus | E10, E11, E11.2, E11.21, E11.22, E11.29, E11.3, E11.31, E11.39, E11.32, E11.321, E11.329, E11.33, E11.331, E11.339, E11.34, E11.341, E11.349, E11.35, E11.351, E11.352, E11.353, E11.354, E11.355, E11.359, E11.36, E11.37, E11.39, E11.4, E11.40, E11.41, E11.42, E11.43, E11.44, E11.49, E11.5, E11.51, E11.52, E11.59, E11.6, E11.61, E11.610, E11.618, E11.62, E11.620, E11.621, E11.622, E11.628, E11.63, E11.630, E11.638, E11.64, E11.641, E11.649, E11.65, E11.69, E11.8, E11.9, E13 |
| Chronic Liver Disease/Cirrhosis | K70, K70.0, K70.1, K70.10, K70.11, K70.2, K70.3, K70.30, K70.31, K70.4, K70.40, K70.41, K70.9, K72.1, K72.10, K72.11, K72.9, K72.90, K72.91, K73, K73.0, K73.1, K73.2, K73.8, K73.9, K74, K74.0, K74.1, K74.2, K74.3, K74.4, K74.5, K74.6, K74.60, K74.69, K74.5, K75.9, K76.1, K76.9 |
| Dementia | F01, F01.5, F01.50, F02, F02.8, F02.80, F02.81, F03, F03.9, F03.90, F03.91, F04 |
| Frailty | R54, R53.0, R53.1, R53.2, R53.8, R53.81, R53.82, R41.81, R53.83 |
| Inability to Walk | R26, R26.0, R26.1, R26.2, R26.8, R26.9, R27, R27.0, R27.8, R27.9, R29.3, R29.4, R29.6 |
| Depression | F32, F32.0, F32.1, F32.2, F32.3, F32.4, F32.5, F32.9, F33, F33.0, F33.1, F33.2, F33.3, F33.4, F33.40, F33.41, F33.42, F33.8, F33.9 |
| Malnutrition | E40, E41, E42, E43, E44, E44.0, E44.1, E45, E46 |
| Obesity | E66, E66.0, E66.01, E66.09, E66.1, E66.2, E66.3, E66.8, E66.9 |
References
- Nathaniel, S.; Saligram, S.; Innasimuthu, A.L. Aortic stenosis: An update. World J. Cardiol. 2010, 2, 135–139. [Google Scholar] [CrossRef]
- Beydoun, H.A.; Beydoun, M.A.; Liang, H.; Dore, G.A.; Shaked, D.; Zonderman, A.B.; Eid, S.M. Sex, Race, and Socioeconomic Disparities in Patients With Aortic Stenosis (from a Nationwide Inpatient Sample). Am. J. Cardiol. 2016, 118, 860–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Gössl, M.; Ahmed, A.; Garberich, R.; Bradley, S.M.; Niikura, H.; Witt, D.; Pedersen, W.R.; Bae, R.; Lesser, J.R.; et al. Contemporary Reasons and Clinical Outcomes for Patients with Severe, Symptomatic Aortic Stenosis Not Undergoing Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2018, 11, e007220. [Google Scholar] [CrossRef] [PubMed]
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapadia, S.R.; Leon, M.B.; Makkar, R.R.; Miller, C.; Moses, W.J.; Cleveland, C.C.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; Kodali, S.K.; et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2485–2491. [Google Scholar] [CrossRef]
- Brown, J.M.; O’Brien, S.M.; Wu, C.; Sikora, J.A.; Griffith, B.P.; Gammie, J.S. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J. Thorac. Cardiovasc. Surg. 2009, 137, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, S.J.; Daimon, M.; Miyazaki, S.; Kawata, T.; Morimoto-Ichikawa, R.; Maruyama, M.; Ohmura, H.; Miyauchi, K.; Lee, S.L.; Daida, H. When and how aortic stenosis is first diagnosed: A single-center observational study. J. Cardiol. 2016, 68, 324–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellikka, P.A.; Sarano, M.E.; Nishimura, R.A.; Malouf, J.F.; Bailey, K.R.; Scott, C.G.; Barnes, M.E.; Tajik, A.J. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005, 111, 3290–3295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Généreux, P.; Stone, G.W.; O’Gara, P.T.; Marquis-Gravel, G.; Redfors, B.; Giustino, G.; Pibarot, P.; Bax, J.J.; Bonow, R.O.; Leon, M.B. Natural History, Diagnostic Approaches, and Therapeutic Strategies for Patients with Asymptomatic Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2016, 67, 2263–2288. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 372–392. [Google Scholar] [CrossRef] [PubMed]
- Secretariat, M.A. Ultrasound screening for abdominal aortic aneurysm: An evidence-based analysis. Ont. Health Technol. Assess Ser. 2006, 6, 1–67. [Google Scholar]
- US Preventive Services Task Force. Screening for abdominal aortic aneurysm: Recommendation statement. Ann. Intern. Med. 2005, 142, 198–202. [Google Scholar] [CrossRef] [Green Version]
- Criss, S.D.; Cao, P.; Bastani, M.; Ten Haaf, K.; Chen, Y.; Sheehan, D.F.; Blom, E.F.; Toumazis, I.; Jeon, J.; de Koning, H.J.; et al. Cost-Effectiveness Analysis of Lung Cancer Screening in the United States: A Comparative Modeling Study. Ann. Intern. Med. 2019, 171, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Tastet, L.; Capoulade, R.; Arsenault, M.; Bédard, E.; Clavel, M.-A.; Pibarot, P. Effect of bicuspid aortic valve phenotype on progression of aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Vavilis, G.; Bäck, M.; Occhino, G.; Trevisan, M.; Bellocco, R.; Evans, M.; Lindholm, B.; Szummer, K.; Carrero, J.J. Kidney Dysfunction and the Risk of Developing Aortic Stenosis. J. Am. Coll. Cardiol. 2019, 73, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Green, K.D.; Fudim, M.; Harrell, F.E.; Wang, T.J.; Robbins, M.A. Racial differences in the prevalence of severe aortic stenosis. J. Am. Heart Assoc. 2014, 3, e000879. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.S.; Katz, R.; Takasu, J.; Kronmal, R.; Budoff, M.J.; O’Brien, K.D. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA). Am. J. Cardiol. 2010, 105, 701–708. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).