Variant in the PLCG2 Gene May Cause a Phenotypic Overlap of APLAID/PLAID: Case Series and Literature Review
Abstract
1. Introduction
2. Material and Methods
2.1. Case Series
2.2. Literature Review
3. Results
3.1. Case Series
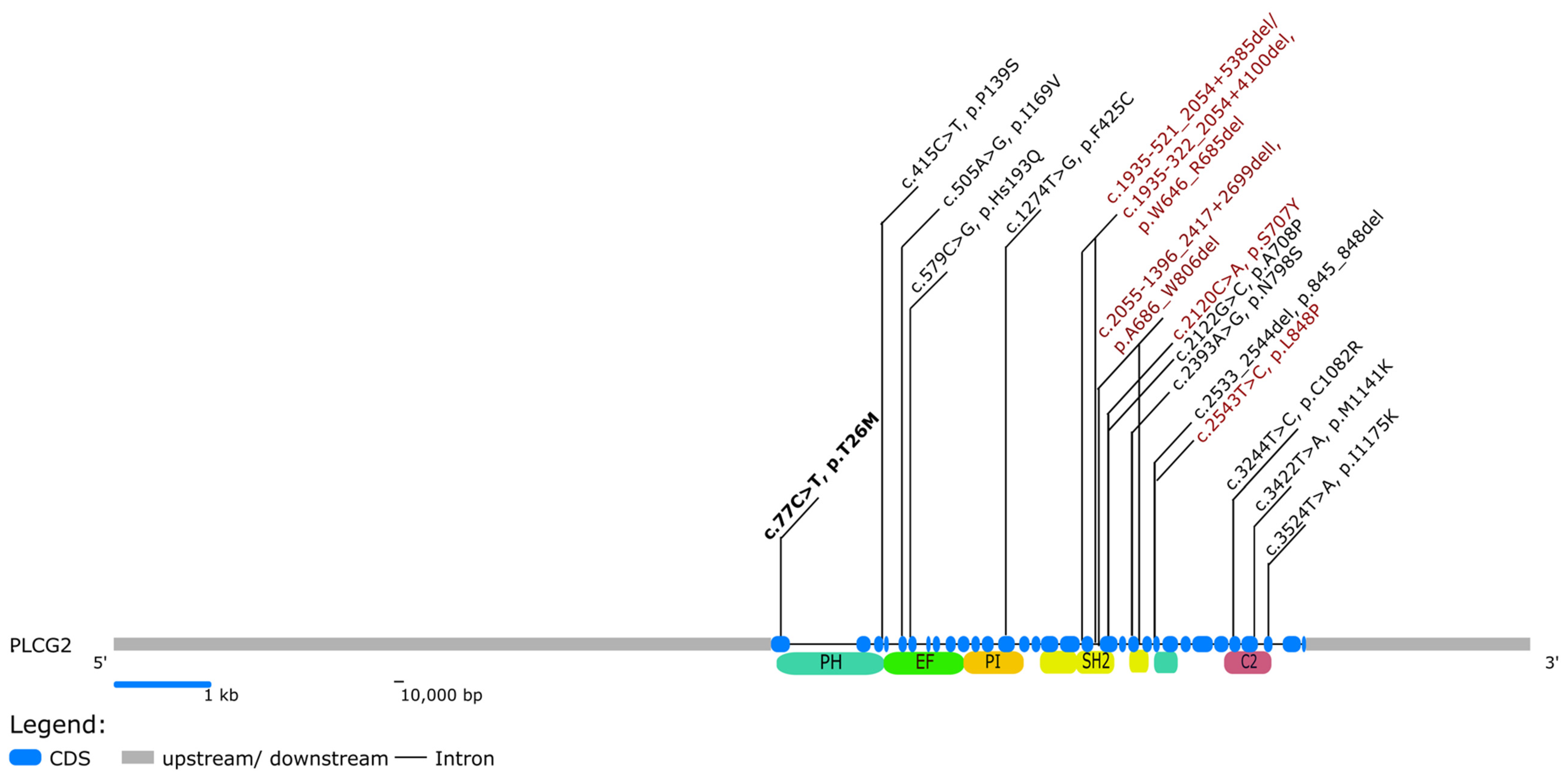
3.2. Literature Review
3.3. Management
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ombrello, M.J. Monogenic Autoinflammatory Diseases Associated with Immunodeficiency. In Textbook of Autoinflammation; Hashkes, P.J., Laxer, R.M., Simon, A., Eds.; Springer Nature AG: Cham, Switzerland, 2019; pp. 499–514. [Google Scholar]
- Giannelou, A.; Zhou, Q.; Kastner, D.L. When less is more: Primary immunodeficiency with an autoinflammatory kick. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 491–500. [Google Scholar] [CrossRef]
- Ombrello, M.J.; Remmers, E.F.; Sun, G.; Freeman, A.F.; Datta, S.; Torabi-Parizi, P.; Subramanian, N.; Bunney, T.D.; Baxendale, R.W.; Martins, M.S.; et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N. Engl. J. Med. 2012, 366, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.D. PLAID: A Syndrome of Complex Patterns of Disease and Unique Phenotypes. J. Clin. Immunol. 2015, 35, 527–530. [Google Scholar] [CrossRef][Green Version]
- Zhou, Q.; Lee, G.-S.; Brady, J.; Datta, S.; Katan, M.; Sheikh, A.; Martins, M.; Bunney, T.; Santich, B.; Moir, S.; et al. A Hypermorphic Missense Mutation in PLCG2, Encoding Phospholipase Cγ2, Causes a Dominantly Inherited Autoinflammatory Disease with Immunodeficiency. Am. J. Hum. Genet. 2012, 91, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Boursier, G.; Rittore, C.; Georgin-Lavialle, S.; Belot, A.; Galeotti, C.; Hachulla, E.; Hentgen, V.; Rossi-Semerano, L.; Sarrabay, G.; Touitou, A.I. Positive Impact of Expert Reference Center Validation on Performance of Next-Generation Sequencing for Genetic Diagnosis of Autoinflammatory Diseases. J. Clin. Med. 2019, 8, 1729. [Google Scholar] [CrossRef]
- Milhavet, F.; Cuisset, L.; Hoffman, H.M.; Slim, R.; El-Shanti, H.; Aksentijevich, I.; Lesage, S.; Waterham, H.; Wise, C.; De Menthiere, C.S.; et al. The infevers autoinflammatory mutation online registry: Update with new genes and functions. Hum. Mutat. 2008, 29, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Martín-Nalda, A.; Fortuny, C.; Rey, L.; Bunney, T.D.; Alsina, L.; Esteve-Sole, A.; Bull, D.; Anton, M.C.; Basagaña, M.; Casals, F.; et al. Severe Autoinflammatory Manifestations and Antibody Deficiency Due to Novel Hypermorphic PLCG2 Mutations. J. Clin. Immunol. 2020, 40, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.F.; Doffinger, R.; Barcena-Morales, G.; Martins, C.; Papapietro, O.; Plagnol, V.; Curtis, J.; Martins, M.; Kumararatne, D.; Cordeiro, A.I.; et al. Novel PLCG2 Mutation in a Patient with APLAID and Cutis Laxa. Front. Immunol. 2018, 9, 2863. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Z.; Gou, L.; Zhong, L.; Li, J.; Ma, M.; Wang, C.; Zhou, Y.; Ru, Y.; Sun, Z.; et al. Single-Center Overview of Pediatric Monogenic Autoinflammatory Diseases in the Past Decade: A Summary and Beyond. Front. Immunol. 2020, 11, 565099. [Google Scholar] [CrossRef]
- Park, H.S.; Oh, A.; Keum, C.W.; Lee, J.; Lee, J.K.; Son, B.R.; Shin, K.S.; Hahn, Y.-S. A novel likely pathogenic PLCG2 variant in a patient with a recurrent skin blistering disease and B-cell lymphopenia. Eur. J. Med. Genet. 2021, 65, 104387. [Google Scholar] [CrossRef]
- Moran-Villasenor, E.; Saez-de-Ocariz, M.; Torrelo, A.; Arostegui, J.I.; Yamazaki-Nakashimada, M.A.; Alcantara-Ortigoza, M.A.; Gonzalez del Angel, A.; Velazquez Aragon, A.; Lopez Herrera, G.; Berron Ruiz, M.; et al. Expanding the clinical features of autoinflammation and phospholipase Cgamma2-associated antibody deficiency and immune dysregulation by description of a novel patient. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2334–2339. [Google Scholar] [CrossRef] [PubMed]
- Karacan, İ.; Balamir, A.; Uğurlu, S.; Aydın, A.K.; Everest, E.; Zor, S.; Önen, M.Ö.; Daşdemir, S.; Özkaya, O.; Sözeri, B.; et al. Diagnostic utility of a targeted next-generation sequencing gene panel in the clinical suspicion of systemic autoinflammatory diseases: A multi-center study. Rheumatol. Int. 2019, 39, 911–999. [Google Scholar] [CrossRef]
- Novice, T.; Kariminia, A.; Del Bel, K.L.; Lu, H.; Sharma, M.; Lim, C.J.; Read, J.; Vander Lugt, M.; Hannibal, M.C.; O’Dwyer, D.; et al. A Germline Mutation in the C2 Domain of PLCgamma2 Associated with Gain-of-Function Expands the Phenotype for PLCG2-Related Diseases. J. Clin. Immunol. 2020, 40, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Khabbazi, A.; Kafshboran, H.R.; Aghdam, M.N.; Nojadeh, J.N.; Daghagh, H.; Danshmandpour, Y.; Kazemzadeh, M.; Hamzeiy, H.; Sakhinia, E. A new report of autoinflammation and PLCG2-associated antibody deficiency and immune dysregulation (APLAID) with a homozygous pattern from Iran. Immunol. Lett. 2020, 221, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Suri, D.; Rawat, A.; Jindal, A.K.; Vignesh, P.; Gupta, A.; Pilania, R.K.; Joshi, V.; Arora, K.; Kumrah, R.; Anjani, G.; et al. Spectrum of Systemic Auto-Inflammatory Diseases in India: A Multi-Centric Experience. Front. Immunol. 2021, 12, 630691. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, B.; Wang, T.; Shen, M.; Zeng, X. Case Report: A Rare Case of Autoinflammatory Phospholipase Cgamma2 (PLCgamma2)-Associated Antibody Deficiency and Immune Dysregulation Complicated with Gangrenous Pyoderma and Literature Review. Front. Immunol. 2021, 12, 667430. [Google Scholar] [CrossRef] [PubMed]
- Kutukculer, N.; Topyildiz, E.; Berdeli, A.; Guven Bilgin, B.; Aykut, A.; Durmaz, A.; Cogulu, O.; Aksu, G.; Karaca, N.E. Four diseases, PLAID, APLAID, FCAS3 and CVID and one gene (PHOSPHOLIPASE C, GAMMA-2; PLCG2): Striking clinical phenotypic overlap and difference. Clin. Case Rep. 2021, 9, 2023–2031. [Google Scholar] [CrossRef]
- Deza, G.; Mensa-Vilaró, A.; March-Rodriguez, A.; Sánchez, S.; Pujol, R.; Aróstegui, J.; Giménez-Arnau, A. Acquired Cold Urticaria vs. Autoinflammatory Diseases, Genetic and Clinical Profile and Differential Diagnosis: Study of a Cohort of Patients in a Tertiary Reference Centre. Acta Derm. Venereol. 2019, 99, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, M.; Offersen, R.; Jensen, J.M.B.; Petersen, M.S.; Larsen, C.S.; Mogensen, T.H. Identification of Novel Genetic Variants in CVID Patients with Autoimmunity, Autoinflammation, or Malignancy. Front. Immunol. 2020, 10, 3022. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Bishnoi, A.; Manjunath, S.; Vignesh, P.; Suri, D.; Gopal, M.; Chatterjee, D.; Jamwal, M.; De, D.; Das, R.; et al. Severe epidermolysis bullosa/Kindler syndrome-like phenotype of an autoinflammatory syndrome in a child. Clin. Exp. Dermatol. 2021, 46, 795–799. [Google Scholar] [CrossRef]
- Aderibigbe, O.M.; Priel, D.L.; Lee, C.-C.R.; Ombrello, M.J.; Prajapati, V.H.; Liang, M.G.; Lyons, J.J.; Kuhns, D.B.; Cowen, E.W.; Milner, J.D. Distinct Cutaneous Manifestations and Cold-Induced Leukocyte Activation Associated with PLCG2Mutations. JAMA Dermatol. 2015, 151, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Shea, J.; Huynh, T.; Milner, J.; Chamlin, S. PLAID syndrome: Characteristic presentation and a novel therapeutic option. Pediatr. Dermatol. 2020, 37, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Demirkaya, E.; Erer, B.; Livneh, A.; Ben-Chetrit, E.; Giancane, G.; Ozdogan, H.; Abu, I.; Gattorno, M.; Hawkins, P.N.; et al. EULAR recommendations for the management of familial Mediterranean fever. Ann. Rheum. Dis. 2016, 75, 644–651. [Google Scholar] [CrossRef]
- Welzel, T.; Wildermuth, A.L.; Deschner, N.; Benseler, S.M.; Kuemmerle-Deschner, J.B. Colchicine—An effective treatment for children with a clinical diagnosis of autoinflammatory diseases without pathogenic gene variants. Pediatr. Rheumatol. 2021, 19, 142. [Google Scholar] [CrossRef] [PubMed]
- Papa, R.; Rusmini, M.; Volpi, S.; Caorsi, R.; Picco, P.; Grossi, A.; Caroli, F.; Bovis, F.; Musso, V.; Obici, L.; et al. Next generation sequencing panel in undifferentiated autoinflammatory diseases identifies patients with colchicine-responder recurrent fevers. Rheumatology 2020, 59, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Chae, J.J.; Park, Y.H.; Park, C.; Hwang, I.Y.; Hoffmann, P.; Kehrl, J.H.; Aksentijevich, I.; Kastner, D.L. Connecting two pathways through Ca 2+ signaling: NLRP3 inflammasome activation induced by a hypermorphic PLCG2 mutation. Arthritis Rheumatol. 2015, 67, 563–567. [Google Scholar] [CrossRef]
- Nakanishi, H.; Kawashima, Y.; Kurima, K.; Chae, J.J.; Ross, A.M.; Pinto-Patarroyo, G.; Patel, S.K.; Muskett, J.A.; Ratay, J.S.; Chattaraj, P.; et al. NLRP3 mutation and cochlear autoinflammation cause syndromic and nonsyndromic hearing loss DFNA34 responsive to anakinra therapy. Proc. Natl. Acad. Sci. USA 2017, 114, E7766–E7775. [Google Scholar] [CrossRef] [PubMed]
- Walliser, C.; Retlich, M.; Harris, R.; Everett, K.L.; Josephs, M.B.; Vatter, P.; Esposito, D.; Driscoll, P.C.; Katan, M.; Gierschik, P.; et al. Rac regulates its effector phospholipase Cgamma2 through interaction with a split pleckstrin homology domain. J. Biol. Chem. 2008, 283, 30351–30362. [Google Scholar] [CrossRef]
- Schade, A.; Walliser, C.; Wist, M.; Haas, J.; Vatter, P.; Kraus, J.M.; Filingeri, D.; Havenith, G.; Kestler, H.A.; Milnder, J.D.; et al. Cool-temperature-mediated activation of phospholipase C-gamma2 in the human hereditary disease PLAID. Cell Signal. 2016, 28, 1237–1251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuemmerle-Deschner, J.B.; Verma, D.; Endres, T.; Broderick, L.; de Jesus, A.A.; Hofer, F.; Blank, N.; Krause, K.; Rietschel, C.; Horneff, G.; et al. Brief Report: Clinical and Molecular Phenotypes of Low-Penetrance Variants of NLRP3: Diagnostic and Therapeutic Challenges. Arthritis Rheumatol. 2017, 69, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Rowczenio, D.M.; Trojer, H.; Russell, T.; Baginska, A.; Lane, T.; Stewart, N.M.; Gillmore, J.D.; Hawkins, P.N.; Woo, P.; Mikoluc, B.; et al. Clinical characteristics in subjects with NLRP3 V198M diagnosed at a single UK center and a review of the literature. Arthritis Res. Ther. 2013, 15, R30. [Google Scholar] [CrossRef]
- Ravet, N.; Rouaghe, S.; Dode, C.; Bienvenu, J.; Stirnemann, J.; Levy, P.; Delpech, M.; Grateau, G. Clinical significance of P46L and R92Q substitutions in the tumour necrosis factor superfamily 1A gene. Ann. Rheum. Dis. 2006, 65, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Aksentijevich, I.; Galon, J.; Soares, M.; Mansfield, E.; Hull, K.; Oh, H.H.; Goldbach-Mansky, R.; Dean, J.; Athreya, B.; Reginato, A.J.; et al. The tumor-necrosis-factor receptor-associated periodic syndrome: New mutations in TNFRSF1A, ancestral origins, genotype-phenotype studies, and evidence for further genetic heterogeneity of periodic fevers. Am. J. Hum. Genet. 2001, 69, 301–314. [Google Scholar] [CrossRef] [PubMed]
| Clinical and Laboratory Characteristics | Pat 1 (f, 44 y.) | Pat 2 (m, 14 y.) | Pat 3 (m, 11 y.) | Pat 4 (m, 4 y.) | Pat 5 (f, 4 y.) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Symptoms | ||||||||||
| Recurrent fevers | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Headache | ✓ | ✗ | ✓ | ✗ | ✗ | |||||
| High frequency hearing loss | ✓ | ✓ | ✗ | ✗ | ✗ | |||||
| Conjunctivitis | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Painful recurrent lymph nodes | ✓ | ✗ | ✗ | ✓ | ✓ | |||||
| Episodes with abdominal pain and diarrhea | ✓ | ✗ | ✓ | ✓ | ✗ | |||||
| Cold-induced urticaria | ✓ | ✗ | ✓ | ✓ | ✓ | |||||
| Myalgia/ arthralgia | ✓ | ✓ | ✓ | ✗ | ✗ | |||||
| Recurrent upper airway infections, sinusitis | ✗ | ✓ | ✓ | ✓ | ✓ | |||||
| Recurrent swelling of palms and feet | ✗ | ✗ | ✓ | ✓ | ✗ | |||||
| Fatigue | ✓ | ✗ | ✗ | ✓ | ✓ | |||||
| Symptom Onset | ||||||||||
| Symptom Onset | Early Adulthood | Infancy | ||||||||
| Laboratory Parameters | no flare | flare | no flare | flare | no flare | flare | no flare | flare | no flare | flare |
| CRP (mg/dL, reference max 0.5 mg/dL) | 0.25 | - | 0.09 | - | 0.5 | 0.79 | 0.01 | - | 0.02 | 0.6 |
| Soluble IL-2 R (U/mL, reference 158–613 U/mL) | 172.0 | - | 266.0 | - | 644.0 | - | 560.0 | - | 636.0 | - |
| CK (U/L, reference <170 U/L) | 173 | 112 | 190 | - | 186 | - | 166 | - | 157 | - |
| Calprotectin (S100 A8/A9) (µg/mL, reference <3 µg/mL) | 4.8 | - | 1.8 | 5.65 | 3.6 | - | 1.6 | - | 2.1 | - |
| Serum amyloid A (mg/L, reference <10 mg/L) | 5 | 16.0 | - | 24 | 9 | 18 | - | 24 | 6 | 40 |
| Lymphocytes (%) | 16.2 | - | 35.5 | - | 36.3 | 39.3 | 49.4 | - | 54.4 | 28.6 |
| Monocytes (%) | 13.9 | - | 8.0 | - | 8.6 | 12.1 | 7.2 | 10.4 | 5.2 | 8.1 |
| Leucocytes | ||||||||||
| -Abs. neutros. (thousand/µL) | 2.74 (2.1–77) | 2.65 (2.0–6.6) | 1.87 (1.8–6.6) | 2.37 (1.8–7.4) | 1.76 (1.8–6.8) | |||||
| -Abs. lymphos. (thousand/µL) | 0.86 (1.2–3.5) | 1.99 (1.1–3.4) | 1.94 (1.1–3.4) | 3.26 (1.3–4.7) | 2.75 (1.2–7.0) | |||||
| -Abs. monos. (thousand/µL) | 0.58 (0.2–0.6) | 0.45 (0.4–1.3) | 0.38 (0.3–0.9) | 0.48 (0.3–1.2) | 0.26 (0.5–1.1) | |||||
| -Abs. eos. (thousand/µL) | 0.16 (0.03–0.47) | 0.35 (0.0–0.4) | 0.32 (0.0–0.4) | 0.22 (0.0–0.3) | 0.11 (0.0–0.3) | |||||
| Immunology | ||||||||||
| CD19, CD20 (absolute/µL) | 86 | 147 | 288 | 139 | 123 | |||||
| IgD+CD27− naïve B-cells (norm ca. 75% of CD19/CD20) (% d. CD19) | 45 | 57.14 | 34 | 70.19 | 51 | |||||
| IgD+CD27+ unswitched memory B cells (norm >10% of CD19/CD20) (% d. CD19) | 0 | 0 | 0.4 | 0.96 | 0 | |||||
| IgD−CD27+ switched memory B-cells (norm ca. 10% of CD19/CD20) (% d. CD19) | 23 | 32.5 | 48.82 | 20.2 | 33 | |||||
| NK cells | normal | normal | normal | normal | normal | |||||
| IgG (subclasses 1–4 included), IgA, IgM | normal | normal | normal | normal | normal | |||||
| T cell subpopulation | normal | normal | normal | normal | normal | |||||
| INFg expression of stimulated memory CD4+ T cells with PMA/ionomycin | slightly decreased | normal | normal | normal | normal | |||||
| IL-4 expression of stimulated memory CD4+ T cells with PMA/ionomycin | normal | normal | normal | high-normal | high-normal | |||||
| IL-17 expression of stimulated memory CD4+ T cells with PMA/ionomycin | normal | normal | normal | normal | normal | |||||
| IL-2 expression of stimulated memory CD4+ T cells with PMA/ionomycin | normal | normal | normal | normal | normal | |||||
| CD4+ and CD8+ T cell proliferation after PHA/anti-CD3 +/− CD28 stimulation | normal | normal | normal | normal | normal | |||||
| ANA | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| ENA-screen, ANCAs, ds-DNA antibodies | ✗ | n.a. | n.a. | n.a. | ✗ | |||||
| Rheumatoid factor and anti-CCP antibodies | ✗ | n.a. | n.a. | n.a. | n.a. | |||||
| Cryoglobulins and cold aggultinins | ✗ | n.a. | n.a. | n.a. | n.a. | |||||
| Myositis antibodies * | ✗ | n.a. | n.a. | n.a. | n.a. | |||||
| Additional Examinations | ||||||||||
| Complement factors (C3, C4) | ✗ | n.a. | n.a. | n.a. | n.a. | |||||
| TSH (mU/l; reference 0.7–4.17) | 1.26 | 2.25 | 2.96 | 3.12 | 2.35 | |||||
| fT3 (pg/mL; reference 2.79–4.42) | n.a. | 3.9 | 4.0 | 4.4 | 4.4 | |||||
| fT4 (ng/mL) | 11.7 (9.3–17) | 0.9 (0.8–1.2) | 0.8 (0.8–1.2) | 1.1 (0.8–1.2) | n.a. | |||||
| HIV-serology | negative | negative | negative | negative | negative | |||||
| Vaccine Responses | ||||||||||
| Anti-tetanus toxin IgG (IU/mL) | 1.03 | 0.95 | 0.92 | insufficient (0.13) | 0.42 | |||||
| Anti-diphterie toxin IgG (IU/mL) | 1.20 | insufficient (0.22) | 0.33 | insufficient (<0.10) | 0.41 | |||||
| Anti-HBs (m/U/mL) | >1000 | 263 | insufficient (17) | >1000 | >1000 | |||||
| Measles IgG | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Mumps IgG | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Rubella IgG Clia (IU/mL) | n.a. | 129 | 70.3 | 181 | >350 | |||||
| VZV IgG | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Clinical and Immunological Characteristics | PLAID | APLAID | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ombrello et al. [3] | * Wang et al. [10] | Kutukculer et al. [18] | Zhou et al. [5] | Neves et al. [9] | Moran- Villasenor et al. [12] | Novice et al. [14] | Khabbazi et al. [15] | Martin-Nalda et al. [8] | Suri et al. [16] | Wu et al. [17] | |||||||
| n = 27 | n = 1 | n = 1 | n = 1 | n = 1 | n = 2 | n = 1 | n = 1 | n = 3 | n = 1 | n = 1 | n = 1 | n = 1 | n = 1 | n = 1 | |||
| Clinical Symptoms | |||||||||||||||||
| Headache | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ✓ | n.a. | n.a. | n.a. | n.a. | ✓ |
| Sensorineural deafness | n.a. | ✗ | ✗ | n.a. | n.a. | n.a. | ✓ | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ✗ |
| Eye inflammation | n.a. | ✗ | ✗ | n.a. | n.a. | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | n.a. | ✓ | ✓ | ✗ | ✓ | ✗ |
| Cold urticaria | ✓ | ✓ | ✓ | n.a. | ✓ | ✗ | ✗ | ✗ | ✗ | n.a. | ✗ | ✗ | n.a. | ✗ | n.a. | n.a. | n.a. |
| Rashes | urticaria | urti-caria | urti-caria | n.a. | urticaria, erythem, ovaloid scars | erythmatous plaques, vesiculo-pustular lesions | vesiculo- pustular rash | vesiclo- pustular rash | erythematous, non-folliculo- centric papules, urticaria | poly- morphic eruption | ✗ | urticaria | erythematous plaques, vesiculo- pustular | maculo- papular eruption, erythematous plaques, urticaria | erythematous macular rash | photo- sensitive rash | vesiculopustular rashes |
| Cutis laxa | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ✓ | n.a. | n.a. | n.a. | n.a. | n.a. | ✓ | ✓ | n.a. | n.a. | n.a. |
| Cutaneous granulomas | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ✓ | ✓ | n.a. | n.a. | n.a. | n.a. | ✓ | n.a. | n.a. | n.a. | n.a. |
| Inflammatory bowel disease | n.a. | n.a. | n.a. | n.a. | n.a. | ✓ | ✓ | ✓ | ✓ | n.a. | n.a. | ✗ | ✗ | ✗ | n.a. | n.a. | ✗ |
| Abdominal pain | n.a. | ✗ | ✗ | n.a. | n.a. | ✓ | n.a. | n.a. | n.a. | n.a. | n.a. | ✓ | ✗ | ✗ | n.a. | n.a. | ✗ |
| Arthralgia/myalgia | n.a. | ✗ | ✗ | n.a. | n.a. | ✓ | n.a. | n.a. | n.a. | ✓ | n.a. | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ |
| Allergic disease | ✓(15/27) | n.a. | n.a. | n.a. | ✗ | n.a. | ✗ | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ✗ |
| Autoimmunity | ✓ | ✗ | ✗ | n.a. | ✗ | ✗ | ✗ | ✗ | ✗ | n.a. | n.a. | ✗ | ✗ | ✗ | n.a. | n.a. | ✗ |
| Recurrent (chest) infection | ✓ | ✗ | ✗ | ✓ | n.a. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | n.a. | ✓ | ✓ | ✓ | n.a. | ✗ |
| Interstitial pneumonitis | n.a. | n.a. | n.a. | n.a. | n.a. | ✓ | ✓ | n.a. | ✗ | n.a. | ✓ | n.a. | n.a. | n.a. | ✓ | n.a. | ✗ |
| Immunology | |||||||||||||||||
| T cells | norm | n.a. | n.a. | norm | norm | norm, INF-y/IL-17 prod. norm | norm, INF-y/IL-17 prod. ↓ | norm | norm | n.a. | norm | n.a. | norm | ↓ | n.a. | n.a. | n.a. |
| Class-switched memory B cells | ↓ | n.a. | n.a. | n.a | n.a. | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | n.a. | n.a. | ↓ | n.a. | n.a. | n.a. |
| NK cells | ↓ | n.a. | n.a. | norm | n.a. | norm | norm | ↓ | n.a. | n.a. | norm | n.a. | norm | norm | n.a. | n.a. | ↓ |
| IgG | ↓ | n.a. | n.a. | norm | ↑ | n.a. | ↓ | ↓ | n.a. | n.a. | ↓ | norm | ↓ | norm | ↑ | n.a. | ↓ |
| IgA | ↓ | n.a. | n.a. | norm | ↑ | n.a. | ↓ | ↓ | n.a. | n.a. | ↓ | norm | ↓ | norm | ↑ | ↑ | ↓ |
| IgM | ↓ | n.a. | n.a. | ↓ | norm | n.a. | ↓ | ↓ | ↓ | ↓ | ↓ | norm | ↓ | ↓ | norm | norm | ↓ |
| Circulating auto antibodies | ✓(13/27) | ✓ | ✓ | n.a. | ✗ | ✗ | ✗ | ✗ | ✗ | n.a. | n.a. | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Authors | Disease | PLCG2 Gene Variant | Pathogenicity Classification | Functional Test |
|---|---|---|---|---|
| Ombrello et al. [3] | PLAID | c.1935-521_2054+5385del/c.1935-322_2054+4100del, p.W646_R685del; c.2055-1396_2417+2699del, p.A686_R685.del | not classified 1 | enzymatic activity of PLCy2 in a COS-7 transfected system, measurement of calcium flux, phosphorylation of ERK, and degranulation of natural killer cells |
| Wang et al. [10] * | PLAID | c.3244T>C, p.C1082R | not classified 1,Δ | n.a. |
| PLAID | c.3524T>A, p.I1175K | |||
| Kutukculer et al. [18] | PLAID | c.415C>T, p.P139S | VUS [18] | n.a. |
| APLAID | n.a., p.R268A | benign [18] | ||
| Zhou et al. [5] | APLAID | c.2120C>A, p.S707Y | not classified 1 | transfection of HEK293T/ COS-7 cells, measurement of enzymatic activity of PLCy2, calcium flux, production of IP3/IP1 and ERK phosphorylation |
| Neves et al. [9] | APLAID | c.2543T>C, p.L848P | not classified 1 | transfection of COS-7 cells, measurement of IP and enzymatic activity of PLCy2 |
| Moran- Villasenor et al. [12] | APLAID | c.2543T>C, p.L848P | not classified 1 | n.a. |
| Novice et al. [14] | APLAID | c.3422T>A, p.M1141K | pathogenic [14] | calcium flux, ERK phosphorylation, platelet activation related to PLCy2 and BCR stimulation |
| Khabbazi et al. [15] | APLAID | c.579C>G, p.H193Q | pathogenic (homzy.) [15] | n.a. |
| Martin-Nalda et al. [8] | APLAID | c.2533_2544del, p.L845_L848del | pathogenic 1 | calcium flux, measurement of IP, enzymatic activity of PLC and cytokine measurements after LPS stimulation |
| APLAID | c.2122G>C, p.A708P | |||
| Suri et al. [16] | APLAID | c.2393A>G; p.N798S | not classified 1 | n.a. |
| Wu et al. [17] | APLAID | c.505A>G, p.I169V | VUS 1,□ | n.a. |
| Deza et al. [19] | Christiansen et al. [20] | Mahajan et al. [21] | Park et al. [11] | |||
|---|---|---|---|---|---|---|
| n = 1 | n = 1 | n = 1 | n = 1 | n = 1 | n = 1 | |
| Leading symptom/suggested disease reported | PLAID/acquired cold urticaria | idiopathic thrombocytopenia, pneumococcal meningitis | autoinflammatory epidermolysis bullosa | recurrent skin blistering disease and B-cell lymphopenia | ||
| PLCG2 gene variant | c.3125G>C, p.S1042T | c.1274T>G, p.F425C | c.1565C>G, p.P522R | c.2866C>T, p.R956C | c.2393A>G, p.N798S | c.2119T>C, pS.707P |
| Pathogenecity classification | likely benign | VUS | likely benign | VUS 1 | not classified1 | likely pathogenic [11]/likely benign 1 |
| Comment | n.a | additional Variant TNFRSF1A, c.1343C>T, p.P448L | n.a. | n.a. | ||
| Clinical Symptoms | ||||||
| Headache | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Sensorineural deafness | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Eye inflammation | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Cold urticaria | ✓ | ✓ | ✓ | n.a. | n.a. | n.a. |
| Rashes | urticaria | urticaria | urticaria | n.a. | n.a. | blistering skin lesions |
| Cutis laxa | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Cutaneous granulomas | n.a. | n.a. | n.a. | n.a. | cutaneous erosions, depigmentation | n.a. |
| Inflammatory bowel disease | n.a. | n.a. | n.a. | n.a. | ✗ | n.a. |
| Abdominal pain | ✓ | ✗ | ✗ | n.a. | n.a. | n.a. |
| Arthralgia/myalgia | ✗ | ✗ | ✗ | n.a. | joint destruction | n.a. |
| Allergic disease | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Autoimmunity | n.a. | n.a. | n.a. | n.a. | ✗ | n.a. |
| Recurrent (chest) infection | ✗ | ✗ | ✗ | n.a. | n.a. | ✓ |
| Interstitial pneumonitis | n.a. | n.a. | n.a. | ✓ | ✗ | n.a. |
| Immunology | ||||||
| T cells | n.a. | n.a. | n.a. | norm. | n.a. | norm. |
| Class-switched memory B cells | n.a. | n.a. | n.a. | ↓ | n.a. | n.a. |
| NK cells | n.a. | n.a. | n.a. | norm. | n.a. | norm. |
| IgG | n.a. | n.a. | n.a. | ↓ | n.a. | norm. |
| IgA | n.a. | n.a. | n.a. | ↓ | n.a. | norm. |
| IgM | n.a. | n.a. | n.a. | norm. | n.a. | ↓ |
| Circulating auto antibodies | n.a. | n.a. | n.a. | n.a. | ✗ | ✗ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welzel, T.; Oefelein, L.; Holzer, U.; Müller, A.; Menden, B.; Haack, T.B.; Groβ, M.; Kuemmerle-Deschner, J.B. Variant in the PLCG2 Gene May Cause a Phenotypic Overlap of APLAID/PLAID: Case Series and Literature Review. J. Clin. Med. 2022, 11, 4369. https://doi.org/10.3390/jcm11154369
Welzel T, Oefelein L, Holzer U, Müller A, Menden B, Haack TB, Groβ M, Kuemmerle-Deschner JB. Variant in the PLCG2 Gene May Cause a Phenotypic Overlap of APLAID/PLAID: Case Series and Literature Review. Journal of Clinical Medicine. 2022; 11(15):4369. https://doi.org/10.3390/jcm11154369
Chicago/Turabian StyleWelzel, Tatjana, Lea Oefelein, Ursula Holzer, Amelie Müller, Benita Menden, Tobias B. Haack, Miriam Groβ, and Jasmin B. Kuemmerle-Deschner. 2022. "Variant in the PLCG2 Gene May Cause a Phenotypic Overlap of APLAID/PLAID: Case Series and Literature Review" Journal of Clinical Medicine 11, no. 15: 4369. https://doi.org/10.3390/jcm11154369
APA StyleWelzel, T., Oefelein, L., Holzer, U., Müller, A., Menden, B., Haack, T. B., Groβ, M., & Kuemmerle-Deschner, J. B. (2022). Variant in the PLCG2 Gene May Cause a Phenotypic Overlap of APLAID/PLAID: Case Series and Literature Review. Journal of Clinical Medicine, 11(15), 4369. https://doi.org/10.3390/jcm11154369






