Atopic Dermatitis and Food Allergy: A Complex Interplay What We Know and What We Would Like to Learn
Abstract
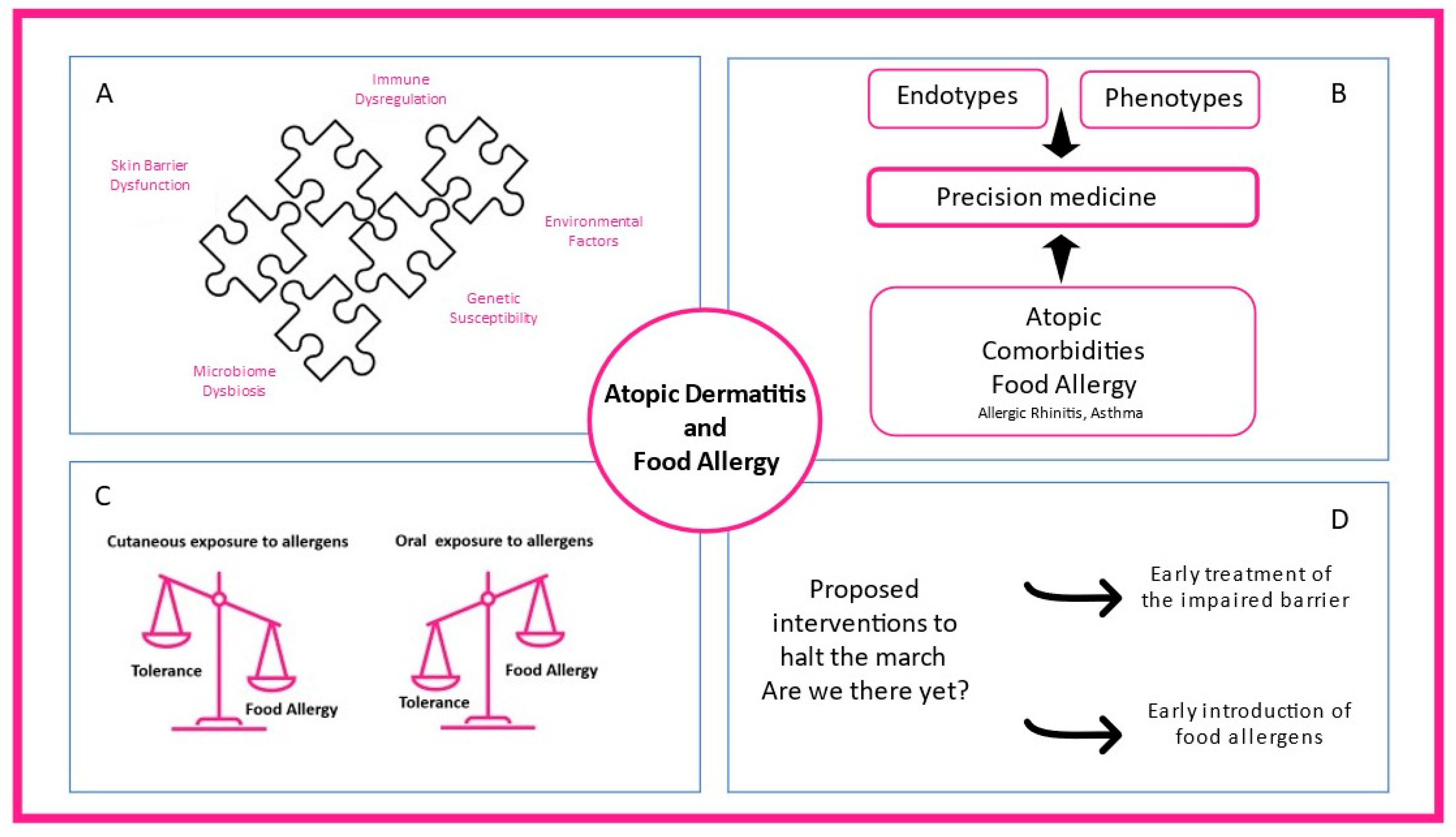
:1. Introduction
2. How Much Atopic Is Atopic Dermatitis?
2.1. Defining Atopy in Atopic Dermatitis
2.2. Phenotypes and Endotypes of AD
2.2.1. Phenotypes of AD
2.2.2. Endotypes of AD
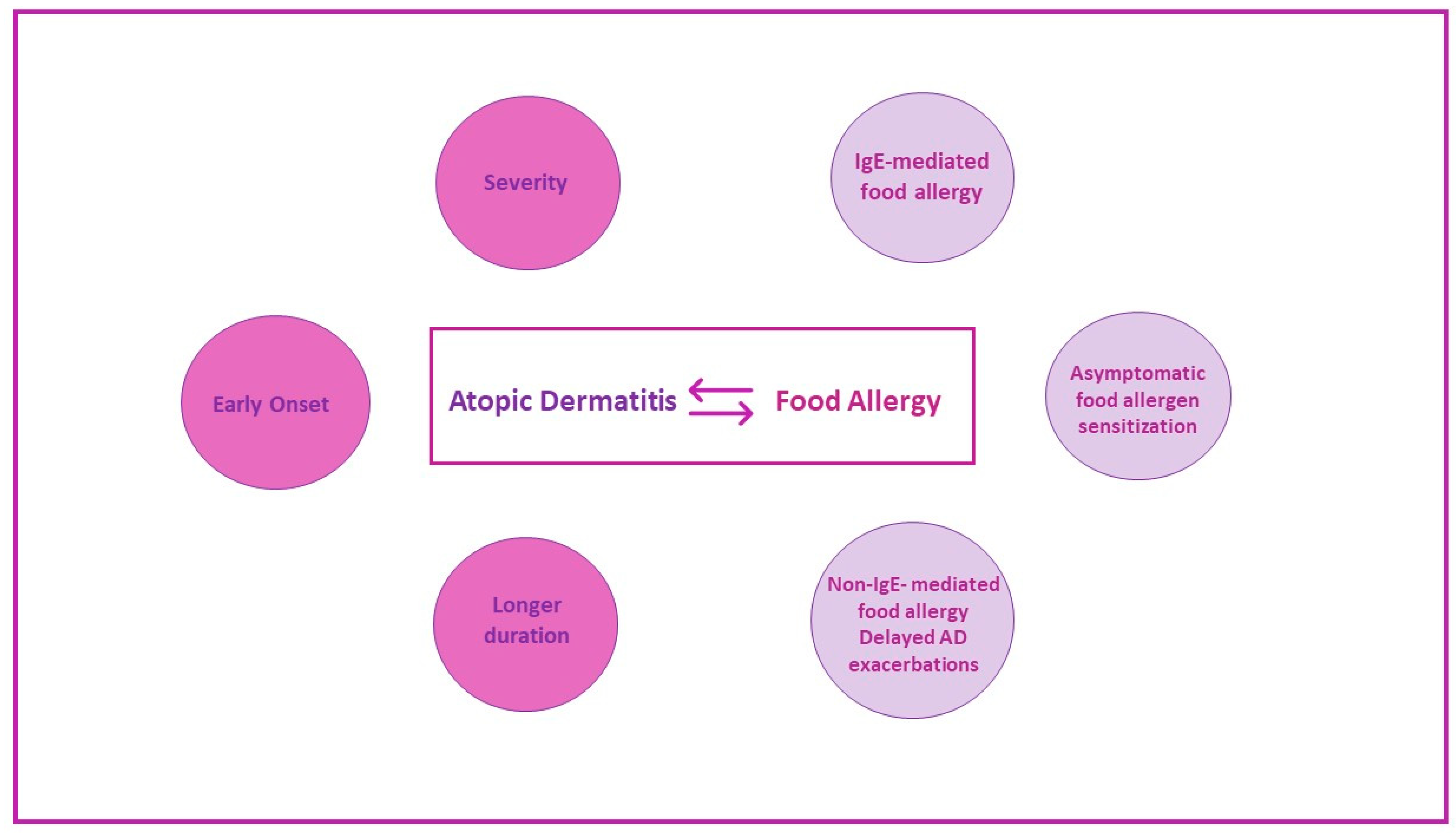
3. Association between Food Sensitization and Food Allergy with Atopic Dermatitis
3.1. Transepidermal Water Loss and Skin Barrier Impairment
3.2. Food Sensitization and Food Allergy among Patients with AD
3.3. Conclusions
4. Mechanisms Explaining how Atopic Dermatitis Promotes Sensitization to Food Allergens: The Dual-Allergen-Exposure Hypothesis
4.1. Introduction
4.2. Allergen Exposure through the Skin
4.3. Allergen Exposure through Gastrointestinal Mucosa
4.4. Conclusions
5. Patterns of Clinical Reactivity to Foods in Children with AD
5.1. Immediate-Type Reactions (IgE-Mediated)
5.2. Delayed Exacerbation of AD (Non-IgE-Mediated)
5.3. Mixed Reactions
6. Diagnosis and Management of Food Sensitization and Food Allergy in Patients with AD
6.1. Sensitization to Food Allergens in AD Patients
6.2. The Role of Skin Prick Tests, Atopy Patch Tests, In Vitro Measurement of Specific IgE, and Measurement of Food Allergens Components in the Diagnosis of Food Allergy
6.3. The Role of Elimination and Reintroduction of Certain Foods in Diagnosis of Food Allergy
7. Elimination Diets in AD: Favor or Harm?
7.1. Elimination Diets May Have a Beneficial Effect on AD Severity
7.2. Elimination Diets May Increase the Risk of Food Allergy in Patients with AD
8. Aiming to Protect: Is There a Way to Interfere in the Relationship between Food Allergy and Atopic Dermatitis?
8.1. Restoring the Skin Barrier and Preventing Atopic Dermatitis Could Reduce Epicutaneous Sensitization and Subsequent Food Allergy
8.2. Early Introduction of Allergenic Foods in High-Risk Infants to Prevent Food Allergy and Atopic Dermatitis
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The Global Burden of Skin Disease in 2010: An Analysis of the Prevalence and Impact of Skin Conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Bieber, T. Atopic dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef]
- Odhiambo, J.A.; Williams, H.C.; Clayton, T.O.; Robertson, C.F.; Asher, M.I. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J. Allergy Clin. Immunol. 2009, 124, 1251–1258.e23. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Hanifin, J.M. Adult eczema prevalence and associations with asthma and other health and demographic factors: A US population–based study. J. Allergy Clin. Immunol. 2013, 132, 1132–1138. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Laughter, M.; Maymone, M.; Mashayekhi, S.; Arents, B.; Karimkhani, C.; Langan, S.; Dellavalle, R.; Flohr, C. The global burden of atopic dermatitis: Lessons from the Global Burden of Disease Study 1990–2017. Br. J. Dermatol. 2020, 184, 304–309. [Google Scholar] [CrossRef]
- Narla, S.; Silverberg, J.I. The Role of Environmental Exposures in Atopic Dermatitis. Curr. Allergy Asthma Rep. 2020, 20, 74. [Google Scholar] [CrossRef]
- Ständer, S. Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1136–1143. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef]
- Pawankar, R.; Baena-Cagnani, C.E.; Bousquet, J.; Canonica, G.W.; Cruz, A.A.; Kaliner, M.A.; Lanier, B.Q.; Henley, K. State of world allergy report 2008: Allergy and chronic respiratory diseases. World Allergy Organ J. 2008, 1 (Suppl. 6), S4–S17. [Google Scholar] [CrossRef]
- Prescott, S.; Allen, K.J. Food allergy: Riding the second wave of the allergy epidemic. Pediatric Allergy Immunol. 2011, 22, 155–160. [Google Scholar] [CrossRef]
- Hill, D.A.; Spergel, J.M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 2018, 120, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Johansson, S.G.; Hourihane, J.O.; Bousquet, J.; Bruijnzeel-Koomen, C.; Dreborg, S.; Haahtela, T.; Kowalski, M.L.; Mygind, N.; Ring, J.; van Cauwenberge, P.; et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2001, 56, 813–824. [Google Scholar] [CrossRef]
- Akdis, C.A.; Agache, I.; Allergy, E.A.; Immunology, C. Global Atlas of Allergy; European Academy of Allergy and Clinical Immunology: Florence, Italy, 2014. [Google Scholar]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatric Allergy Immunol. 2016, 27 (Suppl. 23), 1–250. [Google Scholar] [CrossRef]
- Sur, L.M.; Armat, I.; Duca, E.; Sur, G.; Lupan, I.; Sur, D.; Samasca, G.; Lazea, C.; Lazar, C. Food Allergy a Constant Concern to the Medical World and Healthcare Providers: Practical Aspects. Life 2021, 11, 1204. [Google Scholar] [CrossRef]
- Meyer, R.; Fox, A.T.; Lozinsky, A.C.; Michaelis, L.J.; Shah, N. Non-IgE-mediated gastrointestinal allergies-Do they have a place in a new model of the Allergic March. Pediatric Allergy Immunol. 2018, 30, 149–158. [Google Scholar] [CrossRef]
- Anvari, S.; Miller, J.; Yeh, C.Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef] [Green Version]
- Kramer, O.N.; Strom, M.A.; Ladizinski, B.; Lio, P.A. The history of atopic dermatitis. Clin. Dermatol. 2017, 35, 344–348. [Google Scholar] [CrossRef]
- Flohr, C.; Johansson, S.G.; Wahlgren, C.F.; Williams, H. How atopic is atopic dermatitis? J. Allergy Clin. Immunol. 2004, 114, 150–158. [Google Scholar] [CrossRef]
- Eller, E.; Kjaer, H.F.; Høst, A.; Andersen, K.E.; Bindslev-Jensen, C. Food allergy and food sensitization in early childhood: Results from the DARC cohort. Allergy 2009, 64, 1023–1029. [Google Scholar] [CrossRef]
- Hill, D.J.; Heine, R.G.; Hosking, C.S. The diagnostic value of skin prick testing in children with food allergy. Pediatric Allergy Immunol. 2004, 15, 435–441. [Google Scholar] [CrossRef]
- Tsakok, T.; Marrs, T.; Mohsin, M.; Baron, S.; du Toit, G.; Till, S.; Flohr, C. Does atopic dermatitis cause food allergy? A systematic review. J. Allergy Clin. Immunol. 2016, 137, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- Bieber, T.; D’Erme, A.M.; Akdis, C.A.; Traidl-Hoffmann, C.; Lauener, R.; Schäppi, G.; Schmid-Grendelmeier, P. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J. Allergy Clin. Immunol. 2017, 139, S58–S64. [Google Scholar] [CrossRef] [Green Version]
- Tokura, Y. Extrinsic and intrinsic types of atopic dermatitis. J. Dermatol. Sci. 2010, 58, 1–7. [Google Scholar] [CrossRef]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef]
- Wollenberg, A.; Christen-Zäch, S.; Taieb, A.; Paul, C.; Thyssen, J.; De Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.; et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2717–2744. [Google Scholar] [CrossRef]
- Thyssen, J.; Berents, T.; Bradley, M.; Deleuran, M.; Korhonen, L.; Langeland, T.; Särnhult, T.; Thomsen, S.; Thune, T.; Wahlgren, C.; et al. Clinical Management of Atopic Dermatitis in Adults: Mapping of Expert Opinion in 4 Nordic Countries using a Modified Delphi Process. Acta Derm. Venereol. 2020, 100, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bakker, D.S.; Nierkens, S.; Knol, E.F.; Giovannone, B.; Delemarre, E.M.; van der Schaft, J.; van Wijk, F.; de Bruin-Weller, M.S.; Drylewicz, J.; Thijs, J.L. Confirmation of multiple endotypes in atopic dermatitis based on serum biomarkers. J. Allergy Clin. Immunol. 2021, 147, 189–198. [Google Scholar] [CrossRef]
- Thijs, J.L.; Strickland, I.; Bruijnzeel-Koomen, C.A.; Nierkens, S.; Giovannone, B.; Csomor, E.; Sellman, B.R.; Mustelin, T.; Sleeman, M.A.; de Bruin-Weller, M.S.; et al. Moving toward endotypes in atopic dermatitis: Identification of patient clusters based on serum biomarker analysis. J. Allergy Clin. Immunol. 2017, 140, 730–737. [Google Scholar] [CrossRef] [Green Version]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Czarnowicki, T.; Esaki, H.; Gonzalez, J.; Malajian, D.; Shemer, A.; Noda, S.; Talasila, S.; Berry, A.; Gray, J.; Becker, L.; et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)+ TH2/TH1 cell imbalance, whereas adults acquire CLA+ TH22/TC22 cell subsets. J. Allergy Clin. Immunol. 2015, 136, 941–951.e3. [Google Scholar] [CrossRef] [Green Version]
- Esaki, H.; Brunner, P.M.; Renert-Yuval, Y.; Czarnowicki, T.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; Johnson, D.B.; et al. Early-onset pediatric atopic dermatitis is T H 2 but also T H 17 polarized in skin. J. Allergy Clin. Immunol. 2016, 138, 1639–1651. [Google Scholar] [CrossRef] [Green Version]
- Bakker, D.S.; de Graaf, M.; Nierkens, S.; Delemarre, E.M.; Knol, E.; van Wijk, F.; de Bruin-Weller, M.S.; Drylewicz, J.; Thijs, J.L. Unraveling heterogeneity in pediatric atopic dermatitis: Identification of serum biomarker based patient clusters. J. Allergy Clin. Immunol. 2021, 149, 125–134. [Google Scholar] [CrossRef]
- Kelleher, M.; Dunn-Galvin, A.; Hourihane, J.O.; Murray, D.; Campbell, L.E.; McLean, W.I.; Irvine, A.D. RETRACTED: Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J. Allergy Clin. Immunol. 2015, 135, 930–935.e1. [Google Scholar] [CrossRef] [Green Version]
- Tham, E.H.; Rajakulendran, M.; Lee, B.W.; Van Bever, H.P.S. Epicutaneous sensitization to food allergens in atopic dermatitis: What do we know? Pediatric Allergy Immunol. 2020, 31, 7–18. [Google Scholar] [CrossRef]
- Rustad, A.M.; Nickles, M.A.; Bilimoria, S.N.; Lio, P.A. The Role of Diet Modification in Atopic Dermatitis: Navigating the Complexity. Am. J. Clin. Dermatol. 2021, 23, 27–36. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef]
- Venkataraman, D.; Soto-Ramírez, N.; Kurukulaaratchy, R.J.; Holloway, J.W.; Karmaus, W.; Ewart, S.L.; Arshad, S.H.; Erlewyn-Lajeunesse, M. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. J. Allergy Clin. Immunol. 2014, 134, 876–882.e4. [Google Scholar] [CrossRef] [Green Version]
- Eigenmann, P.A.; Calza, A.-M. Diagnosis of IgE-mediated food allergy among Swiss children with atopic dermatitis. Pediatric Allergy Immunol. 2000, 11, 95–100. [Google Scholar] [CrossRef]
- Eigenmann, P.A.; Sicherer, S.H.; Borkowski, T.A.; Cohen, B.A.; Sampson, H.A. Prevalence of IgE-Mediated Food Allergy Among Children with Atopic Dermatitis. Pediatrics 1998, 101, e8. [Google Scholar] [CrossRef] [Green Version]
- Burks, A.; James, J.M.; Hiegel, A.; Wilson, G.; Wheeler, J.; Jones, S.M.; Zuerlein, N. Atopic dermatitis and food hypersensitivity reactions. J. Pediatrics 1998, 132, 132–136. [Google Scholar] [CrossRef]
- Niggemann, B.; Sielaff, B.; Beyer, K.; Binder, C.; Wahn, U. Outcome of double-blind, placebo-controlled food challenge tests in 107 children with atopic dermatitis. Clin. Exp. Allergy 1999, 29, 91–96. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; The EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef]
- Hill, D.J.; Hosking, C.S.; De Benedictis, F.M.; Oranje, A.P.; Diepgen, T.L.; Bauchau, V.; The EPAAC Study Group. Confirmation of the association between high levels of immunoglobulin E food sensitization and eczema in infancy: An international study. Clin. Exp. Allergy 2007, 38, 161–168. [Google Scholar] [CrossRef]
- Martin, P.E.; Eckert, J.K.; Koplin, J.; Lowe, A.; Gurrin, L.; Dharmage, S.; Vuillermin, P.; Tang, M.L.K.; Ponsonby, A.-L.; Matheson, M.; et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin. Exp. Allergy 2014, 45, 255–264. [Google Scholar] [CrossRef]
- Wood, R.A.; Sicherer, S.H.; Vickery, B.P.; Jones, S.M.; Liu, A.H.; Fleischer, D.M.; Henning, A.K.; Mayer, L.; Burks, A.W.; Grishin, A.; et al. The natural history of milk allergy in an observational cohort. J. Allergy Clin. Immunol. 2013, 131, 805–812.e4. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H. Clinical and Laboratory Predictors of Egg Allergy Resolution in Children. Allergy Asthma Immunol. Res. 2019, 11, 446–449. [Google Scholar] [CrossRef]
- Giannetti, A.; Cipriani, F.; Indio, V.; Gallucci, M.; Caffarelli, C.; Ricci, G. Influence of Atopic Dermatitis on Cow’s Milk Allergy in Children. Medicina 2019, 55, 460. [Google Scholar] [CrossRef] [Green Version]
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef]
- Brough, H.A.; Nadeau, K.C.; Sindher, S.B.; Alkotob, S.; Chan, S.; Bahnson, H.T.; Leung, D.Y.M.; Lack, G. Epicutaneous sensitization in the development of food allergy: What is the evidence and how can this be prevented? Allergy 2020, 75, 2185–2205. [Google Scholar] [CrossRef]
- Lack, G.; Fox, D.; Northstone, K.; Golding, J. Factors Associated with the Development of Peanut Allergy in Childhood. N. Engl. J. Med. 2003, 348, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Brough, H.A.; Liu, A.H.; Sicherer, S.; Makinson, K.; Douiri, A.; Brown, S.J.; Stephens, A.C.; McLean, W.I.; Turcanu, V.; Wood, R.A.; et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J. Allergy Clin. Immunol. 2014, 135, 164–170.e4. [Google Scholar] [CrossRef] [Green Version]
- García-Boyano, M.; Pedrosa, M.; Quirce, S.; Boyano-Martínez, T. Household almond and peanut consumption is related to the development of sensitization in young children. J. Allergy Clin. Immunol. 2015, 137, 1248–1251.e6. [Google Scholar] [CrossRef] [Green Version]
- Tojima, I.; Matsumoto, K.; Kikuoka, H.; Hara, S.; Yamamoto, S.; Shimizu, S.; Kouzaki, H.; Shimizu, T. Evidence for the induction of Th2 inflammation by group 2 innate lymphoid cells in response to prostaglandin D2 and cysteinyl leukotrienes in allergic rhinitis. Allergy 2019, 74, 2417–2426. [Google Scholar] [CrossRef]
- Drislane, C.; Irvine, A.D. The role of filaggrin in atopic dermatitis and allergic disease. Ann. Allergy Asthma Immunol. 2019, 124, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Akdis, C.A.; Arkwright, P.D.; Brüggen, M.-C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 immunity in the skin and lungs. Allergy 2020, 75, 1582–1605. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Calatroni, A.; Zaramela, L.S.; LeBeau, P.K.; Dyjack, N.; Brar, K.; David, G.; Johnson, K.; Leung, S.; Ramirez-Gama, M.; et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci. Transl. Med. 2019, 11, eaav2685. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Ahn, K.; Leung, D.Y.M. Interactions Between Atopic Dermatitis and Staphylococcus aureus Infection: Clinical Implications. Allergy Asthma Immunol. Res. 2019, 11, 593–603. [Google Scholar] [CrossRef]
- Bunikowski, R.; Mielke, M.; Skarabis, H.; Herz, U.; Bergmann, R.L.; Wahn, U.; Renz, H. Prevalence and role of serum IgE antibodies to the StaphylococcuS aureus–derived superantigens SEA and SEB in children with atopic dermatitis. J. Allergy Clin. Immunol. 1999, 103, 119–124. [Google Scholar] [CrossRef]
- Chan, S.M.H.; Turcanu, V.; Stephens, A.C.; Fox, A.T.; Grieve, A.P.; Lack, G. Cutaneous lymphocyte antigen and α4β7 T-lymphocyte responses are associated with peanut allergy and tolerance in children. Allergy 2012, 67, 336–342. [Google Scholar] [CrossRef]
- Weiner, H.L.; da Cunha, A.P.; Quintana, F.; Wu, H. Oral tolerance. Immunol Rev. 2011, 241, 241–259. [Google Scholar] [CrossRef]
- Manam, S.; Tsakok, T.; Till, S.; Flohr, C. The association between atopic dermatitis and food allergy in adults. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 423–429. [Google Scholar] [CrossRef]
- Muraro, A.; Lemanske, R.F., Jr.; Castells, M.; Torres, M.J.; Khan, D.; Simon, H.-U.; Bindslev-Jensen, C.; Burks, W.; Poulsen, L.K.; Sampson, H.A.; et al. Precision medicine in allergic disease-food allergy, drug allergy, and anaphylaxis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma and Immunology. Allergy 2017, 72, 1006–1021. [Google Scholar] [CrossRef] [Green Version]
- Robison, R.; Singh, A.M. Controversies in Allergy: Food Testing and Dietary Avoidance in Atopic Dermatitis. J. Allergy Clin. Immunol. Pract. 2018, 7, 35–39. [Google Scholar] [CrossRef]
- Bergmann, M.M.; Caubet, J.-C.; Boguniewicz, M.; Eigenmann, P. Evaluation of Food Allergy in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. Pract. 2013, 1, 22–28. [Google Scholar] [CrossRef]
- Breuer, K.; Heratizadeh, A.; Wulf, A.; Baumann, U.; Constien, A.; Tetau, D.; Kapp, A.; Werfel, T. Late eczematous reactions to food in children with atopic dermatitis. Clin. Exp. Allergy 2004, 34, 817–824. [Google Scholar] [CrossRef]
- Werfel, T.; Breuer, K. Role of food allergy in atopic dermatitis. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 379–385. [Google Scholar] [CrossRef]
- Boyce, J.A.; Assa’Ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J. Am. Acad. Dermatol. 2011, 64, 175–192. [Google Scholar] [CrossRef] [Green Version]
- Togias, A.; Cooper, S.F.; Acebal, M.L.; Assa’ad, A.; Baker, J.R., Jr.; Beck, L.A.; Block, J.; Byrd-Bredbenner, C.; Chan, E.S.; Eichenfield, L.F.; et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. Ann. Allergy Asthma Immunol. 2017, 118, 166–173.e7. [Google Scholar] [CrossRef] [Green Version]
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 126 (Suppl. 6), S1–S58. [Google Scholar] [CrossRef]
- Sampson, H.A. The evaluation and management of food allergy in atopic dermatitis. Clin. Dermatol. 2003, 21, 183–192. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, G.-Q.; Li, Z.-Y. The diagnostic value of APT for food allergy in children: A systematic review and meta-analysis. Pediatric Allergy Immunol. 2019, 30, 451–461. [Google Scholar] [CrossRef]
- Walter, A.; Seegräber, M.; Wollenberg, A. Food-Related Contact Dermatitis, Contact Urticaria, and Atopy Patch Test with Food. Clin. Rev. Allergy Immunol. 2018, 56, 19–31. [Google Scholar] [CrossRef]
- Frischmeyer-Guerrerio, P.A.; Rasooly, M.; Gu, W.; Levin, S.; Jhamnani, R.D.; Milner, J.D.; Stone, K.; Guerrerio, A.L.; Jones, J.; Borres, M.P.; et al. IgE testing can predict food allergy status in patients with moderate to severe atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 393–400.e2. [Google Scholar] [CrossRef]
- Eigenmann, P.A.; Beyer, K.; Lack, G.; Muraro, A.; Ong, P.Y.; Sicherer, S.H.; Sampson, H.A. Are avoidance diets still warranted in children with atopic dermatitis? Pediatric Allergy Immunol. 2020, 31, 19–26. [Google Scholar] [CrossRef]
- Atherton, D.; Soothill, J.; Sewell, M.; Wells, R.; Chilvers, C. A Double-Blind Controlled Crossover Trial of an Antigen-Avoidance Diet in Atopic Eczema. Lancet 1978, 311, 401–403. [Google Scholar] [CrossRef]
- Lever, R.; Macdonald, C.; Waugh, P.; Aitchison, T. Randomised controlled trial of advice on an egg exclusion diet in young children with atopic eczema and sensitivity to eggs. Pediatric Allergy Immunol. 1998, 9, 13–19. [Google Scholar] [CrossRef]
- David, T.J. Anaphylactic shock during elimination diets for severe atopic eczema. Arch. Dis. Child. 1984, 59, 983–986. [Google Scholar] [CrossRef]
- Flinterman, A.E.; Knulst, A.C.; Meijer, Y.; Bruijnzeel-Koomen, C.A.; Pasmans, S.G. Acute allergic reactions in children with AEDS after prolonged cow’s milk elimination diets. Allergy 2006, 61, 370–374. [Google Scholar] [CrossRef]
- Barbi, E.; Gerarduzzi, T.; Longo, G.; Ventura, A. Fatal allergy as a possible consequence of long-term elimination diet. Allergy 2004, 59, 668–669. [Google Scholar] [CrossRef]
- Nachshon, L.; Goldberg, M.R.; Elizur, A.; Appel, M.Y.; Levy, M.B.; Katz, Y. Food allergy to previously tolerated foods: Course and patient characteristics. Ann. Allergy Asthma Immunol. 2018, 121, 77–81.e1. [Google Scholar] [CrossRef]
- Hobbs, C.B.; Skinner, A.C.; Burks, A.W.; Vickery, B.P. Food allergies affect growth in children. J. Allergy Clin. Immunol. Pract. 2014, 3, 133–134.e1. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, J.A.; Sicherer, S.H. Quality of life in food allergy. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 236–242. [Google Scholar] [CrossRef]
- DunnGalvin, A.; Polloni, L.; Le Bovidge, J.; Muraro, A.; Greenhawt, M.; Taylor, S.; Baumert, J.; Burks, W.; Trace, A.; DunnGalvin, G.; et al. Preliminary Development of the Food Allergy Coping and Emotions Questionnaires for Children, Adolescents, and Young People: Qualitative Analysis of Data on IgE-Mediated Food Allergy from Five Countries. J. Allergy Clin. Immunol. Pract. 2018, 6, 506–513.e11. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Lowe, A.; Su, J.; Allen, K.; Abramson, M.; Cranswick, N.; Robertson, C.; Forster, D.; Varigos, G.; Hamilton, S.; Kennedy, R.; et al. A randomized trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitization: The PEBBLES pilot study. Br. J. Dermatol. 2017, 178, e19–e21. [Google Scholar] [CrossRef]
- Yamamoto-Hanada, K.; Kobayashi, T.; Williams, H.C.; Mikami, M.; Saito-Abe, M.; Morita, K.; Natsume, O.; Sato, M.; Iwama, M.; Miyaji, Y.; et al. Early aggressive intervention for infantile atopic dermatitis to prevent development of food allergy: A multicenter, investigator-blinded, randomized, parallel group controlled trial (PACI Study)—Protocol for a randomized controlled trial. Clin. Transl. Allergy 2018, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Natsume, O.; Kabashima, S.; Nakazato, J.; Yamamoto-Hanada, K.; Narita, M.; Kondo, M.; Saito, M.; Kishino, A.; Takimoto, T.; Inoue, E.; et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): A randomised, double-blind, placebo-controlled trial. Lancet 2016, 389, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Perkin, M.R.; Logan, K.; Bahnson, H.T.; Marrs, T.; Radulovic, S.; Craven, J.; Flohr, C.; Mills, E.; Versteeg, S.A.; van Ree, R.; et al. Efficacy of the Enquiring About Tolerance (EAT) study among infants at high risk of developing food allergy. J. Allergy Clin. Immunol. 2019, 144, 1606–1614.e2. [Google Scholar] [CrossRef] [Green Version]
- Horimukai, K.; Morita, K.; Narita, M.; Kondo, M.; Kitazawa, H.; Nozaki, M.; Shigematsu, Y.; Yoshida, K.; Niizeki, H.; Motomura, K.-I.; et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 824–830.e6. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.L.; Chalmers, J.R.; Hanifin, J.M.; Thomas, K.S.; Cork, M.J.; McLean, W.H.; Brown, S.J.; Chen, Z.; Chen, Y.; Williams, H.C. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J. Allergy Clin. Immunol. 2014, 134, 818–823. [Google Scholar] [CrossRef] [Green Version]
- Skjerven, H.O.; Rehbinder, E.M.; Vettukattil, R.; LeBlanc, M.; Granum, B.; Haugen, G.; Hedlin, G.; Landrø, L.; Marsland, B.J.; Rudi, K.; et al. Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): A factorial, multicentre, cluster-randomised trial. Lancet 2020, 395, 951–961. [Google Scholar] [CrossRef]
- Chalmers, J.R.; Haines, R.H.; Bradshaw, L.E.; Montgomery, A.A.; Thomas, K.S.; Brown, S.J.; Ridd, M.J.; Lawton, S.; Simpson, E.L.; Cork, M.J.; et al. Daily emollient during infancy for prevention of eczema: The BEEP randomised controlled trial. Lancet 2020, 395, 962–972. [Google Scholar] [CrossRef]
- Dissanayake, E.; Tani, Y.; Nagai, K.; Sahara, M.; Mitsuishi, C.; Togawa, Y.; Suzuki, Y.; Nakano, T.; Yamaide, F.; Ohno, H.; et al. Skin Care and Synbiotics for Prevention of Atopic Dermatitis or Food Allergy in Newborn Infants: A 2 × 2 Factorial, Randomized, Non-Treatment Controlled Trial. Int. Arch. Allergy Immunol. 2019, 180, 202–211. [Google Scholar] [CrossRef]
- du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Fisher, H.R.; Feeney, M.; Radulovic, S.; Basting, M.; et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J. Allergy Clin. Immunol. 2018, 141, 1343–1353. [Google Scholar] [CrossRef] [Green Version]
- Du Toit, G.; Sayre, P.H.; Roberts, G.; Sever, M.L.; Lawson, K.; Bahnson, H.T.; Brough, H.A.; Santos, A.F.; Harris, K.M.; Radulovic, S.; et al. Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. N. Engl. J. Med. 2016, 374, 1435–1443. [Google Scholar] [CrossRef]
- Palmer, D.J.; Metcalfe, J.; Makrides, M.; Gold, M.S.; Quinn, P.; West, C.E.; Loh, R.; Prescott, S.L. Early regular egg exposure in infants with eczema: A randomized controlled trial. J. Allergy Clin. Immunol. 2013, 132, 387–392.e1. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papapostolou, N.; Xepapadaki, P.; Gregoriou, S.; Makris, M. Atopic Dermatitis and Food Allergy: A Complex Interplay What We Know and What We Would Like to Learn. J. Clin. Med. 2022, 11, 4232. https://doi.org/10.3390/jcm11144232
Papapostolou N, Xepapadaki P, Gregoriou S, Makris M. Atopic Dermatitis and Food Allergy: A Complex Interplay What We Know and What We Would Like to Learn. Journal of Clinical Medicine. 2022; 11(14):4232. https://doi.org/10.3390/jcm11144232
Chicago/Turabian StylePapapostolou, Niki, Paraskevi Xepapadaki, Stamatis Gregoriou, and Michael Makris. 2022. "Atopic Dermatitis and Food Allergy: A Complex Interplay What We Know and What We Would Like to Learn" Journal of Clinical Medicine 11, no. 14: 4232. https://doi.org/10.3390/jcm11144232
APA StylePapapostolou, N., Xepapadaki, P., Gregoriou, S., & Makris, M. (2022). Atopic Dermatitis and Food Allergy: A Complex Interplay What We Know and What We Would Like to Learn. Journal of Clinical Medicine, 11(14), 4232. https://doi.org/10.3390/jcm11144232






