Repeated Femtosecond Laser-Assisted Astigmatic Keratotomies in Post-Keratoplasty Eyes
Abstract
1. Introduction
2. Methods
Ethical Principles
3. Patients
3.1. The FLAK Technique
3.2. Outcome Measurements
4. Statistical Analysis
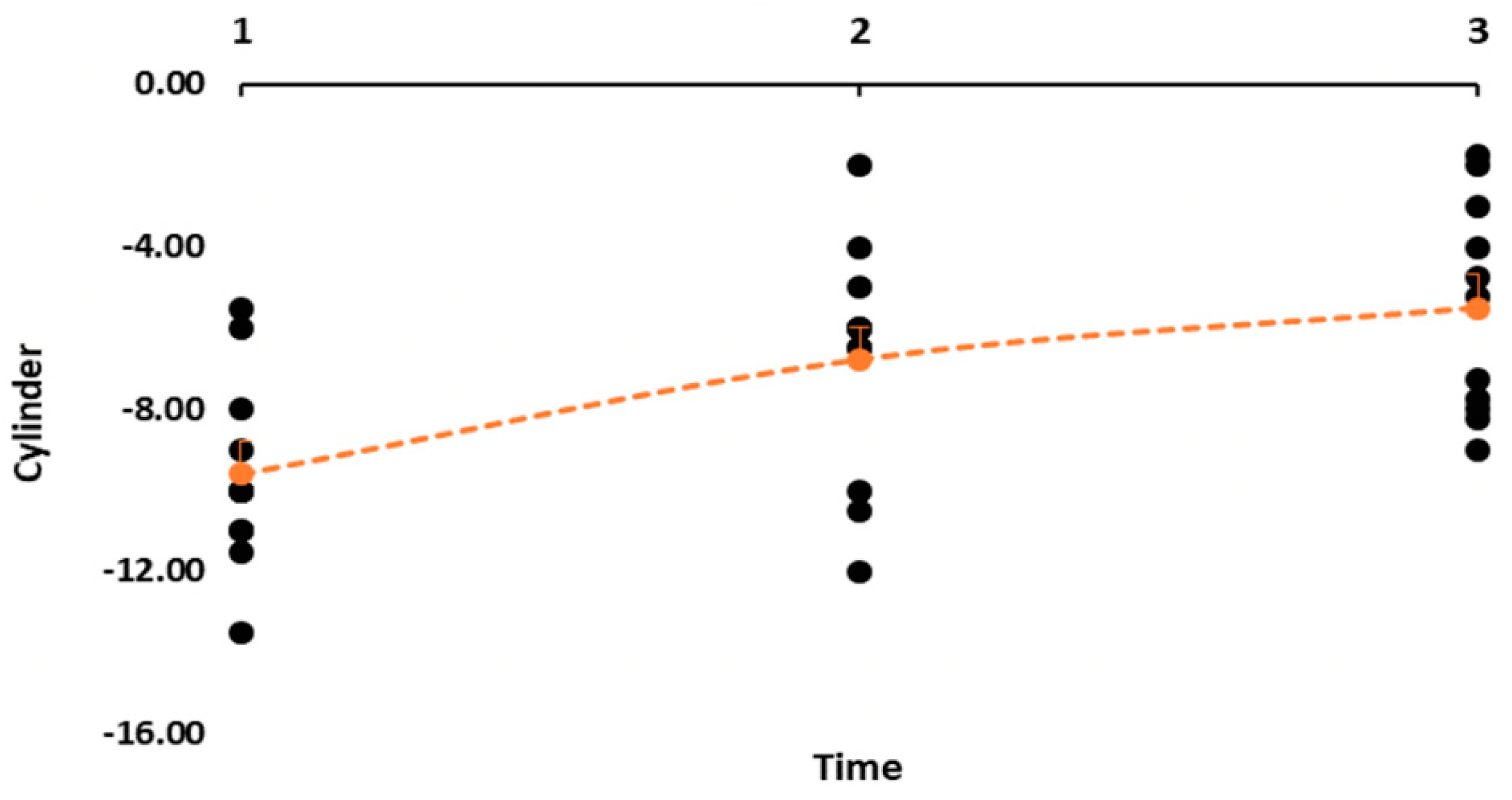
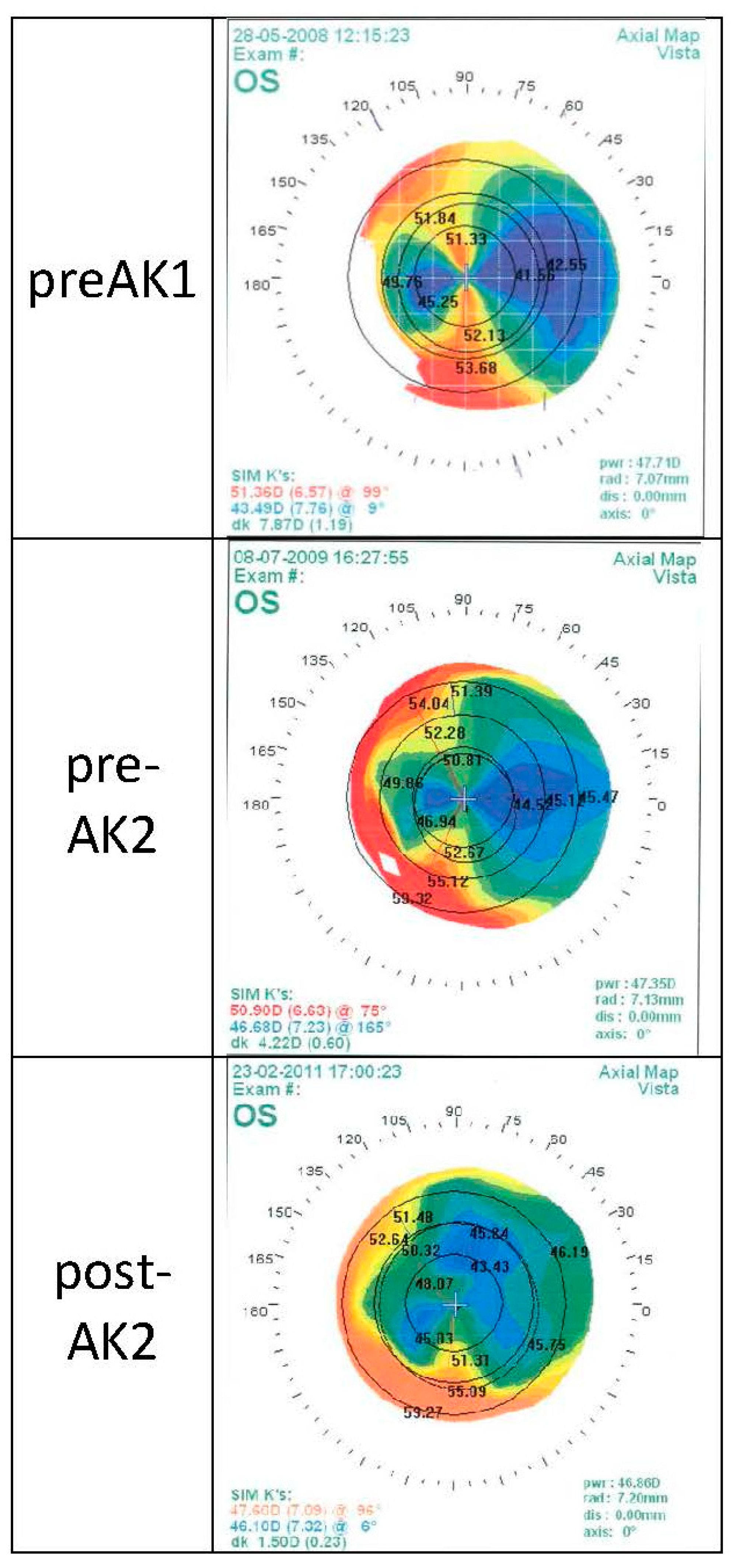
5. Results
5.1. Patient Characteristics
5.2. Visual Outcome
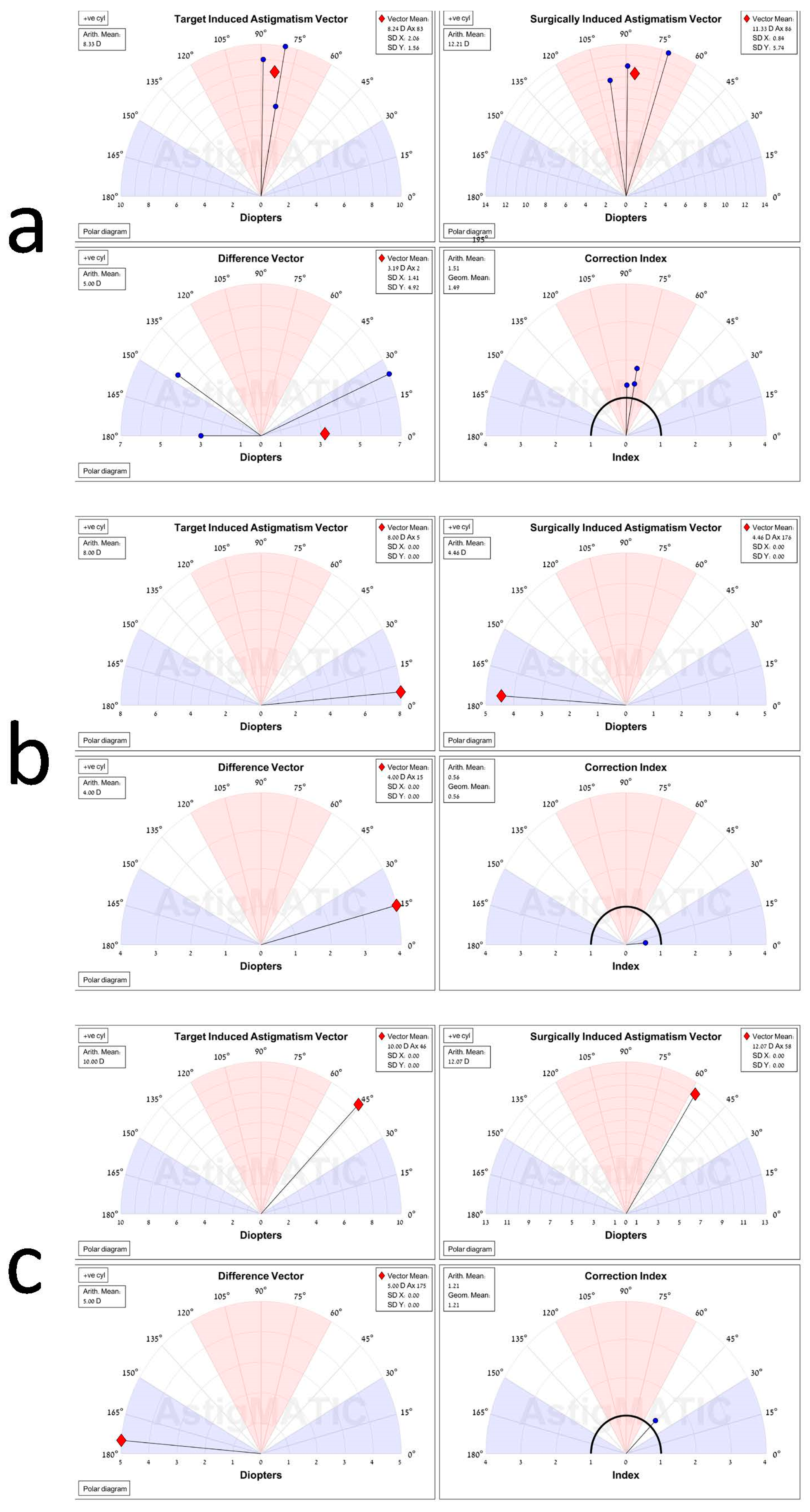
5.3. Vector Analysis
6. Complications
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirshahi, A.; Latz, C. Femtosecond laser-assisted astigmatic keratotomy. Der Ophthalmol. Z. Der Dtsch. Ophthalmol. Ges. 2020, 117, 415–423. [Google Scholar]
- Byun, Y.-S.; Kim, S.; Lazo, M.Z.; Choi, M.-H.; Kang, M.-J.; Lee, J.-H.; Yoo, Y.-S.; Whang, W.-J.; Joo, C.-K. Astigmatic correction by intrastromal astigmatic keratotomy during femtosecond laser–assisted cataract surgery: Factors in outcomes. J. Cataract Refract. Surg. 2018, 44, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Ash, J.; Pararajasegaram, P.; Harris, S.; Coster, D. Long-term outcome after corneal transplantation: Visual result and patient perception of success. Ophthalmology 1991, 98, 651–657. [Google Scholar] [CrossRef]
- Kari Krootila, O.W.; Holopainen, J. Post-keratoplasty Astigmatism. In Corneal Transplantation; Hjortdal, J., Ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Noh, H.; Yoo, Y.-S.; Shin, K.Y.; Lim, D.H.; Chung, T.-Y. Comparison of penetrating femtosecond laser-assisted astigmatic keratotomy and toric intraocular lens implantation for correction of astigmatism in cataract surgery. Sci. Rep. 2021, 11, 7340. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Xu, J.; Zhang, J. Evaluation of the effectiveness of combined femtosecond laser-assisted cataract surgery and femtosecond laser astigmatic keratotomy in improving post-operative visual outcomes. BMC Ophthalmol. 2018, 18, 161. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S. Femtosecond laser-assisted astigmatic keratotomy: A review. Eye Vis. 2018, 5, 6. [Google Scholar] [CrossRef]
- Wu, E. Femtosecond-assisted astigmatic keratotomy. Int. Ophthalmol. Clin. 2011, 51, 77–85. [Google Scholar] [CrossRef]
- AnNakhli, F.; Khattak, A. Vector analysis of femtosecond laser-assisted astigmatic keratotomy after deep anterior lamellar keratoplasty and penetrating keratoplasty. Int. Ophthalmol. 2019, 39, 189–198. [Google Scholar] [CrossRef]
- Callou, T.P.; Garcia, R.; Mukai, A.; Giacomin, N.T.; de Souza, R.G.; Bechara, S.J. Advances in femtosecond laser technology. Clin. Ophthalmol. 2016, 10, 697. [Google Scholar] [CrossRef][Green Version]
- Day, A.C.; Stevens, J.D. Stability of keratometric astigmatism after non-penetrating femtosecond laser intrastromal astigmatic keratotomy performed during laser cataract surgery. J. Refract. Surg. 2016, 32, 152–155. [Google Scholar] [CrossRef]
- Visco, D.M.; Bedi, R.; Packer, M. Femtosecond laser–assisted arcuate keratotomy at the time of cataract surgery for the management of preexisting astigmatism. J. Cataract Refract. Surg. 2019, 45, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Hoffart, L.; Touzeau, O.; Borderie, V.; Laroche, L. Mechanized astigmatic arcuate keratotomy with the Hanna arcitome for astigmatism after keratoplasty. J. Cataract Refract. Surg. 2007, 33, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Day, A.C.; Stevens, J.D. Predictors of femtosecond laser intrastromal astigmatic keratotomy efficacy for astigmatism management in cataract surgery. J. Cataract Refract. Surg. 2016, 42, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Clair, R.M.S.; Sharma, A.; Huang, D.; Yu, F.; Goldich, Y.; Rootman, D.; Yoo, S.; Cabot, F.; Jun, J.; Zhang, L. Development of a nomogram for femtosecond laser astigmatic keratotomy for astigmatism after keratoplasty. J. Cataract Refract. Surg. 2016, 42, 556–562. [Google Scholar] [CrossRef]
- Löffler, F.; Böhm, M.; Herzog, M.; Petermann, K.; Kohnen, T. Tomographic analysis of anterior and posterior and total corneal refractive power changes after femtosecond laser–assisted keratotomy. Am. J. Ophthalmol. 2017, 180, 102–109. [Google Scholar] [CrossRef]
- Roberts, H.W.; Wagh, V.K.; Sullivan, D.L.; Archer, T.J.; O’Brart, D.P. Refractive outcomes after limbal relaxing incisions or femtosecond laser arcuate keratotomy to manage corneal astigmatism at the time of cataract surgery. J. Cataract Refract. Surg. 2018, 44, 955–963. [Google Scholar] [CrossRef]
- Elzarga, A.A.; Osman, A.A.; Gamal, M.; Khafagy, M.M.; Osman, I.S. Vector analysis of femtosecond laser-assisted arcuate keratotomy for post-keratoplasty astigmatic correction. Ophthalmic Res. 2019, 62, 150–156. [Google Scholar] [CrossRef]
- González-Cruces, T.; Cano-Ortiz, A.; Sánchez-González, M.C.; Sánchez-González, J.-M. Cataract surgery astigmatism incisional management. Manual relaxing incision versus femtosecond laser-assisted arcuate keratotomy. A systematic review. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 261, 1–16. [Google Scholar] [CrossRef]
- Nubile, M.; Carpineto, P.; Lanzini, M.; Calienno, R.; Agnifili, L.; Ciancaglini, M.; Mastropasqua, L. Femtosecond laser arcuate keratotomy for the correction of high astigmatism after keratoplasty. Ophthalmology 2009, 116, 1083–1092. [Google Scholar] [CrossRef]
- Wetterstrand, O.; Holopainen, J.M.; Krootila, K. Treatment of post-operative keratoplasty astigmatism using femtosecond laser-assisted intrastromal relaxing incisions. J. Refract. Surg. 2013, 29, 378–382. [Google Scholar] [CrossRef]
- Kumar, N.L.; Kaiserman, I.; Shehadeh-Mashor, R.; Sansanayudh, W.; Ritenour, R.; Rootman, D.S. IntraLase-enabled astigmatic keratotomy for post-keratoplasty astigmatism: On-axis vector analysis. Ophthalmology 2010, 117, 1228–1235.e1. [Google Scholar] [CrossRef] [PubMed]
- Trivizki, O.; Levinger, E.; Levinger, S. Correction ratio and vector analysis of femtosecond laser arcuate keratotomy for the correction of post-mushroom profile keratoplasty astigmatism. J. Cataract Refract. Surg. 2015, 41, 1973–1979. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krysik, K.; Miklaszewski, P.; Dobrowolski, D.; Lyssek-Boron, A.; Grabarek, B.O.; Wylegala, E. Ocular Surface Preparation Before Keratoprosthesis Implantation. Ophthalmology 2022, 11, 249–259. [Google Scholar] [CrossRef]
- Hoffart, L.; Proust, H.; Matonti, F.; Conrath, J.; Ridings, B. Correction of postkeratoplasty astigmatism by femtosecond laser compared with mechanized astigmatic keratotomy. Am. J. Ophthalmol. 2009, 147, 779–787.e1. [Google Scholar] [CrossRef] [PubMed]
- Ho Wang Yin, G.; Hoffart, L. Post-keratoplasty astigmatism management by relaxing incisions: A systematic review. Eye Vis. 2017, 4, 29. [Google Scholar] [CrossRef]
- Vickers, L.A.; Gupta, P.K. Femtosecond laser-assisted keratotomy. Curr. Opin. Ophthalmol. 2016, 27, 277–284. [Google Scholar] [CrossRef]
- Deshmukh, R.; Nair, S.; Vaddavalli, P.K.; Agrawal, T.; Rapuano, C.J.; Beltz, J.; Vajpayee, R.B. Post-penetrating keratoplasty astigmatism. Surv. Ophthalmol. 2021, 67, 1200–1228. [Google Scholar] [CrossRef]
- Anis, M.; Howaidy, A.; Azzam, S. Femtosecond laser-assisted arcuate keratotomy for correction of postkeratoplasty astigmatism. Delta J. Ophthalmol. 2021, 22, 111. [Google Scholar]
- Moussa, S.; Dietrich, M.; Lenzhofer, M.; Ruckhofer, J.; Reitsamer, H.A. Femtosecond laser in refractive corneal surgery. Photochem. Photobiol. Sci. 2019, 18, 1669–1674. [Google Scholar] [CrossRef]
- Mainguy, A.; Vabres, B.; Orignac, I. Vector analysis of astigmatism correction in femtosecond laser-assisted arcuate keratotomy for extreme astigmatism after penetrating keratoplasty. J. Fr. D’ophtalmologie 2022, 45, 640–646. [Google Scholar] [CrossRef]
- Yu, A.C.; Spena, R.; Pellegrini, M.; Bovone, C.; Busin, M. Deep Anterior Lamellar Keratoplasty: Current Status and Future Directions. Cornea 2022, 41, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Elkadim, M.; Myerscough, J.; Bovone, C.; Busin, M. A novel blunt dissection technique to treat modified deep anterior lamellar keratoplasty (DALK)-associated high astigmatism. Eye 2020, 34, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Gauvin, M.; Wallerstein, A. AstigMATIC: An automatic tool for standard astigmatism vector analysis. BMC Ophthalmol. 2018, 18, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.W., Jr.; Price, M.O.; Bowers, P.J.; Price, F.W., Jr. Long-term graft survival after penetrating keratoplasty. Ophthalmology 2003, 110, 1396–1402. [Google Scholar] [CrossRef]
- Seitz, B.; Hager, T.; Langenbucher, A.; Naumann, G.O. Reconsidering sequential double running suture removal after penetrating keratoplasty: A prospective randomized study comparing excimer laser and motor trephination. Cornea 2018, 37, 301–306. [Google Scholar] [CrossRef]
- Tourabaly, M.; Knoeri, J.; Georgeon, C.; Borderie, M.; Bouheraoua, N.; Borderie, V. Long-term results and refractive error after cataract surgery with a scleral incision in eyes with deep anterior lamellar keratoplasty. Cornea 2021, 40, 1466–1473. [Google Scholar] [CrossRef]
- Elkadim, M.; Myerscough, J.; Bovone, C.; Busin, M. Astigmatism orientation after deep anterior lamellar keratoplasty for keratoconus and its correlation with preoperative peripheral corneal astigmatism. Cornea 2020, 39, 192–195. [Google Scholar] [CrossRef]
- Davies, E.; Roberto Pineda, I. Corneal tomography changes and refractive outcomes after Descemet stripping without endothelial keratoplasty. Cornea 2019, 38, 817–819. [Google Scholar] [CrossRef]
- Agha, B.; Forster, R.; Kohnen, T.; Schmack, I. Influence of rebubbling on anterior segment parameters and refractive outcomes in eyes with DMEK for Fuchs endothelial dystrophy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 3175–3183. [Google Scholar] [CrossRef]
- Levinger, E.; Trivizki, O.; Levinger, S.; Kremer, I. Outcome of “mushroom” pattern femtosecond laser-assisted keratoplasty versus conventional penetrating keratoplasty in patients with keratoconus. Cornea 2014, 33, 481–485. [Google Scholar] [CrossRef]
- Javadi, M.A.; Feizi, S.; Rahmani, S.; Khajuee-Kermani, P. Refractive Stability After Deep Anterior Lamellar Keratoplasty for Keratoconus. Cornea 2018, 37, 1506–1510. [Google Scholar] [CrossRef] [PubMed]
- Tzelikis, P.F.; Jácome, A.H.G.; Rocha, G.A.N.; Hida, W.T.; de Souza, L.B. Clinical outcomes after femtosecond laser–assisted implantation of an intrastromal corneal ring segment with a 340-degree arc length in postkeratoplasty patients: 12-month follow-up. J. Cataract Refract. Surg. 2020, 46, 78–85. [Google Scholar] [PubMed]
- Janiszewska-Bil, D.; Czarnota-Nowakowska, B.; Krysik, K.; Lyssek-Boron, A.; Dobrowolski, D.; Grabarek, B.O.; Wylegala, E. Comparison of Long-Term Outcomes of the Lamellar and Penetrating Keratoplasty Approaches in Patients with Keratoconus. J. Clin. Med. 2021, 10, 2421. [Google Scholar] [CrossRef] [PubMed]
- Jafarinasab, M.-R.; Hadi, Y.; Espandar, G. Femtosecond Laser-assisted Allogenic Additive Stromal Keratoplasty With or Without Excimer Laser Donor Keratomileusis for Management of Keratoconus. J. Ophthalmic Vis. Res. 2021, 16, 691. [Google Scholar] [CrossRef] [PubMed]
- Poole, T.R.; Ficker, L.A. Astigmatic keratotomy for post-keratoplasty astigmatism. J. Cataract Refract. Surg. 2006, 32, 1175–1179. [Google Scholar] [CrossRef]
- Hashemian, M.N.; Ojaghi, H.; Mohammadpour, M.; Jabbarvand, M.; Rahimi, F.; Abtahi, M.A.; Mazloumi, M.; Abtahi, S.H. Femtosecond laser arcuate keratotomy for the correction of postkeratoplasty high astigmatism in keratoconus. J. Res. Med. Sci. 2017, 22, 17. [Google Scholar] [CrossRef]
- Fadlallah, A.; Mehanna, C.; Saragoussi, J.J.; Chelala, E.; Amari, B.; Legeais, J.M. Safety and efficacy of femtosecond laser-assisted arcuate keratotomy to treat irregular astigmatism after penetrating keratoplasty. J. Cataract Refract. Surg. 2015, 41, 1168–1175. [Google Scholar] [CrossRef]
- Nichamin, L.D. Astigmatism control. Ophthalmol. Clin. N. Am. 2006, 19, 485–493. [Google Scholar] [CrossRef]
- Hjortdal, J.O.; Ehlers, N. Paired arcuate keratotomy for congenital and post-keratoplasty astigmatism. Acta Ophthalmol. Scand. 1998, 76, 138–141. [Google Scholar] [CrossRef]
- Hovding, G. Transverse keratotomy in postkeratoplasty astigmatism. Acta Ophthalmol. 1994, 72, 464–468. [Google Scholar] [CrossRef]
- Buzzonetti, L.; Petrocelli, G.; Laborante, A.; Mazzilli, E.; Gaspari, M.; Valente, P. Arcuate keratotomy for high postoperative keratoplasty astigmatism performed with the intralase femtosecond laser. J. Refract. Surg. 2009, 25, 709–714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Böhringer, D.; Dineva, N.; Maier, P.; Birnbaum, F.; Kirschkamp, T.; Reinhard, T.; Eberwein, P. Long-term follow-up of astigmatic keratotomy for corneal astigmatism after penetrating keratoplasty. Acta Ophthalmol. 2016, 94, e607–e611. [Google Scholar] [CrossRef] [PubMed]
- Barequet, I.S.; Hirsh, A.; Levinger, S. Femtosecond thin-flap LASIK for the correction of ametropia after penetrating keratoplasty. J. Refract. Surg. 2010, 26, 191–196. [Google Scholar] [CrossRef]
- Wade, M.; Steinert, R.F.; Garg, S.; Farid, M.; Gaster, R. Results of toric intraocular lenses for post-penetrating keratoplasty astigmatism. Ophthalmology 2014, 121, 771–777. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, F.; Alio, J.L.; Martinez-Abad, A.; Izquierdo, L., Jr.; Larco, P., Jr.; Abdelghany, A.A. Astigmatic change as a predictor of intrastromal corneal ring segment late extrusion. J. Cataract Refract. Surg. 2022, 48, 401–407. [Google Scholar] [CrossRef]
- D’Oria, F.; Abdelghany, A.A.; Ledo, N.; Barraquer, R.I.; Alio, J.L. Incidence and Reasons for Intrastromal Corneal Ring Segment Explantation. Am. J. Ophthalmol. 2021, 222, 351–358. [Google Scholar] [CrossRef]
| Age | Spherical Refraction | Anterior Graft Diameter | |
|---|---|---|---|
| AVG | 25.5 | 1.5 | 8.107142857 |
| SD | 10.5 | 6.36 | 0.536523199 |
| Min | 15 | −3 | 7.25 |
| Max | 51 | 6 | 8.6 |
| Astigmatism | LogMAR UCVA | LogMAR BCVA | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| Pre-AK 1 | −9.59 (2.36) | −5.50 to −13.50 | 1.03 (0.29) | 0.52–1.40 | 0.36 (0.25) | 0.17–0.53 |
| Pre-AK 2 | −6.59 (1.99) | −2.00 to −13.00 | 0.96 (0.31) | 0.30–1.30 | 0.49 (0.31) | 0.22–0.70 |
| p-value | Pre-AK 1 Vs. Pre-AK 2 0.0043 | Pre-AK 1 Vs. Pre-AK 2 0.5905 | Pre-AK 1 Vs. Pre-AK 2 0.2827 | |||
| Post-AK 2 | −5.38 (1.79) | −3.00 to −9.00 | 1.00 (0.26) | 0.60–1.30 | 0.41 (0.41) | 0.12–0.53 |
| p-value | Pre-AK 2 Vs. Post-AK 2 0.1494 | Pre-AK 2 Vs. Post-AK 2 0.7464 | Pre-AK 2 Vs. Post-AK 2 0.6072 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levinger, N.; Levinger, S.; Erdinest, N.; Achiron, A.; London, N.; Trivizki, O.; Levinger, E.; Barequet, I.S. Repeated Femtosecond Laser-Assisted Astigmatic Keratotomies in Post-Keratoplasty Eyes. J. Clin. Med. 2022, 11, 4221. https://doi.org/10.3390/jcm11144221
Levinger N, Levinger S, Erdinest N, Achiron A, London N, Trivizki O, Levinger E, Barequet IS. Repeated Femtosecond Laser-Assisted Astigmatic Keratotomies in Post-Keratoplasty Eyes. Journal of Clinical Medicine. 2022; 11(14):4221. https://doi.org/10.3390/jcm11144221
Chicago/Turabian StyleLevinger, Nadav, Shmuel Levinger, Nir Erdinest, Asaf Achiron, Naomi London, Omer Trivizki, Eliya Levinger, and Irina S. Barequet. 2022. "Repeated Femtosecond Laser-Assisted Astigmatic Keratotomies in Post-Keratoplasty Eyes" Journal of Clinical Medicine 11, no. 14: 4221. https://doi.org/10.3390/jcm11144221
APA StyleLevinger, N., Levinger, S., Erdinest, N., Achiron, A., London, N., Trivizki, O., Levinger, E., & Barequet, I. S. (2022). Repeated Femtosecond Laser-Assisted Astigmatic Keratotomies in Post-Keratoplasty Eyes. Journal of Clinical Medicine, 11(14), 4221. https://doi.org/10.3390/jcm11144221







