Comprehensibility of Contraindications in German, UK and US Summaries of Product Characteristics/Prescribing Information—A Comparative Qualitative and Quantitative Analysis
Abstract
:1. Introduction
2. Materials and Methods
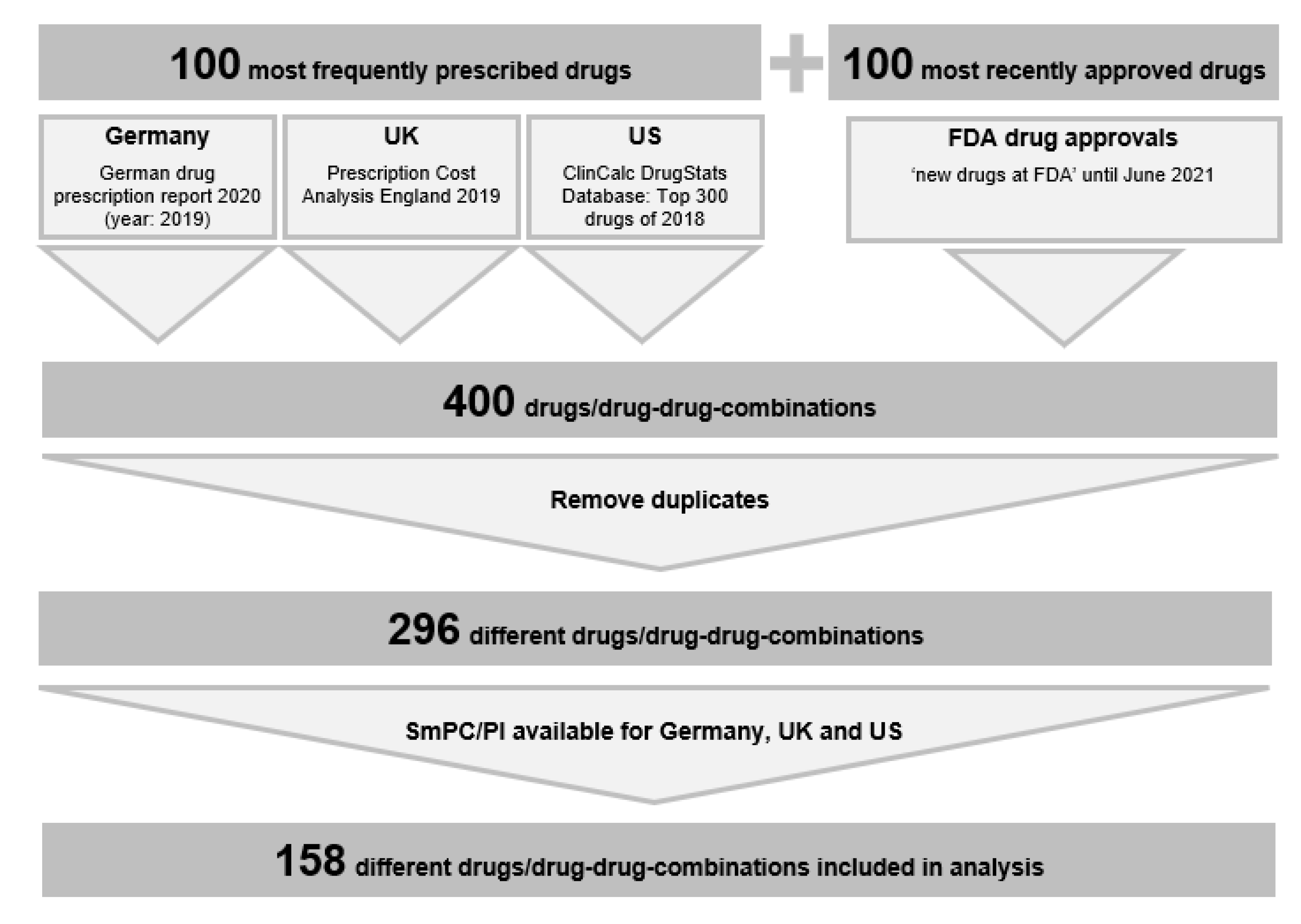
2.1. Drug Selection
2.2. SmPCs/PI
2.3. Extraction of Absolute CIs
2.4. Categorization of the Individual CI Statements
2.5. Analysis of CI Statements
2.6. Clarity
- The term covers a range of diagnoses of which only some can reasonably be assumed to pose a clinically relevant risk when present in a patient taking the drug (‘liver disease’, ‘vascular aneurism’).
- No clear cut off values or definitions are provided (‘impaired renal function’, ‘conditions with increased potassium losses’), or definitions are clear but cover a (too) broad range of conditions of which only some are specific or relevant only for the intended CI (‘vascular disease’).
- The term requires complex clinical judgements, with different prescribers likely coming to different conclusions for the same case because no objective commonly agreed criteria are provided (‘condition if considered a significant risk factor for major bleeding’).
2.7. Codability
2.8. Statistical Analysis
3. Results
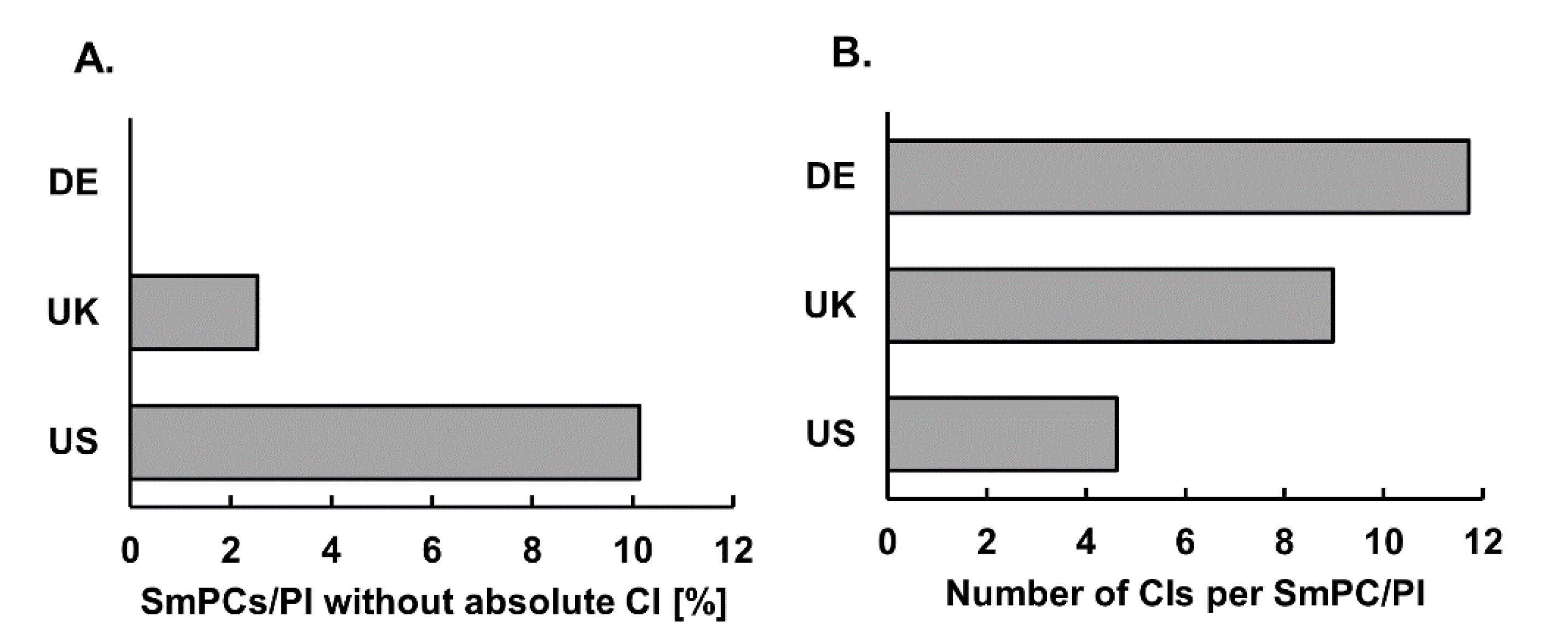
3.1. SmPCs/PI Not Listing any CI
3.2. Number of Individual Absolute CIs
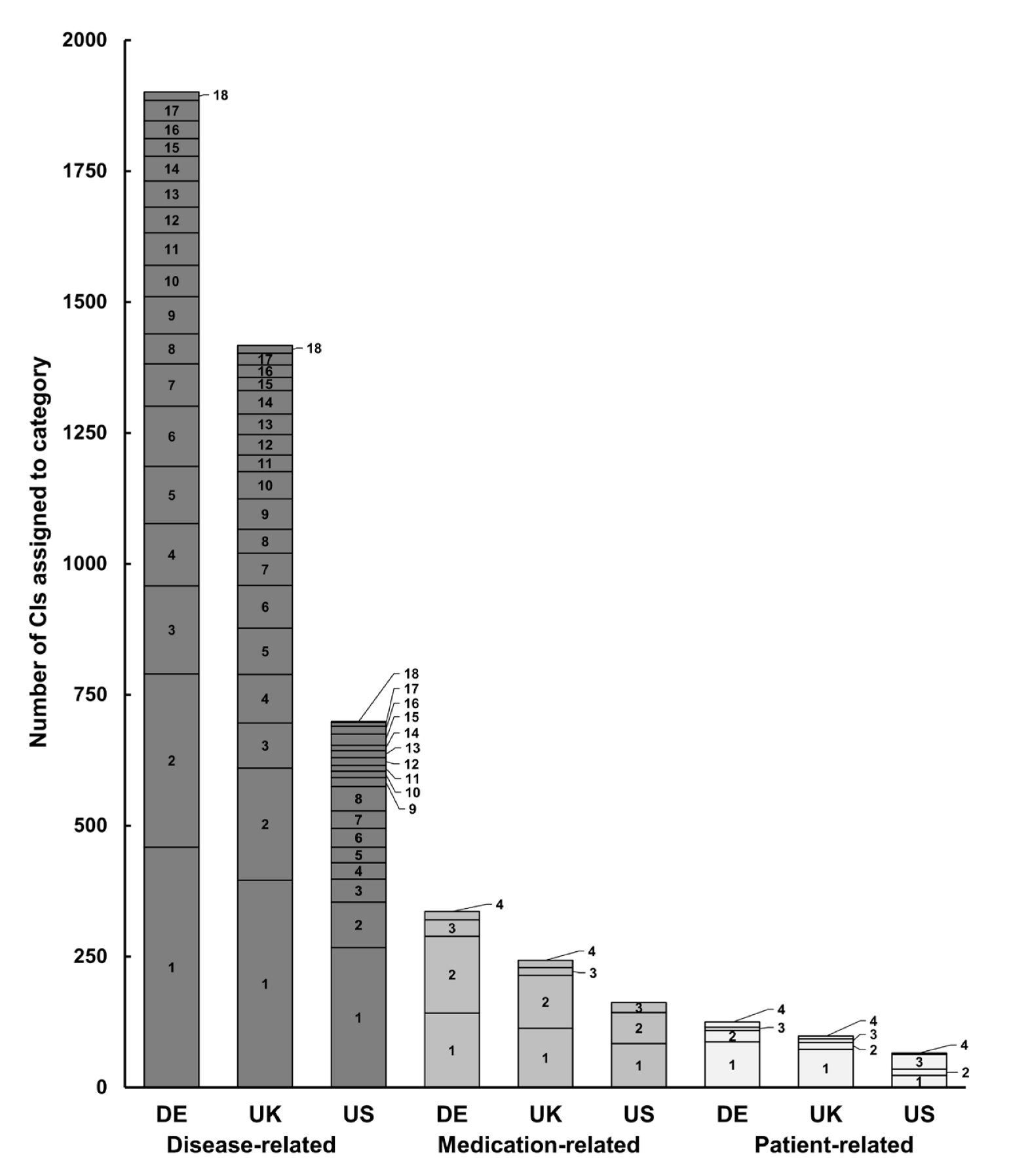
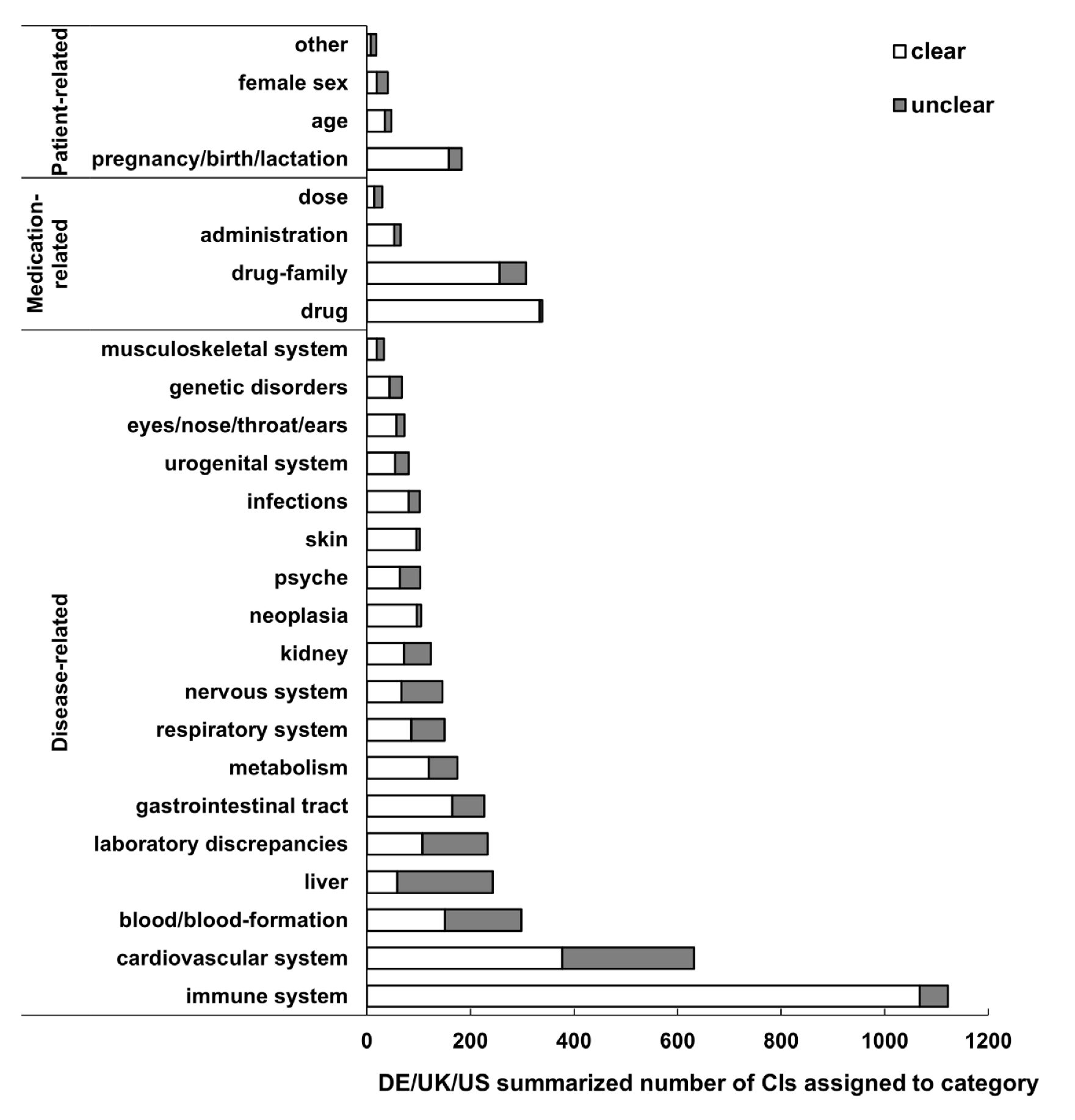
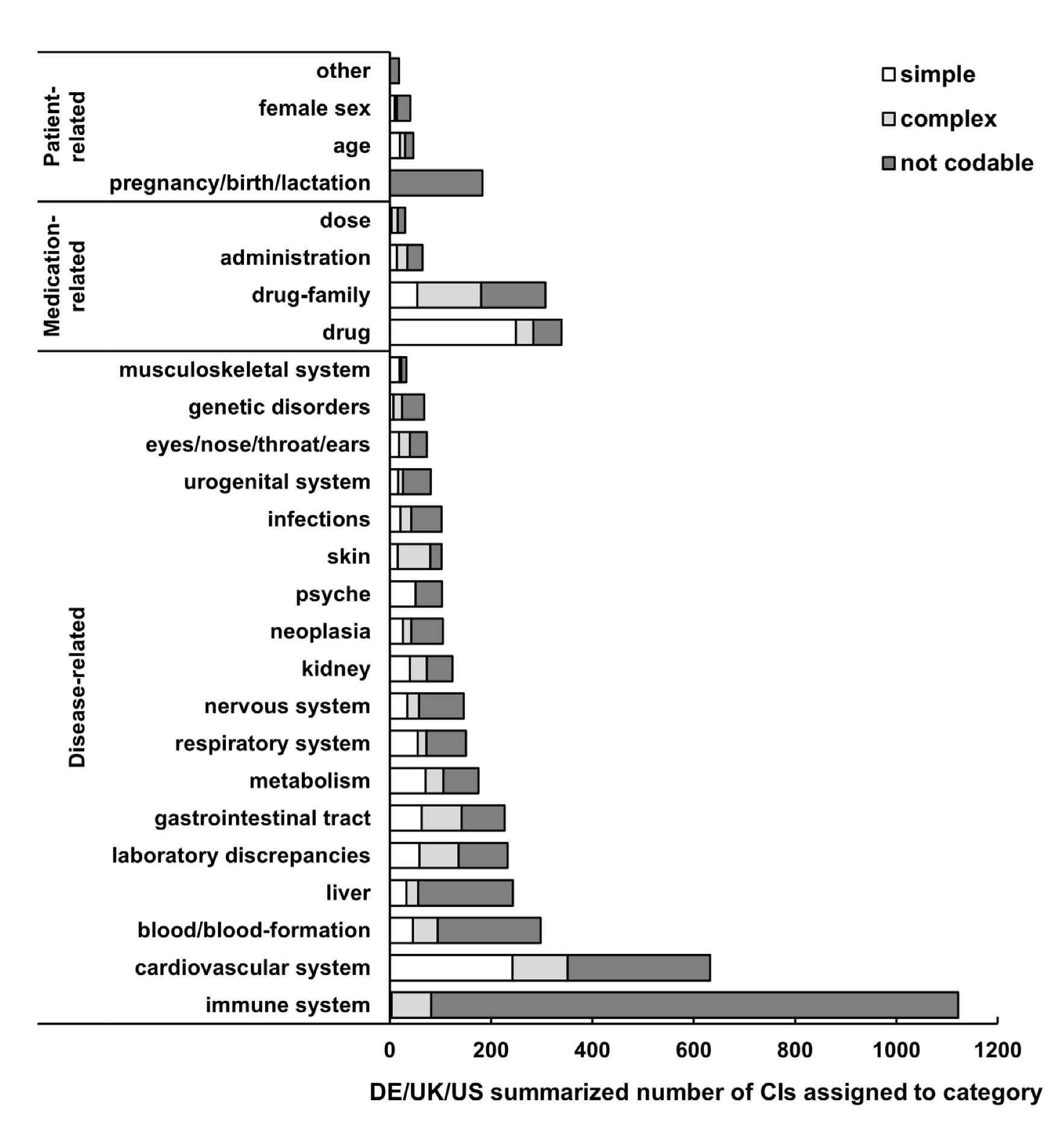
3.3. Categories of CI
3.4. Clarity
3.5. Expressions of CIs
3.6. Codability
3.7. Inter-Rater Reliability
3.8. ICD Codability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vromans, L.; Doyle, G.; Petak-Opel, S.; Rödiger, A.; Röttgermann, M.; Schlüssel, E.; Stetter, E. Shaping medicinal product information: A before and after study exploring physicians’ perspectives on the Summary of Product Characteristics. BMJ Open 2013, 3, e003033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gahr, M.; Freudenmann, R.W.; Connemann, B.J.; Schönfeldt-Lecuona, C.; Muche, R.; Hiemke, C.; Sillmann, Y.M. Nutzung von Fachinformationen bei Hausärzten und Apothekern im Zusammenhang der Aut-idem-Regelung: Ergebnisse einer explorativen Querschnittsbefragung [Use of Summaries of Product Characteristics among family doctors and pharmacists in the context of the aut-idem regulation: Results of an exploratory cross-sectional survey]. Z. Evid. Fortbild. Qual. Gesundhwes. 2020, 150, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.; Eichler, H.-G.; Bucsics, A.; Haefeli, W.E.; Gustafsson, L.L. e-SPC—Delivering drug information in the 21st century: Developing new approaches to deliver drug information to prescribers. Br. J. Clin. Pharmacol. 2012, 73, 12–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stürzlinger, H.; Hiebinger, C.; Pertl, D.; Traurig, P. Computerized Physician Order Entry—Wirksamkeit und Effizienz Elektronischer Arzneimittelverordnung mit Entscheidungsunterstützungssystemen [Effectiveness and Efficiency of Electronic Drug Prescription with Decision Support Systems]. 2009. Available online: https://portal.dimdi.de/de/hta/hta_berichte/hta228_bericht_de.pdf (accessed on 7 June 2022).
- U.S. Food and Drug Administration (FDA). Prescribing Information and Application Holder Responsibilities. Available online: https://www.fda.gov/drugs/laws-acts-and-rules/prescription-drug-labeling-resources#Prescribing%20Information%20Requirements%20and%20Rules (accessed on 7 June 2022).
- EMA. A Guideline on Summary of Product Characteristics (SmPC). 2009. Available online: https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-2/c/smpc_guideline_rev2_en.pdf (accessed on 7 June 2022).
- Pfistermeister, B.; Saß, A.; Criegee-Rieck, M.; Bürkle, T.; Fromm, M.F.; Maas, R. Inconsistencies and misleading information in officially approved prescribing information from three major drug markets. Clin. Pharmacol. Ther. 2014, 96, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Salgado, T.M.; Arguello, B.; Martinez-Martinez, F.; Benrimoj, S.I.; Fernandez-Llimos, F. Clinical relevance of information in the Summaries of Product Characteristics for dose adjustment in renal impairment. Eur. J. Clin. Pharmacol. 2013, 69, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.; Kälvemark, S.; Nilsson, G.; Nilsson, J.L.G. Patient information leaflets—Patients’ comprehension of information about interactions and contraindications. Pharm. World Sci. 2005, 27, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.J.; Lorenzo, S.; Pérez-Jover, V.; Navarro, I.; Martin de Rosales, A.M.; Lara, C. Assessment of the quality of medication information for patients in Spain. Expert Opin. Drug Saf. 2013, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kam, G.; Li, J.; Lee, S.; Lee, H.; Noh, Y.; Shin, J.-Y. Assessment of consistency of drug interaction information in drug labels among the United States, the United Kingdom, China, Japan, and Korea. Clin. Pharmacol. Ther. 2019, 105, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.L.; Kusama, M.; Ono, S. Exploring differences in drug doses between Japan and western countries. Clin. Pharmacol. Ther. 2010, 87, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Weersink, R.A.; Timmermans, L.; Monster-Simons, M.H.; Mol, P.G.M.; Metselaar, H.J.; Borgsteede, S.D.; Taxis, K. Evaluation of information in Summaries of Product Characteristics (SmPCs) on the use of a medicine in patients with hepatic impairment. Front. Pharmacol. 2019, 10, 1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisbach, L.; Schuster, A.K.; Hartmann, M.; Fromm, M.F.; Maas, R.; Farker, K. Inconsistencies and ambiguities in liver-disease-related contraindications—A systematic analysis of SmPCs/PI of major drug markets. J. Clin. Med. 2022, 11, 1933. [Google Scholar] [CrossRef] [PubMed]
- Rubrichi, S.; Quaglini, S.; Spengler, A.; Russo, P.; Gallinari, P. A system for the extraction and representation of Summary of Product Characteristics content. Artif. Intell. Med. 2013, 57, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.; Pamer, C.; Dang, O.; Brajovic, S.; Haider, S.; Botsis, T.; Milward, D.; Winter, A.; Lu, S.; Ball, R. Evaluation of Natural Language Processing (NLP) systems to annotate drug product labeling with MedDRA terminology. J. Biomed. Inform. 2018, 83, 73–86. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Guidance for Industry: Warnings and Precautions, Contraindications, and Boxed Warnings Sections of Labeling for Human Prescription Drug and Biological Products—Content and Format. 2011. Available online: https://www.fda.gov/media/71866/download (accessed on 7 June 2022).
- U.S. Food and Drug Administration (FDA). New Drugs at FDA: CDER’s New Molecular Entities and New Therapeutic Biological Products. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products (accessed on 9 August 2021).
- WiDO. PharMaAnalyst. Available online: https://arzneimittel.wido.de/PharMaAnalyst (accessed on 2 March 2021).
- Schwabe, U.; Ludwig, W.-D. Arzneiverordnungs-Report 2020; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Prescription Cost Analysis 2019. Available online: https://www.nhsbsa.nhs.uk/statistical-collections/prescription-cost-analysis-england/prescription-cost-analysis-england-2019 (accessed on 9 August 2021).
- Kane, S.P. ClinCalc DrugStats Database, Version 2021.10. Available online: https://clincalc.com/DrugStats/Top300Drugs.aspx (accessed on 8 February 2022).
- Fachinfo-Service®. Fachinformationsverzeichnis Deutschland. Available online: https://www.fachinfo.de/ (accessed on 12 August 2021).
- Gelbe Liste Pharmindex. Available online: https://www.gelbe-liste.de/ (accessed on 12 August 2021).
- EMC. Electronic Medicines Compendium. Available online: https://www.medicines.org.uk/emc/ (accessed on 23 August 2021).
- Drugs.com: FDA Professional Drug Information. Available online: https://www.drugs.com/pro (accessed on 23 August 2021).
- U.S. Food and Drug Administration (FDA). Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm (accessed on 23 August 2021).
- World Health Organization (WHO). ICD-10. International Statistical Classification of Diseases and Related Health Problems 10th Revision, Version 2019. Available online: https://icd.who.int/browse10/2019/en (accessed on 7 June 2022).
- Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM). ICD-10-GM German Modification (Version 2021). Available online: https://www.dimdi.de/static/de/klassifikationen/icd/icd-10-gm/kode-suche/htmlgm2021/ (accessed on 7 June 2022).
- Epitools Epidemiological Calculators. Ausvet. Available online: https://epitools.ausvet.com.au/ciproportion (accessed on 7 June 2022).
- Idostatistics. Cohen’s Kappa Free Calculator. Available online: https://idostatistics.com/cohen-kappa-free-calculator/#calculator (accessed on 7 June 2022).
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Barros, J.A.C. One more case of the double standard: Discrepancies between drug information provided to Brazilian and American physicians. Pharmacoepidemiol. Drug. Saf. 2000, 9, 281–287. [Google Scholar] [CrossRef]
- Yoon, D.; Song, I.; Noh, Y.; Li, J.; Shin, J.-Y. Consistency of listed indications and contraindications between the U.S.; the U.K.; Japan, and Korea on prescription drug labels. Regul. Toxicol. Pharmacol. 2018, 98, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, T.M.; Devadasu, V.R.; Rathnam, R.P. Comparison of the safety information on drug labels in three developed countries: The USA, UK and Canada. Saudi Pharm. J. 2017, 25, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- FDA Prescribing Information. Delestrogen (Estradiol). 2020. Available online: https://www.drugs.com/pro/delestrogen.html (accessed on 23 August 2021).
- FDA Prescribing Information. Nordette (Levonogestrel/Ethinylestradiol). 2020. Available online: https://www.drugs.com/pro/nordette.html (accessed on 23 August 2021).
- UK Summary of Product Characteristics. Aspirin. Available online: https://www.medicines.org.uk/emc/product/11864/smpc (accessed on 23 August 2021).
- UK Summary of Product Characteristics. Tenormin (Atenolol). Available online: https://www.medicines.org.uk/emc/product/3861/smpc (accessed on 23 August 2021).
- Popov, V.V.; Kudryavtseva, E.V.; Kumar Katiyar, N.; Shishkin, A.; Stepanov, S.I.; Goel, S. Industry 4.0 and digitalisation in healthcare. Materials 2022, 15, 2140. [Google Scholar] [CrossRef] [PubMed]
- Kostanjsek, N.F.I.; World Health Organization (WHO). ICD-11 Tooling and Implementation. Available online: https://cdn.who.int/media/docs/default-source/classification/icd-11-webinars/introduction-to-icd-11/icd-11-tooling-implementation.pdf?sfvrsn=4a326dea_5 (accessed on 7 June 2022).
- Fung, K.W.; Xu, J.; Bodenreider, O. The new International Classification of Diseases 11th edition: A comparative analysis with ICD-10 and ICD-10-CM. J. Am. Med. Inf. Assoc. 2020, 27, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Seidling, H.M.; Paterno, M.D.; Haefeli, W.E.; Bates, D.W. Coded entry versus free-text and alert overrides: What you get depends on how you ask. Int J. Med. Inform. 2010, 79, 792–796. [Google Scholar] [CrossRef] [PubMed]
- UpToDate®. Available online: https://www.uptodate.com/contents/search (accessed on 12 July 2022).
| Country | Drug (SmPC/PI) | Expression in SmPC/PI |
|---|---|---|
| DE/UK/US | Alendronic acid (Fosamax) | abnormalities to the esophagus which delay esophageal emptying |
| DE | Amisulpride (Solian) | drugs that can trigger serious cardiac arrhythmias |
| DE/UK | Amlodipine (Norvasc, ISTIN) | high grade aortic stenosis |
| DE/UK | Apixaban (Eliquis) | recent spinal surgery |
| DE/UK | Apixaban (Eliquis) | clinical situation if considered a significant risk factor for major bleeding |
| DE | Aspirin (Aspirin) | previous hypersensitivity after taking salicylates or substances having a similar effect, particularly NSAIDs |
| DE | Bisoprolol (Concor) | late stages of peripheral arterial occlusive disease |
| DE/UK | Bupropion (Elontril, Zyban) | patient undergoing abrupt discontinuation from any medicinal product known to be associated with risk of seizures on withdrawal |
| DE/UK | Buspiron (Anxut, generic) | intoxication with antipsychotic drugs |
| US | Carvedilol (Coreg) | severe bradycardia |
| DE | Chlorthalidone (Hygroton) | conditions with increased potassium losses |
| DE/UK/US | Clopidogrel (Plavix) | active pathological bleeding |
| DE | Codeine phosphate/paracetamol (Gelonida) | approaching birth |
| UK | Digoxin (Lanoxin) | AV bock second degree, especially if there is a history of Stokes-Adams attacks |
| US | Ethinylestradiol/norgestimate (generic) | in women: thrombogenic valvular disease |
| US | Ethinylestradiol/norgestimate (generic) | in women: uncontrolled hypertension |
| DE/UK/US | Fenofibrate (Lipidil, Supralip, Tricor) | unexplained (only US/UK) persistent liver function abnormalities |
| DE/UK | Finasteride (Proscar) | children |
| UK | Folic acid (generic) | malignant diseases unless megaloblastic anemia due to folic acid deficiency |
| DE/US | Levothyroxine sodium (Euthyrox, Unithroid) | untreated (only DE)/uncorrected (only UK) adrenal insufficiency |
| DE/UK | Lisdexamfetamine (Elvanse) | advanced arteriosclerosis |
| US | Metformin/sitagliptin (Janumet) | chronic diabetic ketoacidosis |
| DE | Methotrexate (Lantarel) | increased alcohol consumption |
| DE/UK | Methylphenidate (Ritalin) | potentially life-threatening arrhythmia |
| US | Metoprolol (Lopressor) | moderate cardiac failure |
| US | Morphine (Duramorph), Oxycodone (Roxicodone), Tramadol (Ultram) | significant respiratory depression |
| US | Opicapone (Ongentys) | catecholamine-secreting neoplasms |
| UK | Oxycodone (Lynlor) | elevated carbon dioxide levels in the blood |
| DE | Propranolol (Dociton) | higher grade sino-atrial block |
| UK | Metoprolol (generic) | |
| UK | Rosuvastatin (Crestor) | for 40 mg dosis: situations where an increase in plasma levels may occur |
| DE/UK | Telmisartan (Micardis) | biliary obstructive disorders |
| US | Trimethoprim/sulfamethoxazole (Bactrim) | marked hepatic damage |
| DE/UK/US | Valproic acid (Orfiril, Convulex, Depakene) | women of childbearing potential |
| US | Warfarin (Coumadin) | major regional anesthesia |
| US | Warfarin (Coumadin) | traumatic surgery resulting in large open surfaces |
| Codable Terms | Not Codable Terms | ||
|---|---|---|---|
| Expression in SmPC/PI | ICD-10 | ICD-10 Description | Expression in SmPC/PI |
| Breast cancer | C50 | Malignant neoplasm of breast | Estrogen-dependent tumor |
| Sex hormone dependent malignant tumors | |||
| Diseases of the hematopoietic system | D50-D77 | Diseases of the blood and blood-forming organs | Condition if considered a significant risk factor for major bleeding |
| Preexisting blood dyscrasias | |||
| Hemorrhagic diathesis | D69 | Purpura and other hemorrhagic conditions | Blood clotting disorders |
| Thrombophilic disorders | |||
| Sleep apnea syndrome | G47.3 | Sleep apnea | Complex sleep behaviour |
| Unusual sleep behaviour | |||
| Myasthenia gravis | G70.0 | Myasthenia gravis | Patients with predisposing factors for myopathy |
| Esophageal varices | I85 | Esophageal varices | Diseases of the esophagus which delay esophageal emptying |
| Asthma | J45 | Asthma | Reactive airway disease |
| Respiratory disease with bronchospastic component | |||
| Bronchial hyperreactivity | |||
| Crohn´s disease | K50 | Crohn disease (regional enteritis) | Severe intestinal inflammation associated with symptomatic stenosis |
| Paralytic ileus | K56.0 | Paralytic ileus | Delayed gastric emptying |
| Primary biliary cirrhosis | K74.3 | Primary biliary cirrhosis | Severe hepatic impairment |
| Hepatic diseases | |||
| Hepatic dysfunction | |||
| liver parenchymal damage | |||
| Hepatic diseases associated with coagulopathy and clinically relevant bleeding risk | |||
| Gout | M10 | Gout | Symptomatic hyperuricemia |
| Acute abdomen | R10.0 | Acute abdomen | Abdominal pain of unknown origin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Then, M.I.; Andrikyan, W.; Fromm, M.F.; Maas, R. Comprehensibility of Contraindications in German, UK and US Summaries of Product Characteristics/Prescribing Information—A Comparative Qualitative and Quantitative Analysis. J. Clin. Med. 2022, 11, 4167. https://doi.org/10.3390/jcm11144167
Then MI, Andrikyan W, Fromm MF, Maas R. Comprehensibility of Contraindications in German, UK and US Summaries of Product Characteristics/Prescribing Information—A Comparative Qualitative and Quantitative Analysis. Journal of Clinical Medicine. 2022; 11(14):4167. https://doi.org/10.3390/jcm11144167
Chicago/Turabian StyleThen, Melanie I., Wahram Andrikyan, Martin F. Fromm, and Renke Maas. 2022. "Comprehensibility of Contraindications in German, UK and US Summaries of Product Characteristics/Prescribing Information—A Comparative Qualitative and Quantitative Analysis" Journal of Clinical Medicine 11, no. 14: 4167. https://doi.org/10.3390/jcm11144167
APA StyleThen, M. I., Andrikyan, W., Fromm, M. F., & Maas, R. (2022). Comprehensibility of Contraindications in German, UK and US Summaries of Product Characteristics/Prescribing Information—A Comparative Qualitative and Quantitative Analysis. Journal of Clinical Medicine, 11(14), 4167. https://doi.org/10.3390/jcm11144167






