Prostate Cancer Brain Metastasis: Review of a Rare Complication with Limited Treatment Options and Poor Prognosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aim and Design
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction and Analysis
3. Results
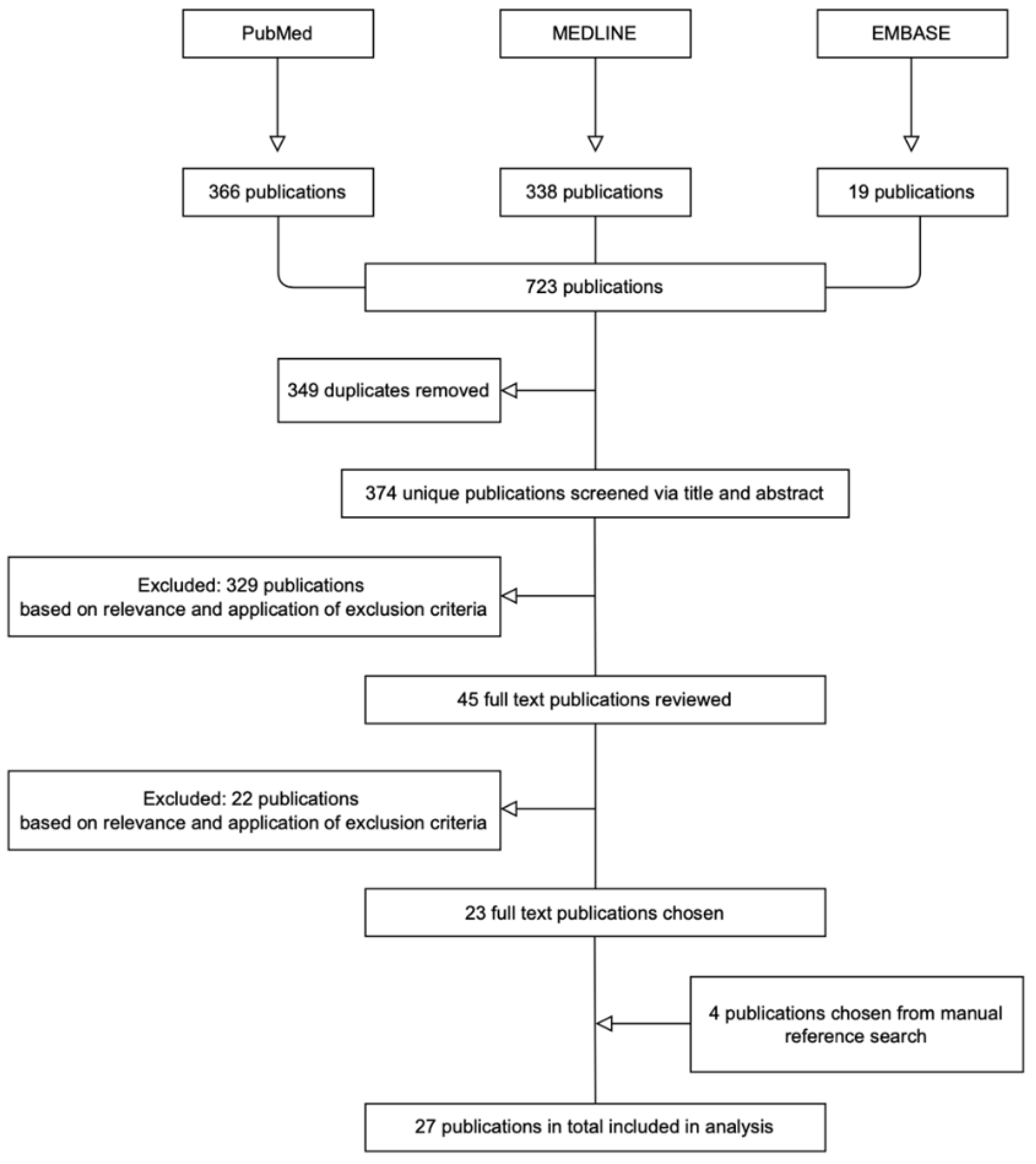
3.1. Literature Search
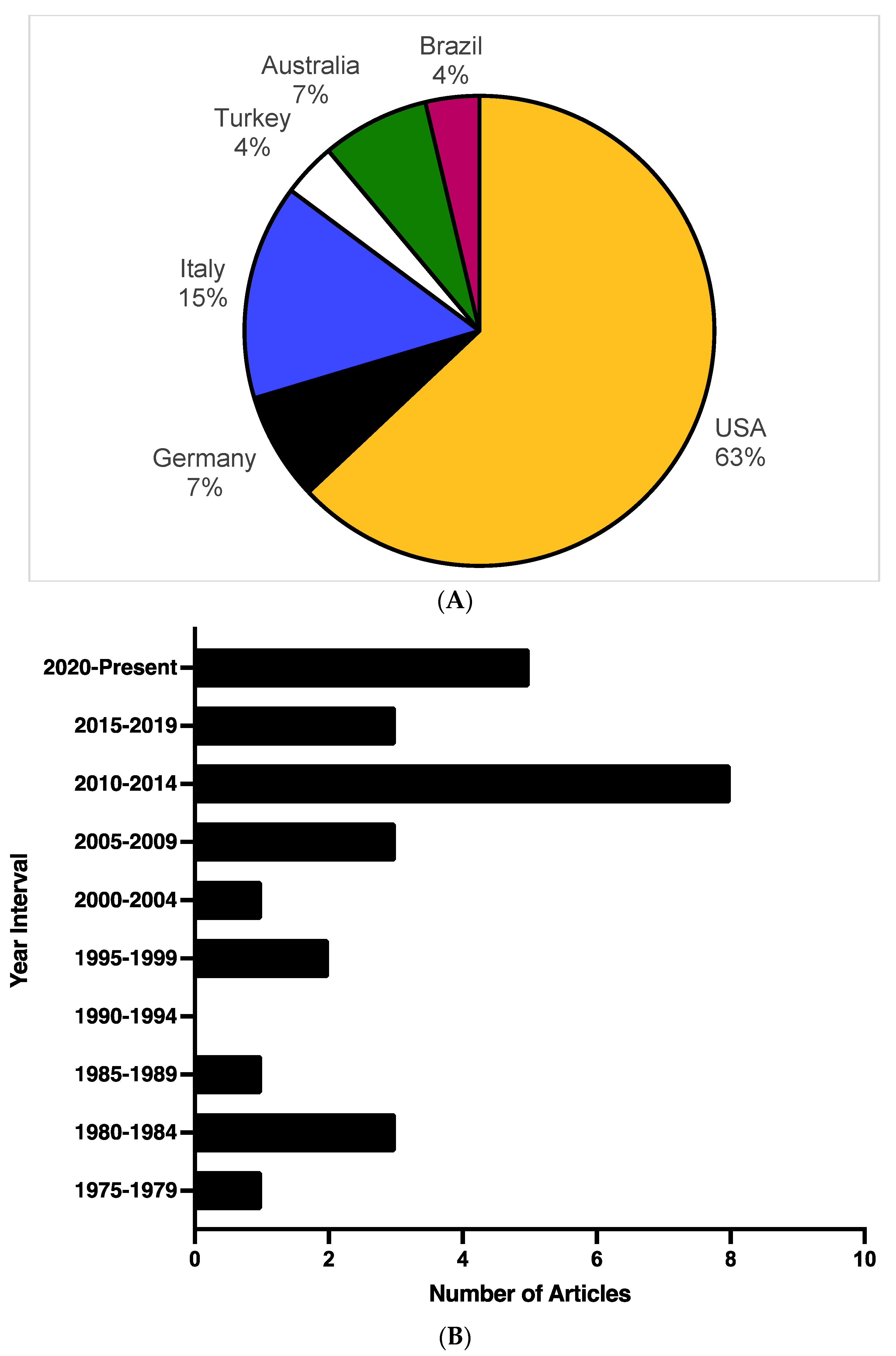
3.2. Study Characteristics
3.3. Disease Characteristics
3.4. Prior Treatment History and Prostate Cancer Brain Metastases Management
3.5. Outcome and Prognostic Factors
3.6. Patient and Disease Characteristics Associated with Brain Metastasis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA-Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef]
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.D.; et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 2014, 74, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Myint, Z.W.; Qasrawi, A.H. Prostate adenocarcinoma with brain metastasis: A surveillance, epidemiology, and end results database analysis 2010-2015. Med. Sci. Monitor. 2021, 27, e930064-1. [Google Scholar] [CrossRef] [PubMed]
- Boxley, P.J.; Smith, D.E.; Gao, D.; Kessler, E.R.; Echalier, B.; Bernard, B.; Ormond, D.R.; Lam, E.T.; Kavanagh, B.D.; Flaig, T.W. Prostate cancer central nervous system metastasis in a contemporary cohort. Clin. Genitourin Canc 2021, 19, 217–222.e1. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Rouah, E.; Ishida, Y. Brain metastasis: Clinical characteristics, pathological findings and molecular subtyping for therapeutic implications. Brain Tumor Pathol. 2016, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Caffo, O.; Veccia, A.; Fellin, G.; Mussari, S.; Russo, L.; Tomio, L.; Galligioni, E. Frequency of brain metastases from prostate cancer: An 18-year single-institution experience. J. Neuro-Oncol. 2013, 111, 163–167. [Google Scholar] [CrossRef]
- Benjamin, R. Neurologic complications of prostate cancer. Am. Fam. Phys. 2002, 65, 1834–1840. [Google Scholar]
- Salvati, M.; Frati, A.; Russo, N.; Ospedaliera, A.; Forlanini, S.C. Brain metastasis from prostate cancer. Report of 13 cases and critical analysis of the literature. Prostate 2005, 2, 10. [Google Scholar]
- Lin, C.; Turner, S.; Gurney, H.; Peduto, A. Increased detections of leptomeningeal presentations in men with hormone refractory prostate cancer: An effect of improved systemic therapy? J. Med. Imag. Radiat. 2008, 52, 376–381. [Google Scholar] [CrossRef]
- Bhambhvani, H.P.; Greenberg, D.R.; Srinivas, S.; Hayden Gephart, M. Prostate cancer brain metastases: A single-institution experience. World Neurosurg. 2020, 138, e445–e449. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Bartscht, T.; Schild, S.E.; Rades, D. Performance status is associated with survival in elderly patients irradiated for cerebral metastases from prostate cancer. Anticancer. Res. 2020, 40, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Kanyılmaz, G.; Aktan, M.; Yavuz, B.B.; Koç, M. Brain metastases from prostate cancer: A single-center experience. Turk. J. Urol. 2019, 45, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Tremont-Lukats, I.W.; Bobustuc, G.; Lagos, G.K.; Lolas, K.; Kyritsis, A.P.; Puduvalli, V.K. Brain metastasis from prostate carcinoma: The M.D. Anderson Cancer Center experience. Cancer 2003, 98, 363–368. [Google Scholar] [CrossRef]
- Steeg, P.S. The blood–tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 696–714. [Google Scholar] [CrossRef]
- Vrignaud, P.; Semiond, D.; Benning, V.; Beys, E.; Bouchard, H.; Gupta, S. Preclinical profile of cabazitaxel. Drug. Des. Dev. Ther. 2014, 8, 1851. [Google Scholar] [CrossRef]
- Markham, A.; Duggan, S. Darolutamide: First approval. Drugs 2019, 79, 1813–1818. [Google Scholar] [CrossRef]
- Scott, L.J. Enzalutamide: A review in castration-resistant prostate cancer. Drugs 2018, 78, 1913–1924. [Google Scholar] [CrossRef]
- Al-Salama, Z.T. Apalutamide: A review in non-metastatic castration-resistant prostate cancer. Drugs 2019, 79, 1591–1598. [Google Scholar] [CrossRef]
- Benoist, G.E.; Hendriks, R.J.; Mulders, P.F.A.; Gerritsen, W.R.; Somford, D.M.; Schalken, J.A.; van Oort, I.M.; Burger, D.M.; van Erp, N.P. Pharmacokinetic aspects of the two novel oral drugs used for metastatic castration-resistant prostate cancer: Abiraterone acetate and enzalutamide. Clin. Pharm. 2016, 55, 1369–1380. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Mikule, K.; Wang, Z.; Poon, G.; Vaidyanathan, A.; Smith, G.; Zhang, Z.Y.; Hanke, J.; Ramaswamy, S.; Wang, J. A Comparative Pharmacokinetic Study of PARP Inhibitors Demonstrates Favorable Properties for Niraparib Efficacy in Preclinical Tumor Models. Oncotarget 2018, 9, 37080–37096. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; van der Burg, E.; Nygren, A.O.H.; Zander, S.A.L.; Derksen, P.W.B.; de Bruin, M.; Zevenhoven, J.; Lau, A.; et al. High Sensitivity of BRCA1-Deficient Mammary Tumors to the PARP Inhibitor AZD2281 Alone and in Combination with Platinum Drugs. Proc. Natl. Acad. Sci. USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawlor, D.; Martin, P.; Busschots, S.; Thery, J.; O’Leary, J.J.; Hennessy, B.T.; Stordal, B. PARP Inhibitors as P-Glyoprotein Substrates. J. Pharm. Sci. 2014, 103, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Durmus, S.; Sparidans, R.W.; van Esch, A.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Breast Cancer Resistance Protein (BCRP/ABCG2) and P-Glycoprotein (P-GP/ABCB1) Restrict Oral Availability and Brain Accumulation of the PARP Inhibitor Rucaparib (AG-014699). Pharm. Res. 2015, 32, 37–46. [Google Scholar] [CrossRef]
- Parrish, K.E.; Cen, L.; Murray, J.; Calligaris, D.; Kizilbash, S.; Mittapalli, R.K.; Carlson, B.L.; Schroeder, M.A.; Sludden, J.; Boddy, A.V.; et al. Efficacy of PARP Inhibitor Rucaparib in Orthotopic Glioblastoma Xenografts Is Limited by Ineffective Drug Penetration into the Central Nervous System. Mol. Cancer Ther. 2015, 14, 2735–2743. [Google Scholar] [CrossRef] [Green Version]
- Kizilbash, S.H.; Gupta, S.K.; Chang, K.; Kawashima, R.; Parrish, K.E.; Carlson, B.L.; Bakken, K.K.; Mladek, A.C.; Schroeder, M.A.; Decker, P.A.; et al. Restricted Delivery of Talazoparib Across the Blood-Brain Barrier Limits the Sensitizing Effects of PARP Inhibition on Temozolomide Therapy in Glioblastoma. Mol. Cancer. Ther. 2017, 16, 2735–2746. [Google Scholar] [CrossRef] [Green Version]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Fendler, W.P.; Reinhardt, S.; Ilhan, H.; Delker, A.; Böning, G.; Gildehaus, F.J.; Stief, C.; Bartenstein, P.; Gratzke, C.; Lehner, S.; et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget 2017, 8, 3581. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Schlenkhoff, C.; Schwarz, B.; Essler, M.; Ahmadzadehfar, H. Combination of 177Lu-PSMA-617 and external radiotherapy for the treatment of cerebral metastases in patients with castration-resistant metastatic prostate cancer. Clin. Nucl. Med. 2017, 42, 704–706. [Google Scholar] [CrossRef]
- Bartscht, T.; Rades, D. Predicting survival after whole-brain irradiation for cerebral metastases from prostate cancer. Anticancer Res. 2014, 34, 4357–4360. [Google Scholar]
- Flannery, T.; Kano, H.; Niranjan, A.; Monaco, E.A.; Flickinger, J.C.; Lunsford, L.D.; Kondziolka, D. Stereotactic radiosurgery as a therapeutic strategy for intracranial metastatic prostate carcinoma. J. Neuro-Oncol. 2010, 96, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chao, S.T.; Toms, S.A.; Vogelbaum, M.A.; Barnett, G.H.; Suh, J.H.; Weil, R.J. Stereotactic radiosurgical treatment of parenchymal brain metastases from prostate adenocarcinoma. Surg. Neurol. 2008, 69, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.G.; Lefkowitz, M.; Skoog, S.J.; Miles, B.J.; Mcleod, D.G.; Coggin, J.T. Intracranial metastases in prostate cancer. Cancer 1984, 53, 2728–2730. [Google Scholar] [CrossRef]
- McCutcheon, I.E.; Eng, D.Y.; Logothetis, C.J. Brain metastasis from prostate carcinoma antemortem recognition and outcome after treatment. Cancer 1999, 86, 2301–2311. [Google Scholar] [CrossRef]
- Sarma, D.P.; Godeau, L. Brain metastasis from prostatic cancer. J. Surg. Oncol. 1983, 23, 173–174. [Google Scholar] [CrossRef]
- Nussbaum, E.S.; Djalilian, H.R.; Cho, K.H.; Hall, W.A. Brain metastases histology, multiplicity, surgery, and survival. Cancer 1996, 78, 1781–1788. [Google Scholar] [CrossRef]
- Guedes, B.D.V.S.; da Rocha, A.J.; Gama, H.P.P.; da Silva, C.J. Dural metastases from prostate carcinoma: A systematic review of the literature apropos of six patients. Eur.J. Radiol. 2011, 80, 236–240. [Google Scholar] [CrossRef]
- Hatzoglou, V.; Patel, G.V.; Morris, M.J.; Curtis, K.; Zhang, Z.; Shi, W.; Huse, J.; Rosenblum, M.; Holodny, A.I.; Young, R.J. Brain metastases from prostate cancer: An 11-year analysis in the MRI era with emphasis on imaging characteristics, incidence, and prognosis. J. Neuroimag. 2014, 24, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Gzell, C.E.; Kench, J.G.; Stockler, M.R.; Hruby, G. Biopsy-proven brain metastases from prostate cancer: A series of four cases with review of the literature. Int. Urol. Nephrol. 2013, 45, 735–742. [Google Scholar] [CrossRef]
- Ganau, M.; Gallinaro, P.; Cebula, H.; Scibilia, A.; Todeschi, J.; Gubian, A.; Nannavecchia, B.; Signorelli, F.; Pop, R.; Coca, H.A.; et al. Intracranial metastases from prostate carcinoma: Classification, management, and prognostication. World Neurosurg. 2020, 134, e559–e565. [Google Scholar] [CrossRef]
- Catane, R.; Kaufman, J.; West, C.; Merrin, C.; Tsukada, Y.; Murphy, G.P. Brain metastasis from prostatic carcinoma. Cancer 1976, 38, 2583–2587. [Google Scholar] [CrossRef]
- Chung, T.; Thannikkary, C. Carcinoma of the prostate with brain metastasis. J. Surg. Oncol. 1986, 33, 103–105. [Google Scholar] [CrossRef]
- Castaldo, J.E.; Bernat, J.L.; Meier, F.A.; Schned, A.R. Intracranial metastases due to prostatic carcinoma. Cancer 1983, 52, 1739–1747. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, J.; Chen, M.; Chen, D.; Ye, S.; Li, X.; Chen, X.; Ren, G.; Yan, S. Sites of synchronous distant metastases and prognosis in prostate cancer patients with bone metastases at initial diagnosis: A population-based study of 16,643 patients. Clin. Translational Med. 2019, 8, 30. [Google Scholar] [CrossRef]
- Lawton, A.; Sudakoff, G.; Dezelan, L.C.; Davis, N. Presentation, treatment, and outcomes of dural metastases in men with metastatic castrate-resistant prostate cancer: A case series. J. Palliat. Med. 2010, 13, 1125–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormond, D.R.; Kleinschmidt-DeMasters, B.K.; Cavalcante, D.; Smith, E.E.; Cramer, S.D.; Lucia, M.S. Prostatic adenocarcinoma CNS parenchymal and dural metastases: Alterations in ERG, CHD1 and MAP3K7 expression. J. Neuro-Oncol. 2019, 142, 319–325. [Google Scholar] [CrossRef]
- Caffo, O.; Gernone, A.; Ortega, C.; Sava, T.; Cartenì, G.; Facchini, G.; Re, G.L.; Amadio, P.; Bortolus, R.; Pagliarulo, V.; et al. Central nervous system metastases from castration-resistant prostate cancer in the docetaxel era. J. Neuro-Oncol 2012, 107, 191–196. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the prostate cancer clinical trials working group 3. J. Clin. Oncol. 2016, 34, 1402. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, R.S.; Turner, B.E.; Asher, A.L.; Marcrom, S.R.; Fiveash, J.B.; Foreman, P.M.; Press, R.H.; Patel, K.R.; Curran, W.J.; Breen, W.G.; et al. A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro-Oncol. 2019, 21, 1049–1059. [Google Scholar] [CrossRef]
- Cagney, D.N.; Lamba, N.; Sinha, S.; Catalano, P.J.; Bi, W.L.; Alexander, B.M.; Aizer, A.A. Association of neurosurgical resection with development of pachymeningeal seeding in patients with brain metastases. JAMA Oncol. 2019, 5, 703–709. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Risbridger, G.P.; Clark, A.K.; Porter, L.H.; Toivanen, R.; Bakshi, A.; Lister, N.L.; Pook, D.; Pezaro, C.J.; Sandhu, S.; Keerthikumar, S.; et al. The MURAL collection of prostate cancer patient-derived xenografts enables discovery through preclinical models of uro-oncology. Nat. Commun. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.M.S.C.; Teo, M.Y.; Knezevic, A.; Bambury, R.M.; Hatzoglou, V.; Autio, K.A.; Abida, W.; Gopalan, A.; Fine, S.; Danila, D.C.; et al. Clinicopathologic and genomic characterization of parenchymal brain metastases (BM) in prostate cancer (PCa). J. Clin. Oncol. 2019, 37, 227. [Google Scholar] [CrossRef]
- Nguyen, B.; Fong, C.; Luthra, A.; Smith, S.A.; DiNatale, R.G.; Nandakumar, S.; Walch, H.; Chatila, W.K.; Madupuri, R.; Kundra, R.; et al. Genomic Characterization of Metastatic Patterns from Prospective Clinical Sequencing of 25,000 Patients. Cell 2022, 185, 563–575.e11. [Google Scholar] [CrossRef]
- Suh, J.H.; Kotecha, R.; Chao, S.T.; Ahluwalia, M.S.; Sahgal, A.; Chang, E.L. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 2020, 17, 279–299. [Google Scholar] [CrossRef]
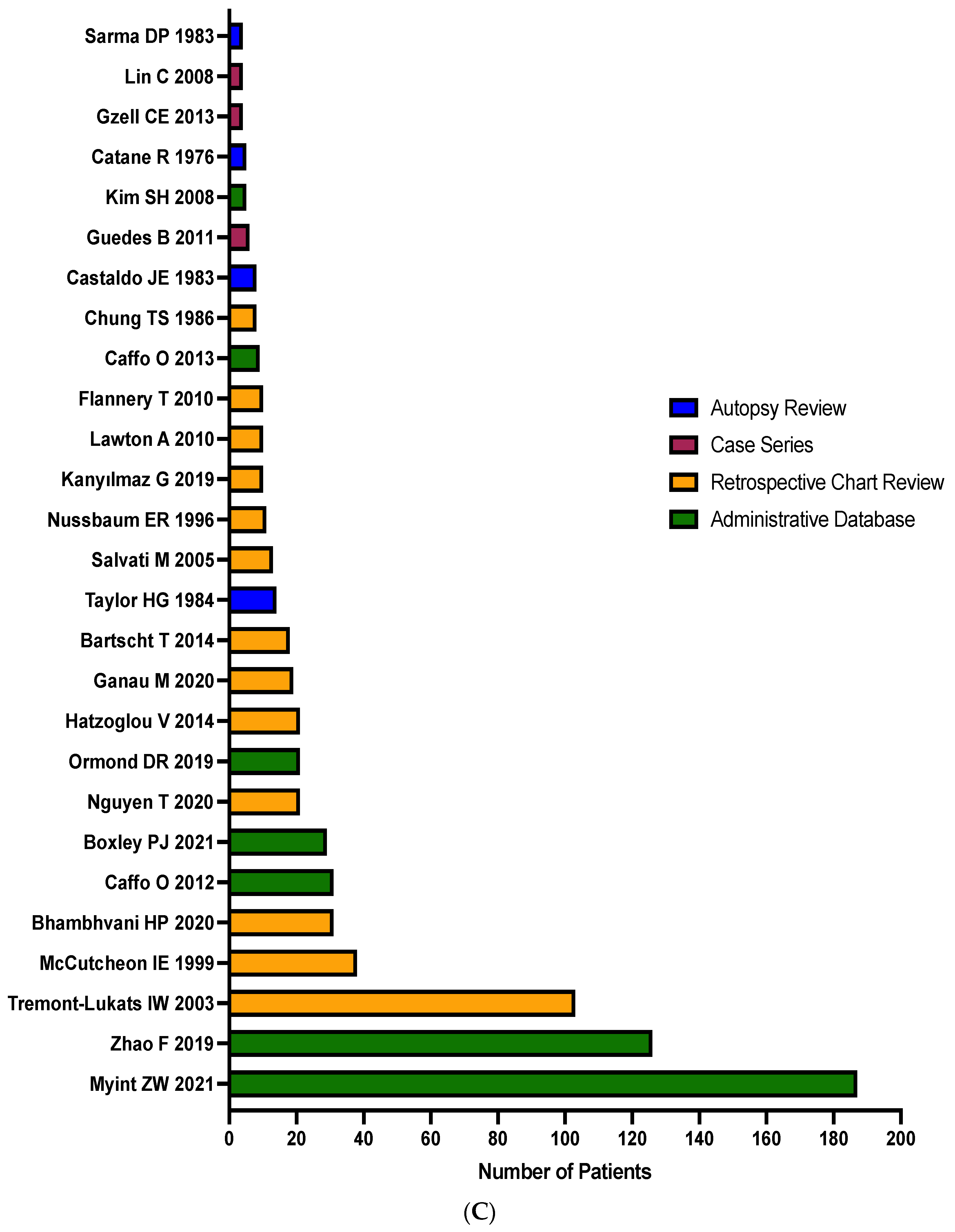
| Reference | N = Total Number of Patients | Patient Selection Method | % Incidence Rate of Brain Metastases | Median Time between Diagnoses in Months (range) a | Prostate Cancer Type b | ||
|---|---|---|---|---|---|---|---|
| CSPC | CRPC | Not Specified | |||||
| Myint ZW 2021 [4] | 187 | AD | 1.26 | 187 | |||
| Boxley PJ 2021 [5] | 29 | AD | 0.44 | 4 | 25 | ||
| Bhambhvani HP 2020 [11] | 31 | RC | 81 (3–195) | 31 | |||
| Nguyen T 2020 [12] | 21 | RC | 21 | ||||
| Ganau M 2020 [40] | 19 | RC | 10 | 9 | |||
| Ormond DR 2019 [46] | 21 | AD | 90 (0–312) | 1 | 20 | ||
| Zhao F 2019 [44] | 126 | AD | 0.76 | 126 | |||
| Kanyılmaz G 2019 [13] | 10 | RC | 10 | ||||
| Hatzoglou V 2014 [38] | 21 | RC | 0.16 | 46 | 21 | ||
| Bartscht T 2014 [30] | 18 | RC | 18 | ||||
| Gzell CE 2013 [39] | 4 | CS | 1 | 3 | |||
| Caffo O 2013 [7] | 9 | AD | 36 (0–111) | 9 | |||
| Caffo O 2012 [47] | 31 | AD | 3.29 | 44 (6–173) | 31 | ||
| Guedes B 2011 [37] | 6 | CS | 3 | 3 | |||
| Lawton A 2010 [45] | 10 | RC | 8.06 | 40 (21–164) | 10 | ||
| Flannery T 2010 [31] | 10 | RC | 1.01 | 36 (12–180) | 1 | 9 | |
| Lin C 2008 [10] | 4 | CS | 4 | ||||
| Kim SH 2008 [32] | 5 | AD | 82 | 5 | |||
| Salvati M 2005 [9] | 13 | RC | 45 (mean) | 13 | |||
| Tremont-Lukats IW 2003 [14] | 103 | RC | 0.63 | 103 | |||
| McCutcheon IE 1999 [34] | 38 | RC | 0.48 | 29 (0–84) | 38 | ||
| Nussbaum ER 1996 [36] | 11 | RC | 22 | 11 | |||
| Chung TS 1986 [42] | 8 | RC | 0.61 | 6 (0–73) | 2 | 6 | |
| Taylor HG 1984 [33] | 14 | AR | 4.13 | 5 | 9 | ||
| Castaldo JE 1983 [43] | 8 | AR | 4.23 | 8 | |||
| Sarma DP 1983 [35] | 4 | AR | 3.31 | 3 | 1 | ||
| Catane R 1976 [41] | 5 | AR | 4.40 | 61 (mean) | 5 | ||
| Overall Median | 13 | N/A | 1.14 | 42 | N/A | N/A | N/A |
| Reference | % Parenchymal Metastasis | % Dural Metastasis | % Leptomeningeal Metastasis | % Bone Metastasis | % Nodal Metastasis | % Liver Metastasis | % Lung Metastasis |
|---|---|---|---|---|---|---|---|
| Myint ZW 2021 [4] | 87 | 13 | 29 | ||||
| Boxley PJ 2021 [5] | 31 | 69 | |||||
| Bhambhvani HP 2020 [11] | 100 | 100 | 35 | 48 | |||
| Nguyen T 2020 [12] | 100 | ||||||
| Ganau M 2020 [40] | 29 | 71 | |||||
| Ormond DR 2019 [46] | 24 | 76 | 100 | ||||
| Zhao F 2019 [44] | 100 | ||||||
| Kanyılmaz G 2019 [13] | 80 | 40 | 90 | 50 | 30 | 30 | |
| Hatzoglou V 2014 [38] | 100 | 95 | 86 | ||||
| Bartscht T 2014 [30] | 100 | 50 | |||||
| Gzell CE 2013 [39] | 100 | 50 | 25 | 25 | |||
| Caffo O 2013 [7] | 100 | ||||||
| Caffo O 2012 [47] | 71 | 29 | |||||
| Guedes B 2011 [37] | 100 | ||||||
| Lawton A 2010 [45] | 100 | 100 | 40 | 10 | 10 | ||
| Flannery T 2010 [31] | 60 | 90 | 60 | 30 | |||
| Lin C 2008 [10] | 100 | 100 | |||||
| Kim SH 2008 [32] | 100 | 60 | |||||
| Salvati M 2005 [9] | 100 | 92 | 23 | 38 | |||
| Tremont-Lukats IW 2003 [14] | 100 | 95 | |||||
| McCutcheon IE 1999 [34] | 100 | 58 | 21 | 18 | 32 | ||
| Nussbaum ER 1996 [36] | 100 | ||||||
| Chung TS 1986 [42] | 100 | ||||||
| Taylor HG 1984 [33] | 43 | 86 | 7 | 100 | 71 | 36 | |
| Castaldo JE 1983 [43] | 75 | 63 | 13 | 100 | 60 | 50 | |
| Sarma DP 1983 [35] | 100 | 67 | 67 | 33 | |||
| Catane R 1976 [41] | 100 | 40 | 100 | 100 | 100 | ||
| Overall Median | N/A | N/A | N/A | 95 | 40 | 28 | 34 |
| Reference | Median Survival in Months (range) a | Surgery (%) | Radiation (%) | Surgery and Radiation (%) | Supportive Care (%) | Other (%) | Type of Radiation Therapy |
|---|---|---|---|---|---|---|---|
| Myint ZW 2021 [4] | 12 | 19 | 13 | ||||
| Boxley PJ 2021 [5] | |||||||
| Bhambhvani HP 2020 [11] | 3 (0.4–25) | 42 | 26 | 32 | SRS | ||
| Nguyen T 2020 [12] | 2 | 100 | WBRT | ||||
| Ganau M 2020 [40] | 100 | WBRT, SRS | |||||
| Ormond DR 2019 [46] | |||||||
| Zhao F 2019 [44] | 10 | ||||||
| Kanyılmaz G 2019 [13] | 4.5 (2–21) | 100 | WBRT | ||||
| Hatzoglou V 2014 [38] | 2.8 | ||||||
| Bartscht T 2014 [30] | 100 | WBRT | |||||
| Gzell CE 2013 [39] | 3.5 (2–24+) | 25 | 75 | WBRT | |||
| Caffo O 2013 [7] | 2 (0.25–35.4) | ||||||
| Caffo O 2012 [47] | 4 | 3 | 32 | 16 | 48 | 35 | WBRT, SRS |
| Guedes B 2011 [37] | |||||||
| Lawton A 2010 [45] | 6.17 (<1–15) | 70 | 20 | 30 | |||
| Flannery T 2010 [31] | 13 | 100 | SRS | ||||
| Lin C 2008 [10] | 75 | 25 | WBRT | ||||
| Kim SH 2008 [32] | 7 (6–22+) | 80 | 20 | WBRT, SRS | |||
| Salvati M 2005 [9] | 13 | 23 | 77 | 8 | WBRT, SRS | ||
| Tremont-Lukats IW 2003 [14] | 1 | 24 | 76 | SRS | |||
| McCutcheon IE 1999 [34] | 4 | 3 | 76 | 21 | WBRT | ||
| Nussbaum ER 1996 [36] | 13 | ||||||
| Chung TS 1986 [42] | 7.6 (mean) | 71 | 29 | WBRT | |||
| Taylor HG 1984 [33] | |||||||
| Castaldo JE 1983 [43] | 7 | 50 | 50 | WBRT | |||
| Sarma DP 1983 [35] | 25 | ||||||
| Catane R 1976 [41] | 40 | WBRT | |||||
| # Studies Reporting | 4.5 (median) | 5 | 15 | 7 | 7 | 4 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajeswaran, K.; Muzio, K.; Briones, J.; Lim-Fat, M.J.; Tseng, C.-L.; Smoragiewicz, M.; Detsky, J.; Emmenegger, U. Prostate Cancer Brain Metastasis: Review of a Rare Complication with Limited Treatment Options and Poor Prognosis. J. Clin. Med. 2022, 11, 4165. https://doi.org/10.3390/jcm11144165
Rajeswaran K, Muzio K, Briones J, Lim-Fat MJ, Tseng C-L, Smoragiewicz M, Detsky J, Emmenegger U. Prostate Cancer Brain Metastasis: Review of a Rare Complication with Limited Treatment Options and Poor Prognosis. Journal of Clinical Medicine. 2022; 11(14):4165. https://doi.org/10.3390/jcm11144165
Chicago/Turabian StyleRajeswaran, Kobisha, Kaitlin Muzio, Juan Briones, Mary Jane Lim-Fat, Chia-Lin Tseng, Martin Smoragiewicz, Jay Detsky, and Urban Emmenegger. 2022. "Prostate Cancer Brain Metastasis: Review of a Rare Complication with Limited Treatment Options and Poor Prognosis" Journal of Clinical Medicine 11, no. 14: 4165. https://doi.org/10.3390/jcm11144165
APA StyleRajeswaran, K., Muzio, K., Briones, J., Lim-Fat, M. J., Tseng, C.-L., Smoragiewicz, M., Detsky, J., & Emmenegger, U. (2022). Prostate Cancer Brain Metastasis: Review of a Rare Complication with Limited Treatment Options and Poor Prognosis. Journal of Clinical Medicine, 11(14), 4165. https://doi.org/10.3390/jcm11144165







