Development and Internal Validation of a New Prognostic Model Powered to Predict 28-Day All-Cause Mortality in ICU COVID-19 Patients—The COVID-SOFA Score
Abstract
1. Introduction
2. Materials and Methods
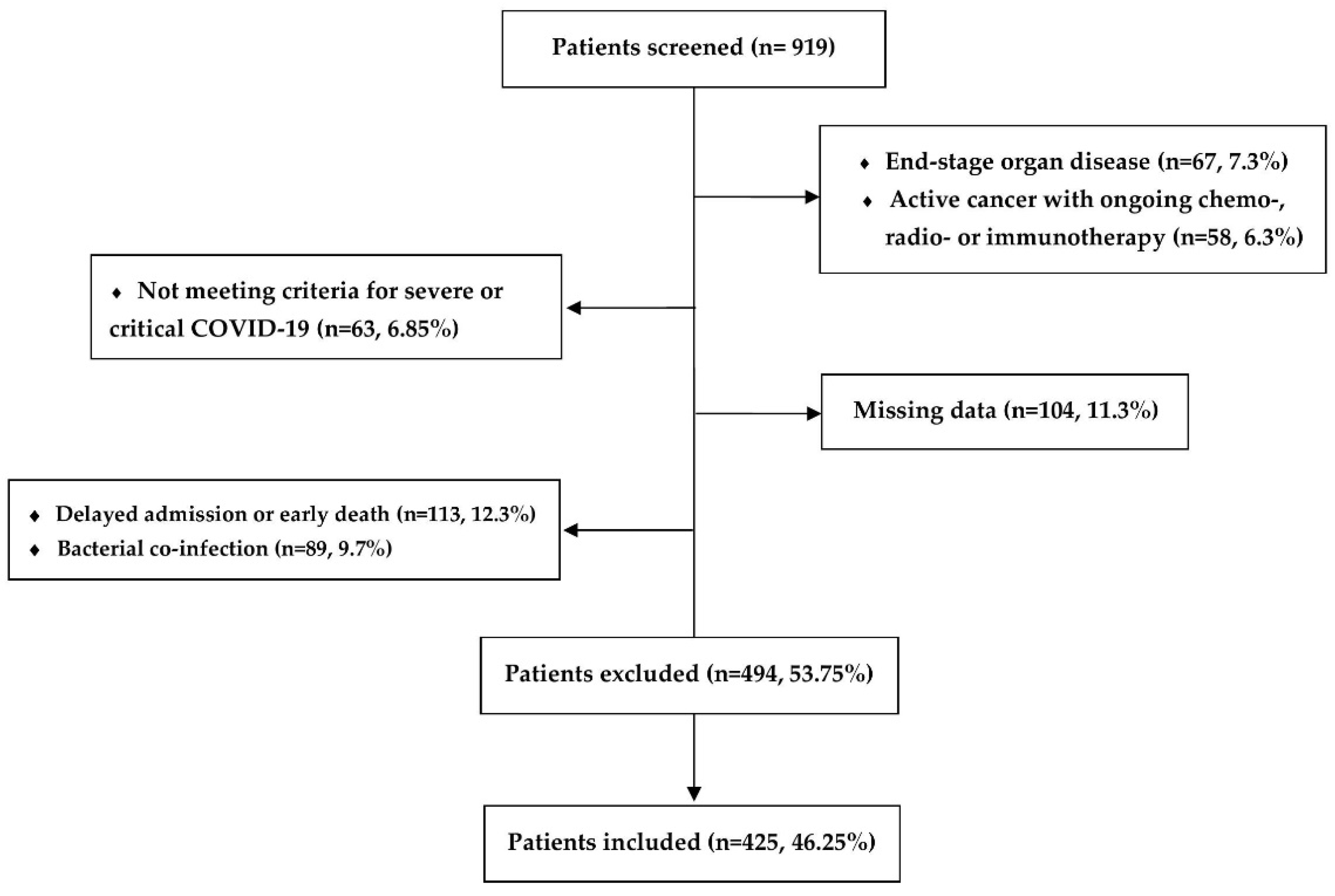
2.1. Study Population
2.2. Data Collection and Study End-Points
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population at ICU Admission
3.1.1. Building the Predictive Model and the COVID-SOFA Score Equation
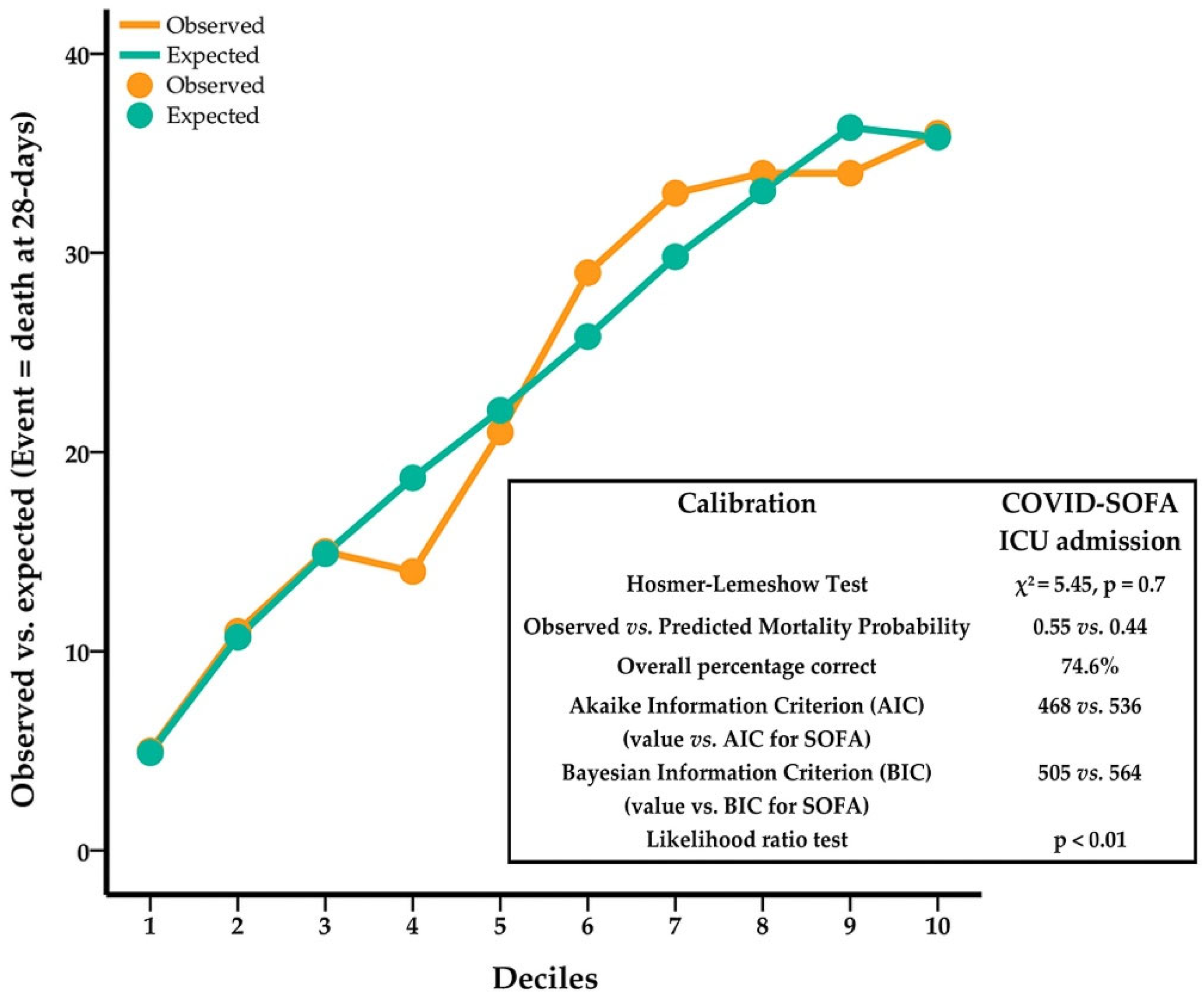
3.1.2. COVID-SOFA Score’s Goodness-Of-Fit
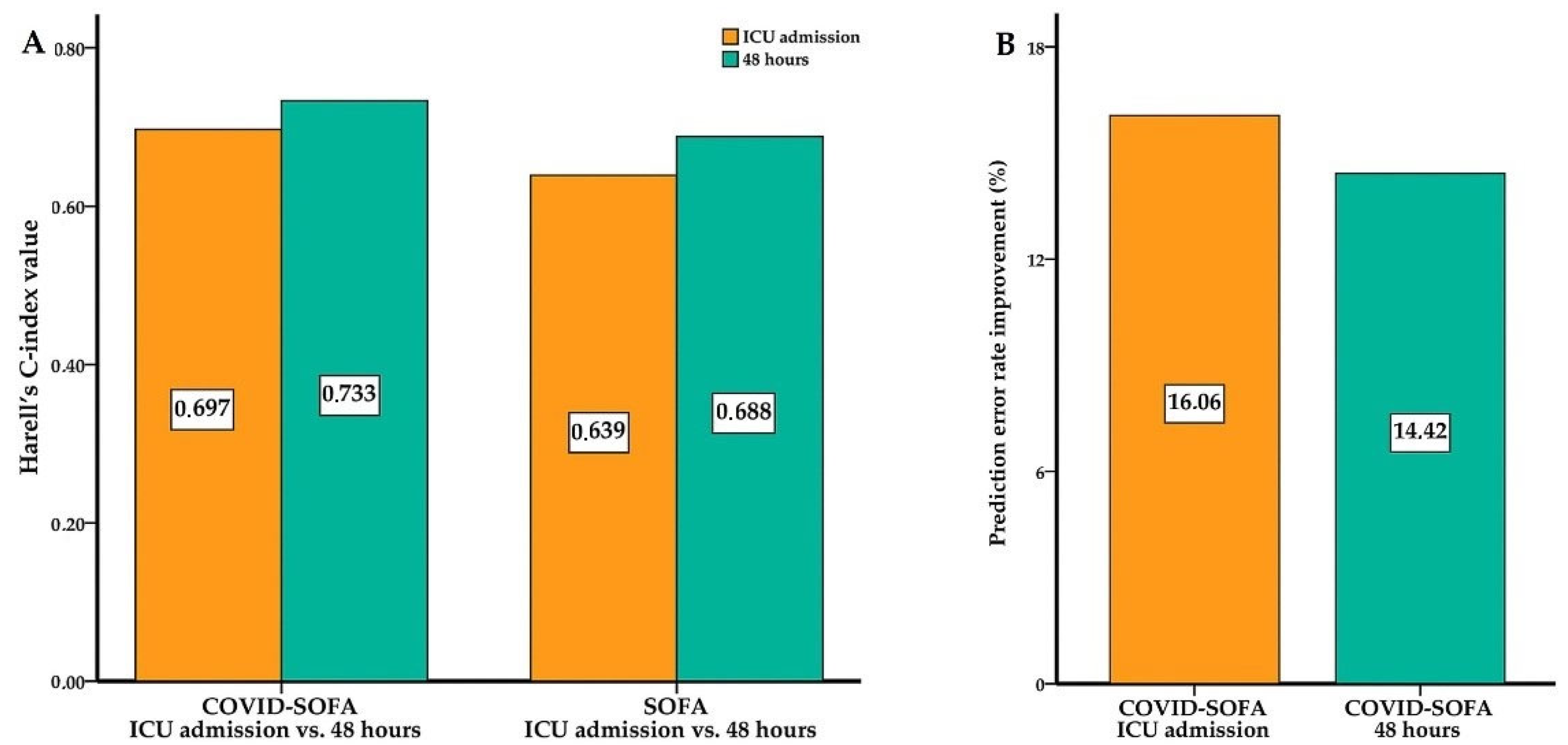
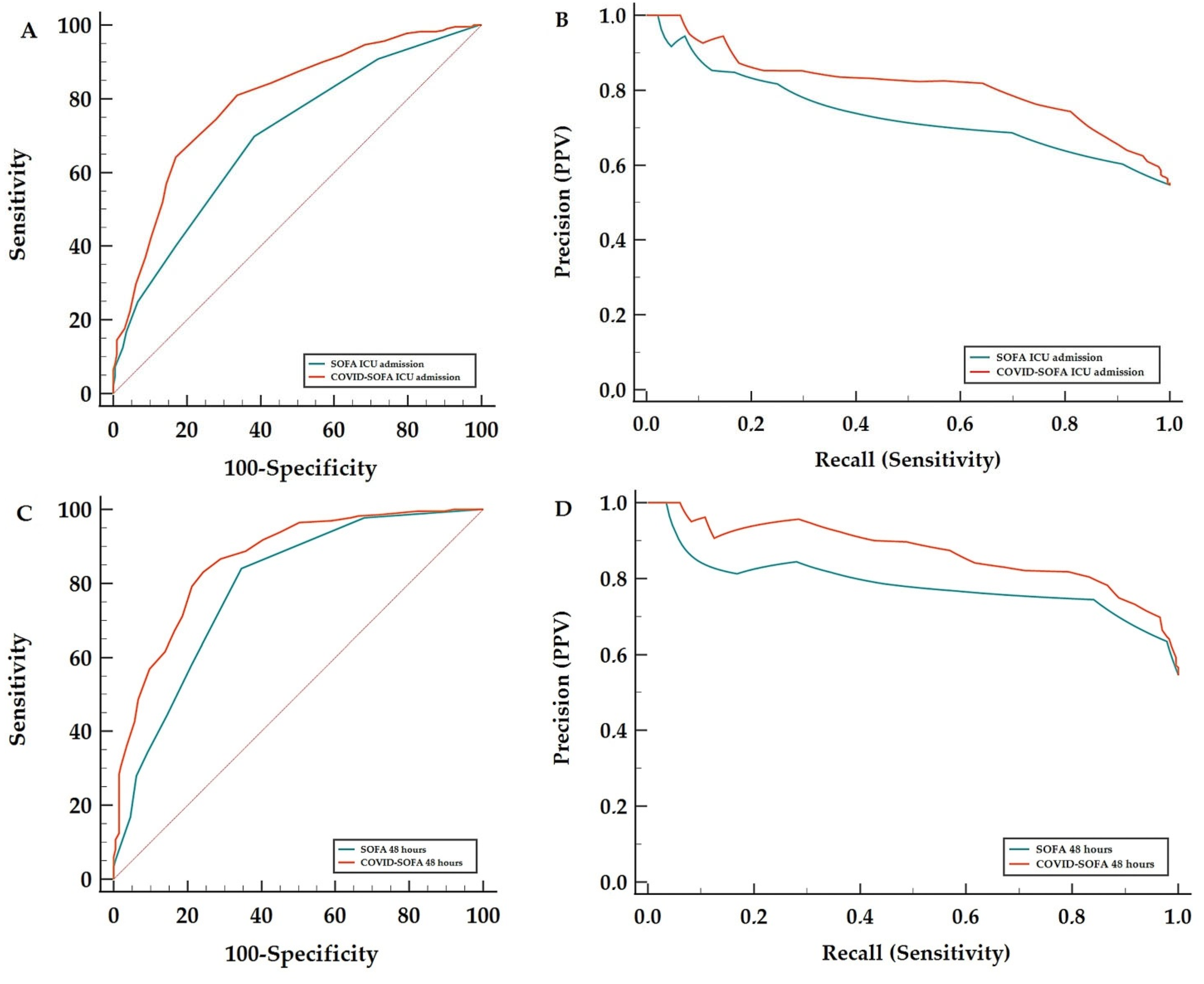
3.2. COVID-SOFA Score Discriminative Power between Survivors and Non-Survivors at 28 Days
3.3. Mortality Probability Calculation at All End-Points Using the COVID-SOFA Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Karakike, E.; Giamarellos-Bourboulis, E.J.; Kyprianou, M.; Fleischmann-Struzek, C.; Pletz, M.W.; Netea, M.G.; Reinhart, K.; Kyriazopoulou, E. Coronavirus Disease 2019 as Cause of Viral Sepsis: A Systematic Review and Meta-Analysis. Crit. Care Med. 2021, 49, 2042. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.B.; Wang, J.; Nason, M.; Warner, S.; Follmann, D.; Kadri, S.S. Preintubation Sequential Organ Failure Assessment Score for Predicting COVID-19 Mortality. Crit. Care Med. 2022, 50, 1051. [Google Scholar] [CrossRef] [PubMed]
- Raschke, R.A.; Agarwal, S.; Rangan, P.; Heise, C.W.; Curry, S.C. Discriminant Accuracy of the SOFA Score for Determining the Probable Mortality of Patients with COVID-19 Pneumonia Requiring Mechanical Ventilation. JAMA 2021, 325, 1469–1470. [Google Scholar] [CrossRef]
- Antommaria, A.H.M.; Gibb, T.S.; McGuire, A.L.; Wolpe, P.R.; Wynia, M.K.; Applewhite, M.K.; Caplan, A.; Diekema, D.S.; Hester, D.M.; Lehmann, L.S.; et al. Ventilator Triage Policies during the COVID-19 Pandemic at U.S. Hospitals Associated with Members of the Association of Bioethics Program Directors. Ann. Intern. Med. 2020, 173, 188–194. [Google Scholar] [CrossRef]
- Bhavani, S.V.; Luo, Y.; Miller, W.D.; Sanchez-Pinto, L.N.; Han, X.; Mao, C.; Sandıkçı, B.; Peek, M.E.; Coopersmith, C.M.; Michelson, K.N.; et al. Simulation of Ventilator Allocation in Critically Ill Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2021, 204, 1224–1227. [Google Scholar] [CrossRef]
- Zhao, W.; Li, H.; Li, J.; Xu, B.; Xu, J. The Mechanism of Multiple Organ Dysfunction Syndrome in Patients with COVID-19. J. Med. Virol. 2022, 94, 1886–1892. [Google Scholar] [CrossRef]
- Sherren, P.B.; Ostermann, M.; Agarwal, S.; Meadows, C.I.S.; Ioannou, N.; Camporota, L. COVID-19-Related Organ Dysfunction and Management Strategies on the Intensive Care Unit: A Narrative Review. Br. J. Anaesth. 2020, 125, 912–925. [Google Scholar] [CrossRef]
- Leisman, D.E.; Mehta, A.; Thompson, B.T.; Charland, N.C.; Gonye, A.L.K.; Gushterova, I.; Kays, K.R.; Khanna, H.K.; LaSalle, T.J.; Lavin-Parsons, K.M.; et al. Alveolar, Endothelial, and Organ Injury Marker Dynamics in Severe COVID-19. Am. J. Respir. Crit. Care Med. 2022, 205, 507–519. [Google Scholar] [CrossRef]
- Jalan, R.; Saliba, F.; Pavesi, M.; Amoros, A.; Moreau, R.; Ginès, P.; Levesque, E.; Durand, F.; Angeli, P.; Caraceni, P.; et al. Development and Validation of a Prognostic Score to Predict Mortality in Patients with Acute-On-Chronic Liver Failure. J. Hepatol. 2014, 61, 1038–1047. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Ministerul Sănătăţii. ORDIN Nr. 533/2021 Din 22 Aprilie 2021 Privind Modificarea Anexei La Ordinul Ministrului Sănătăţii Nr. 487/2020 Pentru Aprobarea Protocolului de Tratament al Infecţiei Cu Virusul SARS-CoV-2; MONITORUL OFICIAL NR. 434/23.04.2021. Available online: https://legislatie.just.ro/Public/DetaliiDocumentAfis/241318 (accessed on 12 April 2022).
- Vincent, J.-L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-Related Organ Failure Assessment) Score to Describe Organ Dysfunction/Failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B (Methodol.) 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Heus, P.; Reitsma, J.B.; Collins, G.S.; Damen, J.A.A.G.; Scholten, R.J.P.M.; Altman, D.G.; Moons, K.G.M.; Hooft, L. Transparent Reporting of Multivariable Prediction Models in Journal and Conference Abstracts: TRIPOD for Abstracts. Ann. Intern. Med. 2020, 173, 42–47. [Google Scholar] [CrossRef]
- Gupta, S.; Hayek, S.S.; Wang, W.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Factors Associated with Death in Critically Ill Patients with Coronavirus Disease 2019 in the US. JAMA Intern. Med. 2020, 180, 1436–1447. [Google Scholar] [CrossRef]
- Andrei, S.; Valeanu, L.; Stefan, M.G.; Longrois, D.; Popescu, M.; Stefan, G.; Balan, C.; Arafat, R.; Corneci, D.; Droc, G.; et al. Outcomes of COVID-19 Critically Ill Extremely Elderly Patients: Analysis of a Large, National, Observational Cohort. J. Clin. Med. 2022, 11, 1544. [Google Scholar] [CrossRef]
- Moisa, E.; Corneci, D.; Negoita, S.; Filimon, C.R.; Serbu, A.; Negutu, M.I.; Grintescu, I.M. Dynamic Changes of the Neutrophil-To-Lymphocyte Ratio, Systemic Inflammation Index, and Derived Neutrophil-To-Lymphocyte Ratio Independently Predict Invasive Mechanical Ventilation Need and Death in Critically Ill COVID-19 Patients. Biomedicines 2021, 9, 1656. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological Findings and Complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef]
- Qian, Z.; Lu, S.; Luo, X.; Chen, Y.; Liu, L. Mortality and Clinical Interventions in Critically Ill Patient with Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 635560. [Google Scholar] [CrossRef]
- Tolchin, B.; Oladele, C.; Galusha, D.; Kashyap, N.; Showstark, M.; Bonito, J.; Salazar, M.C.; Herbst, J.L.; Martino, S.; Kim, N.; et al. Racial Disparities in the SOFA Score among Patients Hospitalized with COVID-19. PLoS ONE 2021, 16, e0257608. [Google Scholar] [CrossRef] [PubMed]
- Beigmohammadi, M.T.; Amoozadeh, L.; Rezaei Motlagh, F.; Rahimi, M.; Maghsoudloo, M.; Jafarnejad, B.; Eslami, B.; Salehi, M.R.; Zendehdel, K. Mortality Predictive Value of APACHE II and SOFA Scores in COVID-19 Patients in the Intensive Care Unit. Can. Respir. J. 2022, 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Krepostman, N.; Collins, M.; Merchant, K.; De Sirkar, S.; Chan, L.; Allen, S.; Newman, J.; Patel, D.; Fareed, J.; Berg, S.; et al. Discriminatory Accuracy of the SOFA Score for Determining Clinical Decompensation in Patients Presenting with COVID-19. Eur. Heart J. 2021, 42 (Suppl. S1), ehab724.2492. [Google Scholar] [CrossRef]
- Christian, M.D. It Is Time to Rethink the Role of the Sequential Organ Failure Assessment Score in Triage Protocols *. Crit. Care Med. 2021, 49, 365–368. [Google Scholar] [CrossRef]
- Camporota, L.; Sanderson, B.; Chiumello, D.; Terzi, N.; Argaud, L.; Rimmelé, T.; Metuor, R.; Verstraete, A.; Cour, M.; Bohé, J.; et al. Prone Position in Coronavirus Disease 2019 and Noncoronavirus Disease 2019 Acute Respiratory Distress Syndrome. Crit. Care Med. 2021. Publish Ahead of Print. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Ho, Y.L.; Besen, B.; Malbouisson, L.; Taniguchi, L.U.; Mendes, P.V.; Costa, E.; Park, M.; Daltro-Oliveira, R.; Roepke, R.; et al. Protective ventilation and outcomes of critically ill patients with COVID-19: A cohort study. Ann. Intensive Care 2021, 11, 92. [Google Scholar] [CrossRef]
- Auld, S.C.; Caridi-Scheible, M.; Blum, J.M.; Robichaux, C.; Kraft, C.; Jacob, J.T.; Jabaley, C.S.; Carpenter, D.; Kaplow, R.; Hernandez-Romieu, A.C.; et al. ICU and Ventilator Mortality among Critically Ill Adults with Coronavirus Disease 2019. Crit. Care Med. 2020, 48, e799–e804. [Google Scholar] [CrossRef]
- Gaudet, A.; Ghozlan, B.; Dupont, A.; Parmentier-Decrucq, E.; Rosa, M.; Jeanpierre, E.; Bayon, C.; Tsicopoulos, A.; Duburcq, T.; Susen, S.; et al. Derivation and Validation of a Predictive Score for Respiratory Failure Worsening Leading to Secondary Intubation in COVID-19: The CERES Score. J. Clin. Med. 2022, 11, 2172. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.X.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-To-Lymphocyte Ratio as an Independent Risk Factor for Mortality in Hospitalized Patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef]
- Riché, F.; Gayat, E.; Barthélémy, R.; Le Dorze, M.; Matéo, J.; Payen, D. Reversal of Neutrophil-To-Lymphocyte Count Ratio in Early versus Late Death from Septic Shock. Crit. Care 2015, 19, 1–10. [Google Scholar] [CrossRef]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The Epidemiology of Sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Bradbury, C.; Abrams, S.T.; Wang, G.; Toh, C. COVID-19 and Immunothrombosis: Emerging Understanding and Clinical Management. Br. J. Haematol. 2021, 194, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Kvietys, P.R.; Fakhoury, H.M.A.; Kadan, S.; Yaqinuddin, A.; Al-Mutairy, E.; Al-Kattan, K. COVID-19: Lung-Centric Immunothrombosis. Front. Cell. Infect. Microbiol. 2021, 11, 679878. [Google Scholar] [CrossRef]
- Fang, X.-Z.; Wang, Y.-X.; Xu, J.-Q.; He, Y.-J.; Peng, Z.-K.; Shang, Y. Immunothrombosis in Acute Respiratory Dysfunction of COVID-19. Front. Immunol. 2021, 12, 651545. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.L. Serial Evaluation of the SOFA Score to Predict Outcome in Critically Ill Patients. JAMA 2001, 286, 1754. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Gupta, S.; Spicer, A.B.; Hayek, S.S.; Srivastava, A.; Chan, L.; Melamed, M.L.; Brenner, S.K.; Radbel, J.; Madhani-Lovely, F.; et al. Machine Learning Prediction of Death in Critically Ill Patients with Coronavirus Disease 2019. Crit. Care Explor. 2021, 3, e0515. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Gu, H.-Q.; Liu, Y.; Zhang, G.; Yang, H.; Hu, H.; Lu, C.; Li, Y.; Wang, L.; Liu, Y.; et al. A Model to Predict the Risk of Mortality in Severely Ill COVID-19 Patients. Comput. Struct. Biotechnol. J. 2021, 19, 1694–1700. [Google Scholar] [CrossRef]
- Lichtner, G.; Balzer, F.; Haufe, S.; Giesa, N.; Schiefenhövel, F.; Schmieding, M.; Jurth, C.; Kopp, W.; Akalin, A.; Schaller, S.J.; et al. Predicting Lethal Courses in Critically Ill COVID-19 Patients Using a Machine Learning Model Trained on Patients with Non-COVID-19 Viral Pneumonia. Sci. Rep. 2021, 11, 13205. [Google Scholar] [CrossRef]
- Schmidt, M.; Guidet, B.; Demoule, A.; Ponnaiah, M.; Fartoukh, M.; Puybasset, L.; Combes, A.; Hajage, D.; Mercat, A.; Asfar, P.; et al. Predicting 90-Day Survival of Patients with COVID-19: Survival of Severely Ill COVID (SOSIC) Scores. Ann. Intensive Care 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Nishikimi, M.; Rasul, R.; Sison, C.P.; Jafari, D.; Shoaib, M.; Shinozaki, K.; Li, T.; Hayashida, K.; Rolston, D.M.; Hirsch, J.S.; et al. Intubated COVID-19 Predictive (ICOP) Score for Early Mortality after Intubation in Patients with COVID-19. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Lombardi, Y.; Azoyan, L.; Szychowiak, P.; Bellamine, A.; Lemaitre, G.; Bernaux, M.; Daniel, C.; Leblanc, J.; Riller, Q.; Steichen, O.; et al. External Validation of Prognostic Scores for COVID-19: A Multicenter Cohort Study of Patients Hospitalized in Greater Paris University Hospitals. Intensive Care Med. 2021, 47, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Tanboğa, I.H.; Canpolat, U.; Çetin, E.H.Ö.; Kundi, H.; Çelik, O.; Çağlayan, M.; Ata, N.; Özeke, Ö.; Çay, S.; Kaymaz, C.; et al. Development and Validation of Clinical Prediction Model to Estimate the Probability of Death in Hospitalized Patients with COVID-19: Insights from a Nationwide Database. J. Med. Virol. 2021, 93, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Liang, H.; Ou, L.; Chen, B.; Chen, A.; Li, C.; Li, Y.; Guan, W.; Sang, L.; Lu, J.; et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients with COVID-19. JAMA Intern. Med. 2020, 180, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Kremer, S.; Merdji, H.; Schenck, M.; Severac, F.; Clere-Jehl, R.; Studer, A.; Radosavljevic, M.; Kummerlen, C.; Monnier, A.; et al. Delirium and Encephalopathy in Severe COVID-19: A Cohort Analysis of ICU Patients. Crit. Care 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Mazeraud, A.; Polito, A.; Sivanandamoorthy, S.; Porcher, R.; Heming, N.; Stoclin, A.; Hissem, T.; Antona, M.; Blot, F.; Gaillard, R.; et al. Association between Anxiety and New Organ Failure, Independently of Critical Illness Severity and Respiratory Status: A Prospective Multicentric Cohort Study. Crit. Care Med. 2020, 48, 1471–1479. [Google Scholar] [CrossRef]
- Flinspach, A.N.; Booke, H.; Zacharowski, K.; Balaban, Ü.; Herrmann, E.; Adam, E.H. High Sedation Needs of Critically Ill COVID-19 ARDS Patients—a Monocentric Observational Study. PLoS ONE 2021, 16, e0253778. [Google Scholar] [CrossRef]
- Brunauer, A.; Koköfer, A.; Bataar, O.; Gradwohl-Matis, I.; Dankl, D.; Dünser, M.W. The Arterial Blood Pressure Associated with Terminal Cardiovascular Collapse in Critically Ill Patients: A Retrospective Cohort Study. Crit. Care 2014, 18, 1–8. [Google Scholar] [CrossRef][Green Version]
- Zakaria, S.; Kwong, H.J.; Sevransky, J.E.; Williams, M.S.; Chandra-Strobos, N. Editor’s Choice-the Cardiovascular Implications of Sedatives in the Cardiac Intensive Care Unit. Eur. Heart J. Acute Cardiovasc. Care 2017, 7, 671–683. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. CHEST 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Saade, A.; Moratelli, G.; Dumas, G.; Mabrouki, A.; Tudesq, J.-J.; Zafrani, L.; Azoulay, E.; Darmon, M. Infectious Events in Patients with Severe COVID-19: Results of a Cohort of Patients with High Prevalence of Underlying Immune Defect. Ann. Intensive Care 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Boulle Geronimi, C.; et al. Relationship between SARS-CoV-2 Infection and the Incidence of Ventilator-Associated Lower Respiratory Tract Infections: A European Multicenter Cohort Study. Intensive Care Med. 2021, 47, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Petty, L.A.; Flanders, S.A.; Vaughn, V.M.; Ratz, D.; O’Malley, M.; Malani, A.N.; Washer, L.; Kim, T.; Kocher, K.E.; Kaatz, S.; et al. Risk Factors and Outcomes Associated with Community-Onset and Hospital-Acquired Coinfection in Patients Hospitalized for Coronavirus Disease 2019 (COVID-19): A Multihospital Cohort Study. Infect. Control. Hosp. Epidemiol. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
| n (%) or Median (IQR: Q1–Q3) | Total Sample | Survivors | Non-Survivors | p Value |
|---|---|---|---|---|
| Demographic and Associated Diseases Data | ||||
| Age | 64 [55–72] | 57 [49.5–65.5] | 68 [62–75] | <0.001 * |
| Gender (Male) | 290 (68.2%) | 135 (46.6%) | 155 (53.4%) | 0.489 ** |
| Obesity | 297 (69.9%) | 127 (42.8%) | 170 (57.2%) | 0.095 ** |
| Cardiac disease | 312 (73.4%) | 120 (38.5%) | 192 (61.5%) | <0.001 ** |
| Respiratory disease | 52 (12.2%) | 13 (25%) | 39 (75%) | 0.025 |
| Diabetes mellitus | 155 (36.5%) | 53 (34.2%) | 102 (65.8%) | <0.001 ** |
| CKD | 39 (9.2%) | 15 (38.5%) | 24 (61.5%) | 0.360 ** |
| Vaccinated | 26 (6.12%) | 11 (42.3%) | 15 (57.7%) | 0.743 ** |
| SOFA Score Parameters | ||||
| PaO2/FiO2 | 110 [87–154.5] | 124 [95–175] | 102.5 [78–140] | <0.001* |
| IMV | 51 (12%) | 6 (11.8%) | 45 (88.2%) | <0.001 ** |
| NIV | 225 (52.9%) | 86 (38.2%) | 139 (61.8%) | |
| HFOT | 149 (35.1%) | 101 (67.8%) | 48 (32.2%) | |
| Creatinine (mg/dL) | 0.8 [0.7–1] | 0.78 [0.68–0.97] | 0.85 [0.7–1.1] | 0.01 * |
| Bilirubin (mg/dL) | 0.6 [0.41–0.86] | 0.6 [0.41–0.8] | 0.6 [0.43–0.87] | 0.793 * |
| Glasgow Coma Scale score | 15 [15–15] | 15 [15–15] | 15 [13–15] | <0.001 * |
| Noradrenaline use | 59 (13.9%) | 14 (23.7%) | 45 (76.3%) | <0.001 ** |
| Platelet count (×103/μL) | 250 [188.5–316.5] | 263 [197.5–331.5] | 236.5 [170–297] | 0.01 * |
| SOFA | 4 [3–5] | 3 [2–4] | 4 [3–5.75] | <0.001 * |
| SOFA IMV patients | 8 [6–9] | 7.5 [7–8] | 8 [6–9] | <0.001 *** |
| SOFA NIV patients | 4 [3–5] | 4 [3–5] | 4 [4–5] | |
| SOFA HFOT patients | 3 [2–3] | 2 [2–3] | 3 [2–3] | |
| Respiratory Outcome | ||||
| Progression to IMV a | 211 (56.41%) | 28 (13.28%) | 183 (86.72%) | <0.001 ** |
| HFOT to IMV b | 53 (35.6%) | 6 (11.33%) | 47 (88.67%) | <0.001 ** |
| NIV TO IMV c | 158 (70.2%) | 22 (13.93%) | 136 (86.07%) | <0.001 ** |
| Hematological and Inflammatory Parameters | ||||
| Lymphocyte count (×103/μL) | 0.74 [0.53–1.01] | 0.88 [0.6–1.2] | 0.67 [0.47–0.87] | <0.001 * |
| Neutrophil count (×103/μL) | 8.53 [6.13–11.65] | 8.04 [5.9–11.06] | 8.91 [6.47–12.58] | 0.01 * |
| NLR | 11.2 [7.21–18.69] | 9.19 [5.9–11.06] | 14.19 [8.98–20.4] | <0.001 * |
| WBC count (×103/μL) | 9.74 [7.3–12.92] | 9.71 [7.16–12.47] | 9.81 [7.42–13.74] | 0.11 * |
| D-dimers (ng/mL) | 431 [263.3–887.5] | 363 [207–684.5] | 511 [320.75–987] | <0.001 |
| C-reactive protein (mg/L) | 121.7 [64.7–206] | 114.5 [63–196.75] | 129.9 [67.5–213.2] | 0.177 |
| Ferritin (ng/mL) | 1018 [572–1483] | 941 [494–1460] | 1063 [606–1500] | 0.229 |
| ICU LoS | 12 [8–17] | 13 [9.5–17] | 11 [7–16] | 0.003 * |
| HAIs | 208 (48.9%) | 54 (26%) | 154 (74%) | <0.001 ** |
| Mortality | ||||
| 28-day all-cause mortality | 232 (54.58%) | |||
| 60-day all-cause mortality | 245 (57.64%) | |||
| 28-Day All-Cause Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|
| C-Index, 95% CI | C-Index Diff. | Error Rate Improvement | AUROC 95% CI | AUROC Diff. | p | AUPRC 95% CI | AURPC Diff. 95% BC(a) CI | |
| COVID-SOFA ICU Admission | 0.697 0.662–0.731 | 0.058 | 16.06% | 0.796 0.755–0.833 | 0.097 | <0.001 * | 0.813 0.757–0.858 | 0.079 |
| SOFA ICU Admission | 0.639 0.605–0.672 | 0.699 0.653–0.742 | 0.734 0.674–0.787 | 0.066–0.094 | ||||
| COVID-SOFA 48 h | 0.733 0.700–0.765 | 0.045 | 14.42% | 0.862 0.826–0.893 | 0.074 | <0.001 * | 0.870 0.820–0.907 | 0.086 |
| SOFA 48 h | 0.688 0.654–0.723 | 0.788 0.746–0.826 | 0.784 0.727–0.832 | 0.07–0.11 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moisa, E.; Corneci, D.; Negutu, M.I.; Filimon, C.R.; Serbu, A.; Popescu, M.; Negoita, S.; Grintescu, I.M. Development and Internal Validation of a New Prognostic Model Powered to Predict 28-Day All-Cause Mortality in ICU COVID-19 Patients—The COVID-SOFA Score. J. Clin. Med. 2022, 11, 4160. https://doi.org/10.3390/jcm11144160
Moisa E, Corneci D, Negutu MI, Filimon CR, Serbu A, Popescu M, Negoita S, Grintescu IM. Development and Internal Validation of a New Prognostic Model Powered to Predict 28-Day All-Cause Mortality in ICU COVID-19 Patients—The COVID-SOFA Score. Journal of Clinical Medicine. 2022; 11(14):4160. https://doi.org/10.3390/jcm11144160
Chicago/Turabian StyleMoisa, Emanuel, Dan Corneci, Mihai Ionut Negutu, Cristina Raluca Filimon, Andreea Serbu, Mihai Popescu, Silvius Negoita, and Ioana Marina Grintescu. 2022. "Development and Internal Validation of a New Prognostic Model Powered to Predict 28-Day All-Cause Mortality in ICU COVID-19 Patients—The COVID-SOFA Score" Journal of Clinical Medicine 11, no. 14: 4160. https://doi.org/10.3390/jcm11144160
APA StyleMoisa, E., Corneci, D., Negutu, M. I., Filimon, C. R., Serbu, A., Popescu, M., Negoita, S., & Grintescu, I. M. (2022). Development and Internal Validation of a New Prognostic Model Powered to Predict 28-Day All-Cause Mortality in ICU COVID-19 Patients—The COVID-SOFA Score. Journal of Clinical Medicine, 11(14), 4160. https://doi.org/10.3390/jcm11144160






