Abstract
Background: The pathophysiology of cancer-related anemia is multifactorial, including that of chemotherapy-induced anemia (CIA). The guidelines are not consistent in their approach to the use of intravenous (IV) iron in patients with cancer as part of the clinical practice. Materials and methods: All randomized controlled trials that compared IV iron with either no iron or iron taken orally for the treatment of CIA were included. We excluded trials if erythropoiesis-stimulating agents (ESAs) were used. The primary outcome was the percentage of patients requiring a red blood cell (RBC) transfusion during the study period. The secondary outcomes included the hematopoietic response (an increase in the Hb level by more than 1 g/dL or an increase above 11 g/dL), the iron parameters and adverse events. For the dichotomous data, risk ratios (RRs) with 95% confidence intervals (Cis) were estimated and pooled. For the continuous data, the mean differences were calculated. A fixed effect model was used, except in the event of significant heterogeneity between the trials (p < 0.10; I2 > 40%), in which we used a random effects model. Results: A total of 8 trials published between January 1990 and July 2021 that randomized 1015 patients fulfilled the inclusion criteria. Of these, 553 patients were randomized to IV iron and were compared with 271 patients randomized to oral iron and 191 to no iron. IV iron decreased the percentage of patients requiring a blood transfusion compared with oral iron (RR 0.72; 95% CI 0.55–0.95) with a number needed to treat of 20 (95% CI 11–100). IV iron increased the hematopoietic response (RR 1.23; 95% CI 1.01–1.5). There was no difference with respect to the risk of adverse events (RR 0.97; 95% CI 0.88–1.07; 8 trials) or severe adverse events (RR 1.09; 95% CI 0.76–1.57; 8 trials). Conclusions: IV iron resulted in a decrease in the need for RBC transfusions, with no difference in adverse events in patients with CIA. IV iron for the treatment of CIA should be considered in clinical practice.
1. Background
Anemia is a common complication across all malignancies. According to a large European survey of 15,367 cancer patients, cancer-associated anemia has a prevalence of 39.3% at presentation and 67% within six months [1]. The pathophysiology of cancer-related anemia, including that of chemotherapy-induced anemia (CIA), is multifactorial and can comprise bleeding, an iron deficiency, an erythropoietin deficiency due to renal disease and tumor involvement of the bone marrow [2].
There is growing evidence that anemia has a negative impact in cancer. Anemia diminishes the functional capacity and is associated with a decrease in the performance status as well as the quality of life [3]. In addition, anemia increases the need for blood transfusions, which have been associated with transfusion reactions and infections [4].
One of the causes of CIA is functional iron deficiency (FID), defined as a defect in supplying iron to the erythroid marrow despite sufficient iron stores. Transferrin saturation (TSAT) is an indicator of iron availability for erythropoiesis. A low TSAT (<20%) and high ferritin (>100 ng/mL) suggest FID [5].
Erythroid-stimulating agents (ESAs) represent a therapeutic option for the treatment of CIA, but only 40–70% of patients with cancer obtain a hematological response. Several pre-clinical trials have identified potential safety problems related to ESAs [6,7,8,9,10,11]. One of the causes of the absence of an ESA response is FID [12]. To avoid FID, it has been suggested that ESAs should be administered with iron support [13,14].
Several pre-clinical trials have identified potential safety problems related to ESA exposure, suggesting that ESAs have a role in augmenting tumorigenesis and metastasis as well as increasing the risk of a thrombosis [9,10,11].
The current American Society of Clinical Oncology (ASH)/American Society of Hematology (ASH) guidelines do not recommend the adjunctive use of ESAs with chemotherapy when chemotherapy is administered with a curative intent. However, ESAs are considered by the guidelines for chemotherapy with a palliative intent [15]. This is based on evidence from two meta-analyses that demonstrated both increased mortality and venous thromboembolic events [16,17].
Oral iron is rarely used nowadays due to low tolerability and efficacy in patients, especially those with FID; no advantage was observed with oral iron when it was added to ESAs [18,19,20]. IV iron has previously been shown to increase the hematopoietic response and to reduce the need for RBC transfusions with no difference in mortality or adverse events in a meta-analysis of RCTs that assessed IV iron as an adjunct therapy with ESAs [21].
Given the fact that there is no consistent approach for IV iron use in cancer patients undergoing chemotherapy in clinical practice, we attempted to assess the effect of IV iron as a monotherapy for the treatment of chemotherapy-induced anemia.
2. Materials and Methods
2.1. Data Sources
We searched PubMed (January 1966 to July 2021), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 8, 12 August 2021) and the following conference proceedings for trials in oncology and hematology (2017–2021): Annual Meeting of the American Society of Hematology (ASH); Annual Meeting of the European Haematology Association (EHA); the American Society of Clinical Oncology (ASCO); and the European Society for Medical Oncology (ESMO). In addition, we searched databases of ongoing and unpublished trials: http://www.controlled-trials.com, http://www.clinicaltrials.gov/ct and http://clinicaltrials.nci.nih.gov.
PubMed was searched using the terms: (iron OR sodium ferric gluconate OR iron dextran OR iron [MeSH] OR Iron-Dextran Complex [MeSH] OR ferric citrate OR Ferric Compounds [MeSH] OR oral* iron OR intravenous iron OR iv iron OR iron-gluconate OR ferrlecit OR iron-gluconate OR venofer OR ferrous sulphate) AND (cancer [MeSH] OR chemotherapy or malignancy or tumor) AND (Anemia or anemia [Mesh]) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double-blind method [mh] OR single-blind method [mh] OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw]) NOT (animals [mh] NOT human [mh]).
We searched the relevant conferences using the term “iron”. The references quoted in all of the included trials and reviews were analyzed in order to identify any additional trials eligible for inclusion.
2.2. Study Selection
We included all randomized controlled trials comparing IV iron with no iron or oral iron for treating anemia in cancer patients undergoing chemotherapy. All types of malignancies were included. Every IV iron preparation was included. Trials were included independently of the publication status, release date and language. Trials were excluded if any ESAs were used for any arm per protocol or off-label.
2.3. Data Extraction and Quality Assessment
Two reviewers independently extracted data from the included trials and evaluated the quality of the methodologies (S.B. and A.G-G). If the two reviewers were not in agreement, a third evaluator (O.I.) extracted the data and the results were obtained by a consensus. We assessed all possible sources of bias that were relevant, including allocation concealment, the generation of the allocation sequence, blinding, incomplete outcome data reporting and selective outcome reporting. We rated each domain as a low risk of bias, an unclear risk (lack of information on or uncertainty over the potential for bias) or a high risk of bias according to the criteria specified in the Cochrane Handbook, version 5.1.0.
2.4. Definition of Outcomes
The primary outcome was the percentage of patients requiring an RBC transfusion during the study period. The secondary outcomes were divided into efficacy and safety outcomes. The efficacy outcomes included: the percentage of patients achieving a hematopoietic response defined as an increase in the hemoglobin (Hb) level by more than 1 g/dL or an increase above 11 g/dL; an absolute Hb concentration or a change from the baseline in the Hb concentration at the end of trial; the absolute ferritin level and transferrin saturation (TSAT) level at the end of the trial; or a change in these values from the baseline if the absolute values were unavailable.
The safety outcomes included: any adverse event; severe adverse events that were considered serious according to the trial investigators or grade 3–5 adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE version 4.03, NCI, Bethesda, MD, USA); gastrointestinal adverse events; or infusion reactions [22].
2.5. Data Synthesis and Analysis
Our primary analysis was IV iron versus a control (no iron or oral iron). Dichotomous data were analyzed by calculating the risk ratio (RR) for each trial with a 95% confidence interval (CI) (Review Manager (RevMan), version 5.4 for Windows, The Cochrane Collaboration). For the continuous variables, we obtained the mean and standard deviation (SD). When the mean or SD values were not available, we calculated them by using data obtained from figures or by recalculating them from other effect estimates and dispersion measures. We calculated the mean difference (MD), which represented the combination of absolute differences between the mean values in the two groups in a clinical trial. This summary statistic had the same unit of measurement as the variable measured. Absolute end values rather than a change from the baseline values were preferentially analyzed. Where unavailable, we combined the end values and changes from the baseline values.
We assessed the heterogeneity of the trial results by calculating a χ2 test of heterogeneity and the I2 measure of inconsistency. We used a fixed effect model with the Mantel–Haenszel method for pooling the trial results throughout the review unless a statistically significant heterogeneity was found (p = 0.10 or I2 > 50%), in which case we chose a random effects model and used the DerSimonian and Laird method [23]. We explored the potential sources of heterogeneity through subgroup analyses of the primary outcome according to the type of malignancy and the type of iron formulation.
3. Results
3.1. Description of Included Studies
The search yielded 1683 potentially relevant publications, of which 42 were considered for a future investigation. In addition, one abstract from conference proceedings was included. The study flow chart according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines showing the flow of the trials included in the meta-analysis and the reasons for exclusions is shown in Figure 1. After applying the inclusion criteria, 8 trials performed between January 1990 and July 2021 that randomized 1015 patients were selected [24,25,26,27,28,29,30,31]. Pooled together, 553 patients treated with IV iron were compared with 271 patients treated with oral iron and 191 treated with no iron.
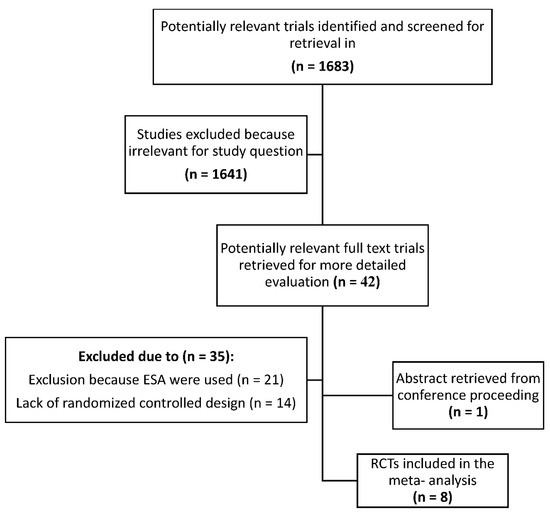
Figure 1.
Trial flow according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines showing the flow of trials included in the meta-analysis. ESA: erythropoiesis-stimulating agents; RCT: randomized controlled trial.
Table 1 presents the characteristics of the included trials. Most trials included patients with solid tumors; three trials included patients with gynecologic cancers [24,25,26], one included esophageal cancer [30], one included lymphoproliferative malignancies [27] and three included all types of cancer [28,29,31].

Table 1.
Characteristics of trials.
All trials included patients with anemia. Each trial applied different inclusion criteria regarding the definitions for anemia and FID (Table 1). Four trials included patients with various degrees of anemia and FID, two trials included patients with anemia according gender (Hb < 12 g/dL for women and Hb < 13 g/dL for men) and two trials included patients with Hb < 10 g/dL. The ferritin level at the baseline was reported in three trials and ranged between 100 and 300 mg/dL [27,28,30]. The following IV iron formulations were used: iron sucrose in four trials, ferric carboxymaltose in two trials and IV iron isomaltoside (currently known as ferric derisomaltose) in two trials. In five trials, a fixed dose of IV iron was given; in three trials, the dose was calculated according to the hemoglobin and weight. The total dose of intravenously administered iron in the trials varied from 400 to 8000 mg. The IV iron schedule varied between the trials. All trials administered the iron after chemotherapy once a week; two trials administered a single dose, three trials administered two doses, two trials administered six doses and one trial administered eight doses. The follow-up ranged from 4 to 24 weeks.
The risk of bias assessment is detailed in Table 2. Regarding the sequence generation, 75% of the trials were found to have a low risk of bias. Regarding the allocation concealment, 87.5% of the trials were of an unclear risk. All included studies were unblinded. All trials were considered to have a low risk of incomplete outcome data.

Table 2.
Risk of bias assessment.
3.2. Primary Outcome: Transfusion Requirements
All eight trials reported the number of patients requiring an RBC transfusion following iron replacement. IV iron decreased the percentage of patients requiring an RBC transfusion compared with oral iron (RR 0.72; 95% CI 0.55–0.95; I2 = 23%), with a number needed to treat of 20 (95% CI 11–100) (Figure 2). The sensitivity analysis was restricted to studies that reported a low risk of sequence generation (n = 6) and did not alter the results (RR 0.78; 95% CI 0.58–1.06; I2 = 22%).
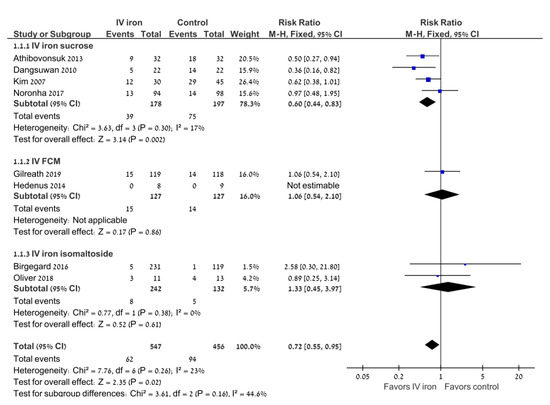
Figure 2.
Intravenous iron versus the control: the need for RBC transfusions. Blue squares represent the point estimate, their sizes represent their weight in the pooled analysis and the horizontal bars represent the 95% CI. The black diamond at the bottom represents the pooled point estimate. CI: confidence interval; IV: intravenous.
Subgroup analyses were performed according to the type of IV iron and the type of malignancy (Table 3). In the subgroup of gynecologic malignancies, the use of IV iron was associated with a decrease in the percentage of patients requiring an RBC transfusion compared with oral iron (RR 0.51; 95% CI 0.36–0.73; I2 = 0%; 5 trials). When analyzed according to the type of IV iron preparation, there was a significant decrease in the percentage of patients requiring an RBC transfusion in the four trials of iron sucrose (RR 0.67; 95% CI 0.47–0.94; I2 = 17%), but not in the two trials of ferric carboxymaltose or in the two trials of iron isomaltose.

Table 3.
Subgroup analyses of the primary outcome: the need for Red Blood Cell (RBC) transfusions.
3.3. Secondary Outcomes
IV iron increased the percentage of patients with a hematopoietic response compared with the control (RR 1.23; 95% CI 1.01–1.5; I2 = 0%).
The absolute Hb level or the change from the baseline in Hb at the end of the study was higher in patients treated with IV iron (MD 0.23; 95% CI 0.01–0.44).
Three trials (including 396 patients) reported on the parameters of iron indices. The ferritin level at the end of the trial was significantly higher in the IV iron arm compared with the standard treatment (MD 260.65; 95% CI 105.79–415.51). There was no difference in TSAT at the end of the trial between the patients treated with IV iron and those without (MD −0.4; 95% CI −5.96–5.17).
3.4. Safety
There was no difference between the study groups in the risk of any adverse events (RR 0.97; 95% CI 0.88–1.07; 8 trials) or severe adverse events (RR 0.98; 95% CI 0.47–2.06; I2 = 69%; random effects model; 8 trials) (Figure 3). There was no difference between the study groups in gastrointestinal adverse events (RR 1.05; 95% CI 0.85–1.3; 4 trials; I2 = 75%). There was no difference in the rate of adverse events requiring treatment discontinuation (RR 0.33; 95% CI 0.07–1.58; I2 = 81.2%; random effects model; 4 trials).
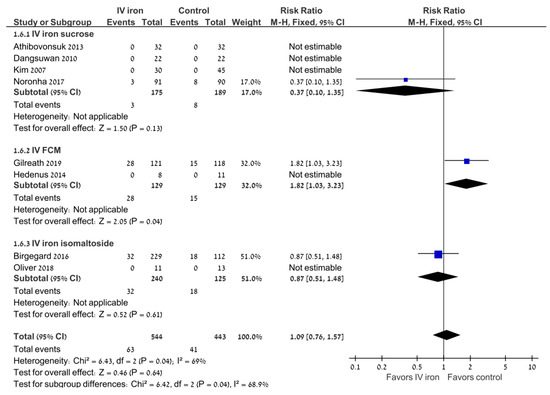
Figure 3.
Intravenous iron versus the control: severe adverse events. Blue squares represent the point estimate, their sizes represent their weight in the pooled analysis and the horizontal bars represent the 95% CI. The black diamond at the bottom represents the pooled point estimate. CI: confidence interval; IV: intravenous.
4. Discussion
In this systematic review and meta-analysis, we included all randomized controlled trials that compared IV iron with no iron or oral iron for chemotherapy-induced anemia (CIA). We found that the intravenous administration of iron for CIA reduced the risk of an RBC transfusion by 28% (95% CI 0.55–0.95; I2 = 23%). In addition, IV iron increased the chance of a hematopoietic response and was associated with an increase in the ferritin level and was not associated with an increase in adverse events (both any and severe).
Our main finding of a decrease in the need for RBC transfusions is of importance to clinical practice. Lowering transfusion requirements can minimize various risks such as a hemolytic transfusion reaction, an acute lung injury and a transfusion infection-related acute lung injury [32,33]. It is a matter of debate if blood transfusions, especially if given peri-operatively, negatively impact cancer outcomes. In a large cohort study of more than 4000 patients with colorectal carcinomas undergoing curative colorectal resections, blood transfusions administered peri-operatively were found to be independently associated with shorter disease-free survival as well as overall survival [34]. This was independent of the anemia status. Although the setting in this study was different from patients undergoing chemotherapy (as in our study), these results further reinforce the need for restrictive transfusion strategies. IV iron, as we have shown, has the advantage of minimizing transfusions.
Our results are in accordance with the current guidelines for blood transfusions, which recommend a restrictive transfusion strategy [35]. The need for fewer RBC transfusions may also potentially reduce hospital visits (either to the hospital ambulatory day clinic or hospitalization), which may have a positive impact on the quality of life [36].
The increase in hematopoietic response is of a clinical relevance. Anemia has been shown to be a negative prognostic factor in cancer [37,38].
IV iron was not associated with an increased risk of adverse events. Similar safety results were shown in a comprehensive meta-analysis that included 103 randomized trials and 14,434 patients in which IV iron was compared with oral iron in many different clinical settings. IV iron was shown to have a comparable safety profile to oral iron with the same risk of SAEs, mortality and serious bacterial infections and fewer GI adverse events [39].
Previous clinical trials using IV iron and a previous meta-analysis support the use of IV iron in CIA. IV iron has been shown to improve the hematopoietic response, to reduce the risk of RBC transfusions and to be well-tolerated [21]. However, this was shown in trials that administered both IV iron and ESAs. This current study is the first meta-analysis to assess the benefit of IV iron supplementation as a monotherapy without ESAs in CIA. As mentioned above, ESAs are controversial because of potential safety problems and are currently only recommended for palliative care [9,10,11].
Our results are in line with most consensus guidelines that recommend IV iron supplementation for the treatment of CIA. The European Society for Medical Oncology (ESMO) originally suggested a treatment with IV iron in their 2010 guidelines and confirmed the utility of IV iron in their 2018 update [40]. The EORTC (European Organisation for Research and Treatment of Cancer) guidelines mention a better response to ESAs with iron IV, but indicate the need to define the optimum dose and timing [9].
Our study has several limitations that merit consideration; first, the included studies were heterogeneous with respect to the various types of malignant tumors and chemotherapy regimens. In addition, there was heterogeneity regarding the iron supplementation, including different iron preparations and schedules. There was not enough information to conduct subgroup analyses according to different baseline hematologic parameters or different malignancies or according to the total administered iron dose. There was only one trial that assessed hematological malignancies. The optimal iron dosage and schedule was not clear. Due to the short follow-up period of up to 24 weeks, there was no long-term follow-up data regarding efficacy, mortality and safety. In addition, we could not collect data regarding the effect of IV iron on cancer-related outcomes. Data regarding the cost-effectiveness of IV iron were not collected as well.
Implications for Practice and Research
Our meta-analysis supports the use of iron intravenously administered for the treatment of CIA. Our results mainly apply to patients with FID. Further research is needed to define the optimal IV iron formulation, dose and schedule and to assess the specific types of malignancies that may benefit from IV iron.
In conclusion, in this meta-analysis we showed that IV iron for the treatment of CIA reduces the need for RBC transfusions and is not associated with adverse events. IV iron for the treatment of CIA should be considered in clinical practice.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ludwig, H.; Van Belle, S.; Barrett-Lee, P.; Birgegård, G.; Bokemeyer, C.; Gascón, P.; Kosmidis, P.; Krzakowski, M.; Nortier, J.; Olmi, P.; et al. The European cancer anaemia survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur. J. Cancer 2004, 40, 2293–2306. [Google Scholar] [CrossRef] [PubMed]
- Gilreath, J.A.; Stenehjem, D.; Rodgers, G.M. Diagnosis and treatment of cancer-related anemia. Am. J. Hematol. 2014, 89, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Harper, P.; Littlewood, T. Anaemia of cancer: Impact on patient fatigue and long-term outcome. Oncology 2005, 69, 2–7. [Google Scholar] [CrossRef]
- Carson, J.L.; Guyatt, G.; Heddle, N.M.; Grossman, B.J.; Cohn, C.S.; Fung, M.K.; Gernsheimer, T.; Holcomb, J.B.; Kaplan, L.J.; Katz, L.M.; et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016, 316, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Gilreath, J.A.; Rodgers, G.M. How I treat cancer-associated anemia. Blood 2020, 136, 801–813. [Google Scholar] [CrossRef]
- Gabrilove, J.L.; Cleeland, C.S.; Livingston, R.B.; Sarokhan, B.; Winer, E.; Einhorn, L.H. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: Improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J. Clin. Oncol. 2001, 19, 2875–2882. [Google Scholar] [CrossRef]
- Österborg, A.; Brandberg, Y.; Molostova, V.; Iosava, G.; Abdulkadyrov, K.; Hedenus, M.; Messinger, D. Randomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignancies. J. Clin. Oncol. 2002, 20, 2486–2494. [Google Scholar] [CrossRef]
- Boogaerts, M.; Coiffier, B.; Kainz, C. Epoetin beta QOL working group. Impact of epoetin beta on quality of life in patients with malignant disease. Br. J. Cancer 2003, 88, 98895. [Google Scholar] [CrossRef]
- Bokemeyer, C.F.; Aapro, M.S.; Courdi, A.; Foubert, J.; Link, H.; Österborg, A.; Repetto, L.; Soubeyran, P. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur. J. Cancer 2007, 43, 258–270. [Google Scholar] [CrossRef]
- Hedley, B.D.; Allan, A.L.; Xenocostas, A. The role of erythropoietin and erythropoiesis-stimulating agents in tumor progression. Clin. Cancer Res. 2011, 17, 6373–6380. [Google Scholar] [CrossRef]
- Todaro, M.; Turdo, A.; Bartucci, M.; Iovino, F.; Dattilo, R.; Biffoni, M.; Stassi, G.; Federici, G.; De Maria, R.; Zeuner, A. Erythropoietin activates cell survival pathways in breast cancer stem–like cells to protect them from chemotherapy. Cancer Res. 2013, 73, 6393–6400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Damrauer, J.S.; Bailey, S.T.; Hadzic, T.; Jeong, Y.; Clark, K.; Fan, C.; Murphy, L.; Lee, C.Y.; Troester, M.A.; et al. Erythropoietin promotes breast tumorigenesis through tumor-initiating cell self-renewal. J. Clin. Investig. 2014, 124, 553–563. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goodnough, L.T. Erythropoietin and iron-restricted erythropoiesis. Exp. Hematol. 2007, 35 (Suppl. S1), 167–172. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.D.; Lichtin, A.E.; Woolf, S.H.; Seidenfeld, J.; Bennett, C.L.; Cella, D.; Djulbegovic, B.; Goode, M.J.; Jakubowski, A.A.; Lee, S.J.; et al. American society of clinical oncology/American society of hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J. Clin. Oncol. 2010, 28, 4996–5010. [Google Scholar] [CrossRef]
- Bohlius, J.; Bohlke, K.; Castelli, R.; Djulbegovic, B.; Lustberg, M.B.; Martino, M.; Mountzios, G.; Peswani, N.; Porter, L.; Tanaka, T.N.; et al. Management of cancer-associated anemia with erythropoiesis-stimulating agents: ASCO/ASH clinical practice guideline update. Blood Adv. 2019, 3, 1197–1210. [Google Scholar] [CrossRef]
- Bohlius, J.; Schmidlin, K.; Brillant, C.; Schwarzer, G.; Trelle, S.; Seidenfeld, J.; Zwahlen, M.; Clarke, M.; Weingart, O.; Kluge, S.; et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet 2009, 373, 1532–1542. [Google Scholar] [CrossRef]
- Bennett, C.L.; Silver, S.M.; Djulbegovic, B.; Samaras, A.T.; Blau, C.A.; Gleason, K.J.; Barnato, S.E.; Elverman, K.M.; Courtney, D.M.; McKoy, J.M.; et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008, 299, 914–924. [Google Scholar] [CrossRef]
- Henry, D.H.; Dahl, N.V.; Auerbach, M.; Tchekmedyian, S.; Laufman, L.R. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 2007, 12, 231–242. [Google Scholar] [CrossRef]
- Aapro, M.; Österborg, A.; Gascón, P.; Ludwig, H.; Beguin, Y. Prevalence and management of cancer related anaemia, iron deficiency and the specific role of i.v. iron. Ann. Oncol. 2012, 23, 1954–1962. [Google Scholar] [CrossRef]
- Steinmetz, H.T. The role of intravenous iron in the treatment of anemia in cancer patients. Ther. Adv. Hematol. 2012, 3, 177–191. [Google Scholar] [CrossRef]
- Gafter-Gvili, A.; Rozen-Zvi, B.; Vidal, L.; Leibovici, L.; Vansteenkiste, J.; Gafter, U.; Shpilberg, O. Intravenous iron supplementation for the treatment of chemotherapy-induced anaemia—Systematic review and meta-analysis of randomised controlled trials. Acta Oncol. 2012, 52, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed on 14 June 2010).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kim, S.W.; Yoon, B.S.; Cho, H.J.; Nahm, E.J.; Kim, S.H.; Kim, J.H.; Kim, J.W. Effect of intravenously administered iron sucrose on the prevention of anemia in the cervical cancer patients treated with concurrent chemoradiotherapy. Gynecol. Oncol. 2007, 105, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Dangsuwan, P.; Manchana, T. Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. Gynecol. Oncol. 2010, 116, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Athibovonsuk, P.; Manchana, T.; Sirisabya, N. Prevention of blood transfusion with intravenous iron in gynecologic cancer patients receiving platinum-based chemotherapy. Gynecol. Oncol. 2013, 131, 679–682. [Google Scholar] [CrossRef]
- Hedenus, M.; Karlsson, T.; Ludwig, H.; Rzychon, B.; Felder, M.; Roubert, B.; Birgegård, G. Intravenous iron alone resolves anemia in patients with functional iron deficiency and lymphoid malignancies undergoing chemotherapy. Med. Oncol. 2014, 31, 302. [Google Scholar] [CrossRef]
- Birgegård, G.; Henry, D.; Glaspy, J.; Chopra, R.; Thomsen, L.L.; Auerbach, M. A Randomized Noninferiority Trial of Intravenous Iron Isomaltoside versus Oral Iron Sulfate in Patients with Nonmyeloid Malignancies and Anemia Receiving Chemotherapy: The PROFOUND Trial. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2016, 36, 402–414. [Google Scholar] [CrossRef]
- Noronha, V.; Joshi, A.; Patil, V.M.; Banavali, S.D.; Gupta, S.; Parikh, P.M.; Marfatia, S.; Punatar, S.; More, S.; Goud, S.; et al. Phase III randomized trial comparing intravenous to oral iron in patients with cancer-related iron deficiency anemia not on erythropoiesis stimulating agents. Asia-Pacific J. Clin. Oncol. 2017, 14, e129–e137. [Google Scholar] [CrossRef]
- Oliver, N.; Keeler, B.; Simpson, J.A.; Madhusudan, S.; Brookes, M.; Acheson, A. Feasibility of intravenous iron isomaltoside to improve anemia and quality of life during palliative chemotherapy for esophagogastric adenocarcinoma. Nutr. Cancer 2018, 70, 1106–1117. [Google Scholar] [CrossRef]
- Gilreath, J.A.; Makharadze, T.; Boccia, R.V.; Krupa, A.; Henry, D.H. Efficacy and Safety of Ferric Carboxymaltose Injection in Reducing Anemia in Patients Receiving Chemotherapy for Non-Myeloid Malignancies: A Phase 3, Placebo-Controlled Study (IRON CLAD). Blood 2019, 134, 3535. [Google Scholar] [CrossRef]
- Sahu, S.; Hemlata; Verma, A. Adverse events related to blood transfusion. Indian J. Anaesth. 2014, 58, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Tobian, A.A.R.; Shaz, B.H. Noninfectious transfusion-associated adverse events and their mitigation strategies. Blood 2019, 133, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-L.; Tai, Y.-H.; Lin, S.-P.; Chan, M.-Y.; Chen, H.-H.; Chang, K.-Y. The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: A propensity score analysis of 4030 patients. Sci. Rep. 2018, 8, 13345. [Google Scholar] [CrossRef] [PubMed]
- Estcourt, L.J.; Malouf, R.; Trivella, M.; Fergusson, D.A.; Hopewell, S.; Murphy, M.F. Restrictive versus liberal red blood cell transfusion strategies for people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support. Cochrane Database Syst. Rev. 2017, 2017, CD011305. [Google Scholar] [CrossRef]
- Fortner, B.V.; Tauer, K.; Zhu, L.; Okon, T.A.; Moore, K.; Templeton, D.; Schwartzberg, L. Medical visits for chemotherapy and chemotherapy-induced neutropenia: A survey of the impact on patient time and activities. BMC Cancer 2004, 4, 22. [Google Scholar] [CrossRef][Green Version]
- Tokunaga, R.; Nakagawa, S.; Miyamoto, Y.; Ohuchi, M.; Izumi, D.; Kosumi, K.; Taki, K.; Higashi, T.; Miyata, T.; Yoshida, N.; et al. The impact of preoperative anaemia and anaemic subtype on patient outcome in colorectal cancer. Color. Dis. 2018, 21, 100–109. [Google Scholar] [CrossRef]
- Clarke, H.; Pallister, C.J. The impact of anaemia on outcome in cancer. Int. J. Lab. Hematol. 2005, 27, 1–13. [Google Scholar] [CrossRef]
- Avni, T.; Bieber, A.; Grossman, A.; Green, H.; Leibovici, L.; Gafter-Gvili, A. The Safety of Intravenous Iron Preparations. Mayo Clin. Proc. 2015, 90, 12–23. [Google Scholar] [CrossRef]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascón, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwig, H.; et al. Management of anemia and iron deficiency in patients with cancer: ESMO clinical practice guidelines. Ann. Oncol. 2018, 29 (Suppl. S4), iv96–iv110. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).