The Potential Role of Renal Denervation in the Management of Heart Failure
Abstract
1. Introduction
2. Sympathetic Activation in Heart Failure
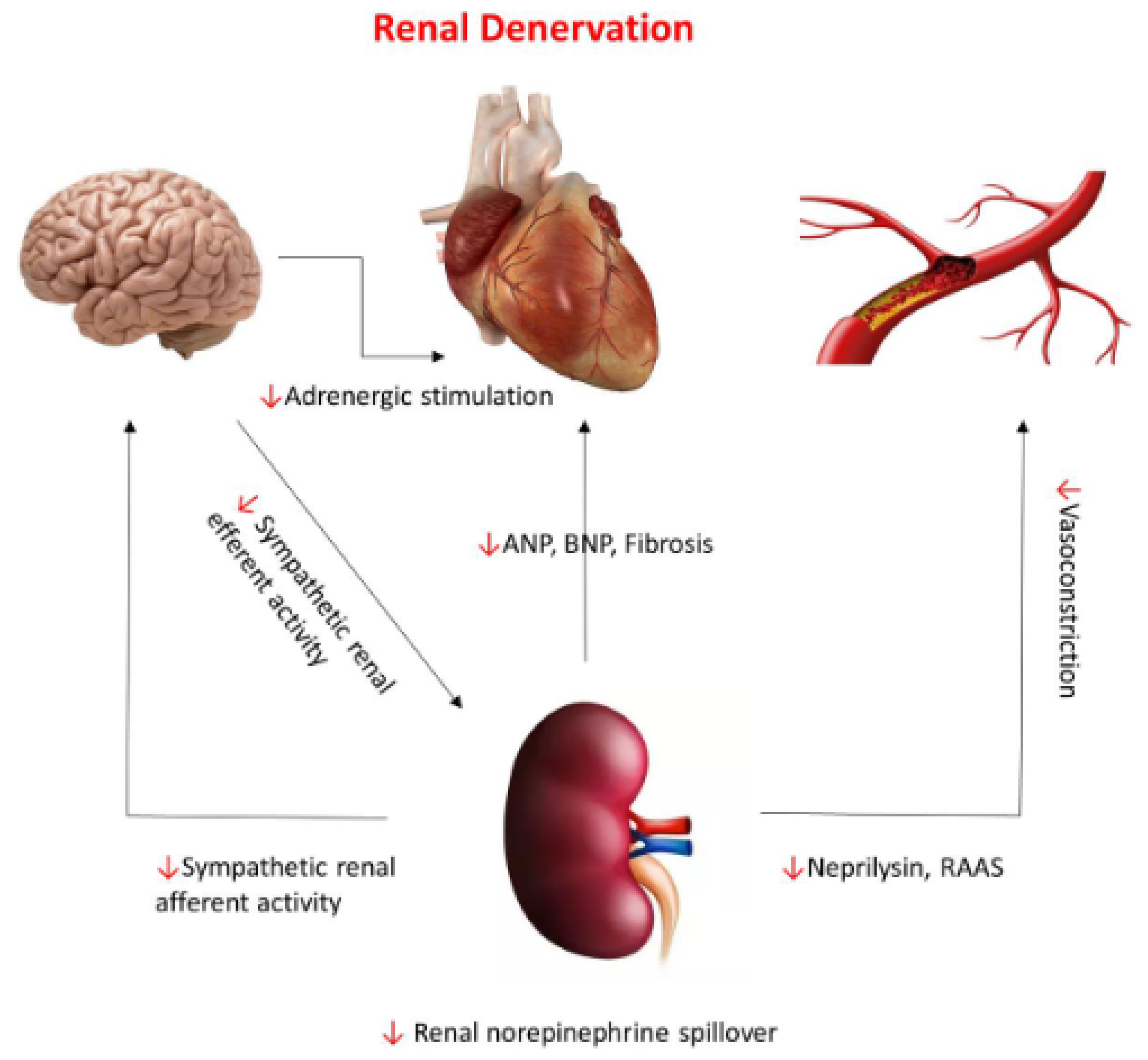
3. Renal Denervation
3.1. Renal Denervation in Heart Failure with Reduced Ejection Fraction
3.2. Renal denervation in Heart Failure with Preserved Ejection Fraction
3.3. Renal Denervation and Cardiac Dysrhythmias in Heart Failure
3.4. Future Directives and Challenges
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.; Judge, A.; Canoy, D.; Tran, J.; Pinho-Gomes, A.C.; Millett, E.R.C.; Salimi-Khorshidi, G.; Cleland, J.G.; McMurray, J.J.V.; Rahimi, K. Temporal Trends and Patterns in Mortality After Incident Heart Failure: A Longitudinal Analysis of 86,000 Individuals. JAMA Cardiol. 2019, 4, 1102–1111. [Google Scholar] [CrossRef]
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 2017, 14, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.E., 3rd; Lefer, D.J. Renal Denervation to Treat Heart Failure. Annu. Rev. Physiol. 2021, 83, 39–58. [Google Scholar] [CrossRef]
- Converse, R.L., Jr.; Jacobsen, T.N.; Toto, R.D.; Jost, C.M.; Cosentino, F.; Fouad-Tarazi, F.; Victor, R.G. Sympathetic overactivity in patients with chronic renal failure. N. Engl. J. Med. 1992, 327, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Esler, M. The 2009 Carl Ludwig Lecture: Pathophysiology of the human sympathetic nervous system in cardiovascular diseases: The transition from mechanisms to medical management. J. Appl. Physiol. 2010, 108, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.; Ewen, S.; Mahfoud, F. Renal Denervation for Chronic Heart Failure: Background and Pathophysiological Rationale. Korean Circ. J. 2017, 47, 9–15. [Google Scholar] [CrossRef]
- Pepper, G.S.; Lee, R.W. Sympathetic activation in heart failure and its treatment with beta-blockade. Arch. Intern. Med. 1999, 159, 225–234. [Google Scholar] [CrossRef]
- Francis, G.S.; Benedict, C.; Johnstone, D.E.; Kirlin, P.C.; Nicklas, J.; Liang, C.S.; Kubo, S.H.; Rudin-Toretsky, E.; Yusuf, S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation 1990, 82, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Petersson, M.; Friberg, P.; Eisenhofer, G.; Lambert, G.; Rundqvist, B. Long-term outcome in relation to renal sympathetic activity in patients with chronic heart failure. Eur. Heart J. 2005, 26, 906–913. [Google Scholar] [CrossRef]
- Bohm, M.; Ewen, S.; Kindermann, I.; Linz, D.; Ukena, C.; Mahfoud, F. Renal denervation and heart failure. Eur. J. Heart Fail. 2014, 16, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Stella, A.; Zanchetti, A. Functional role of renal afferents. Physiol. Rev. 1991, 71, 659–682. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.S.; McDonald, K.M.; Cohn, J.N. Neurohumoral activation in preclinical heart failure. Remodeling and the potential for intervention. Circulation 1993, 87, IV90–IV96. [Google Scholar] [PubMed]
- Mann, D.L.; Kent, R.L.; Parsons, B.; Cooper, G.T. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 1992, 85, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Kandala, J.; Camm, A.J. Non-pharmacological modulation of the autonomic tone to treat heart failure. Eur. Heart J. 2014, 35, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.; Beuckelmann, D.; Brown, L.; Feiler, G.; Lorenz, B.; Nabauer, M.; Kemkes, B.; Erdmann, E. Reduction of beta-adrenoceptor density and evaluation of positive inotropic responses in isolated, diseased human myocardium. Eur. Heart J. 1988, 9, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Kandzari, D.E.; Bohm, M.; Mahfoud, F.; Townsend, R.R.; Weber, M.A.; Pocock, S.; Tsioufis, K.; Tousoulis, D.; Choi, J.W.; East, C.; et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018, 391, 2346–2355. [Google Scholar] [CrossRef]
- Azizi, M.; Schmieder, R.E.; Mahfoud, F.; Weber, M.A.; Daemen, J.; Davies, J.; Basile, J.; Kirtane, A.J.; Wang, Y.; Lobo, M.D.; et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): A multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018, 391, 2335–2345. [Google Scholar] [CrossRef]
- Liang, B.; Liang, Y.; Li, R.; Gu, N. Effect of renal denervation on long-term outcomes in patients with resistant hypertension. Cardiovasc. Diabetol. 2021, 20, 117. [Google Scholar] [CrossRef]
- Patel, H.C.; Hayward, C.; Vassiliou, V.; Patel, K.; Howard, J.P.; Di Mario, C. Renal denervation for the management of resistant hypertension. Integr. Blood Press. Control 2015, 8, 57–69. [Google Scholar] [CrossRef][Green Version]
- Nawar, K.; Mohammad, A.; Johns, E.J.; Abdulla, M.H. Renal denervation for atrial fibrillation: A comprehensive updated systematic review and meta-analysis. J. Hum. Hypertens. 2022. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.S.; Shabanov, V.; Ponomarev, D.; Losik, D.; Ivanickiy, E.; Kropotkin, E.; Polyakov, K.; Ptaszynski, P.; Keweloh, B.; Yao, C.J.; et al. Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension: The ERADICATE-AF Randomized Clinical Trial. JAMA 2020, 323, 248–255. [Google Scholar] [CrossRef]
- Sharp, T.E., 3rd; Polhemus, D.J.; Li, Z.; Spaletra, P.; Jenkins, J.S.; Reilly, J.P.; White, C.J.; Kapusta, D.R.; Lefer, D.J.; Goodchild, T.T. Renal Denervation Prevents Heart Failure Progression Via Inhibition of the Renin-Angiotensin System. J. Am. Coll. Cardiol. 2018, 72, 2609–2621. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.J.; Gao, J.; Scarborough, A.L.; Trivedi, R.; McDonough, K.H.; Goodchild, T.T.; Smart, F.; Kapusta, D.R.; Lefer, D.J. Radiofrequency Renal Denervation Protects the Ischemic Heart via Inhibition of GRK2 and Increased Nitric Oxide Signaling. Circ. Res. 2016, 119, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.J.; Trivedi, R.K.; Gao, J.; Li, Z.; Scarborough, A.L.; Goodchild, T.T.; Varner, K.J.; Xia, H.; Smart, F.W.; Kapusta, D.R.; et al. Renal Sympathetic Denervation Protects the Failing Heart Via Inhibition of Neprilysin Activity in the Kidney. J. Am. Coll. Cardiol. 2017, 70, 2139–2153. [Google Scholar] [CrossRef]
- Xia, Z.; Han, L.; Pellegrino, P.R.; Schiller, A.M.; Harrold, L.D.; Lobato, R.L.; Lisco, S.J.; Zucker, I.H.; Wang, H.J. Safety and efficacy of renal denervation in patients with heart failure with reduced ejection fraction (HFrEF): A systematic review and meta-analysis. Heliyon 2022, 8, e08847. [Google Scholar] [CrossRef]
- Kresoja, K.P.; Rommel, K.P.; Fengler, K.; von Roeder, M.; Besler, C.; Lucke, C.; Gutberlet, M.; Desch, S.; Thiele, H.; Bohm, M.; et al. Renal Sympathetic Denervation in Patients With Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2021, 14, e007421. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, S.H.; Fendrick, A.M.; Baicker, K. Variation in medication adherence in heart failure. JAMA Intern. Med. 2013, 173, 468–470. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Powers, J.D.; Ho, P.M.; Maddox, T.M.; Peterson, P.N.; Allen, L.A.; Masoudi, F.A.; Magid, D.J.; Havranek, E.P. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J. Card. Fail. 2011, 17, 664–669. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, X.; Sharma, N.M.; Patel, K.P. Renal denervation improves cardiac function in rats with chronic heart failure: Effects on expression of beta-adrenoceptors. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H337–H346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Song, L.; Li, C.; Feng, Q.; Xu, M.; Li, Z.; Lu, C. Renal denervation improves cardiac function by attenuating myocardiocyte apoptosis in dogs after myocardial infarction. BMC Cardiovasc. Disord. 2018, 18, 86. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Manisty, C.H.; Petraco, R.; Barron, A.J.; Unsworth, B.; Mayet, J.; Hamady, M.; Hughes, A.D.; Sever, P.S.; Sobotka, P.A.; et al. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: Primary outcome from REACH-Pilot study. Int. J. Cardiol. 2013, 162, 189–192. [Google Scholar] [CrossRef]
- Chen, W.; Ling, Z.; Xu, Y.; Liu, Z.; Su, L.; Du, H.; Xiao, P.; Lan, X.; Shan, Q.; Yin, Y. Preliminary effects of renal denervation with saline irrigated catheter on cardiac systolic function in patients with heart failure: A Prospective, Randomized, Controlled, Pilot Study. Catheter. Cardiovasc. Interv. 2017, 89, E153–E161. [Google Scholar] [CrossRef]
- Gao, J.Q.; Yang, W.; Liu, Z.J. Percutaneous renal artery denervation in patients with chronic systolic heart failure: A randomized controlled trial. Cardiol. J. 2019, 26, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Drozdz, T.; Jastrzebski, M.; Moskal, P.; Kusiak, A.; Bednarek, A.; Styczkiewicz, K.; Jankowski, P.; Czarnecka, D. Renal denervation in patients with symptomatic chronic heart failure despite resynchronization therapy—A pilot study. Postepy Kardiol. Interwencyjnej 2019, 15, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Q.; Xie, Y.; Yang, W.; Zheng, J.P.; Liu, Z.J. Effects of percutaneous renal sympathetic denervation on cardiac function and exercise tolerance in patients with chronic heart failure. Rev. Port. Cardiol. 2017, 36, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hopper, I.; Gronda, E.; Hoppe, U.C.; Rundqvist, B.; Marwick, T.H.; Shetty, S.; Hayward, C.; Lambert, T.; Hering, D.; Esler, M.; et al. Sympathetic Response and Outcomes Following Renal Denervation in Patients with Chronic Heart Failure: 12-Month Outcomes From the Symplicity HF Feasibility Study. J. Card. Fail. 2017, 23, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Lu, J.; Wang, B.; Ma, G. Effect of percutaneous renal sympathetic nerve radiofrequency ablation in patients with severe heart failure. Int. J. Clin. Exp. Med. 2015, 8, 9779–9785. [Google Scholar]
- Borlaug, B.A.; Paulus, W.J. Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. Eur. Heart J. 2011, 32, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, S.E.; von Lueder, T.G.; Smiseth, O.A.; Wachtell, K.; Mistry, N.; Westheim, A.S.; Hopper, I.; Julius, S.; Pitt, B.; Reid, C.M.; et al. Medical Therapies for Heart Failure With Preserved Ejection Fraction. Hypertension 2020, 75, 23–32. [Google Scholar] [CrossRef]
- Perlini, S.; Palladini, G.; Ferrero, I.; Tozzi, R.; Fallarini, S.; Facoetti, A.; Nano, R.; Clari, F.; Busca, G.; Fogari, R.; et al. Sympathectomy or doxazosin, but not propranolol, blunt myocardial interstitial fibrosis in pressure-overload hypertrophy. Hypertension 2005, 46, 1213–1218. [Google Scholar] [CrossRef]
- Brandt, M.C.; Mahfoud, F.; Reda, S.; Schirmer, S.H.; Erdmann, E.; Bohm, M.; Hoppe, U.C. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J. Am. Coll. Cardiol. 2012, 59, 901–909. [Google Scholar] [CrossRef]
- Mahfoud, F.; Urban, D.; Teller, D.; Linz, D.; Stawowy, P.; Hassel, J.H.; Fries, P.; Dreysse, S.; Wellnhofer, E.; Schneider, G.; et al. Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: Data from a multi-centre cardiovascular magnetic resonance imaging trial. Eur. Heart J. 2014, 35, 2224–2231b. [Google Scholar] [CrossRef]
- Fudim, M.; Sobotka, P.A.; Piccini, J.P.; Patel, M.R. Renal Denervation for Patients with Heart Failure: Making a Full Circle. Circ. Heart Fail. 2021, 14, e008301. [Google Scholar] [CrossRef]
- Bhave, P.D. Renal Denervation Therapy for the Treatment of Arrhythmias: Is the Sky the Limit? J. Am. Heart Assoc. 2017, 6, e005342. [Google Scholar] [CrossRef]
- Bradfield, J.S.; Vaseghi, M.; Shivkumar, K. Renal denervation for refractory ventricular arrhythmias. Trends Cardiovasc. Med. 2014, 24, 206–213. [Google Scholar] [CrossRef]
- Dai, Z.; Yu, S.; Zhao, Q.; Meng, Y.; He, H.; Tang, Y.; Wang, X.; Xiao, J.; Wang, X.; Huang, C. Renal sympathetic denervation suppresses ventricular substrate remodelling in a canine high-rate pacing model. EuroIntervention 2014, 10, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Wirth, K.; Ukena, C.; Mahfoud, F.; Poss, J.; Linz, B.; Bohm, M.; Neuberger, H.R. Renal denervation suppresses ventricular arrhythmias during acute ventricular ischemia in pigs. Heart Rhythm 2013, 10, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Zhou, Q.N.; Lu, Y.M.; Li, Y.D.; Zhang, L.; Zhang, J.H.; Xing, Q.; Lv, W.K.; Cheng, X.C.; Zhang, G.G.; et al. Renal Denervation Reduced Ventricular Arrhythmia After Myocardial Infarction by Inhibiting Sympathetic Activity and Remodeling. J. Am. Heart Assoc. 2018, 7, e009938. [Google Scholar] [CrossRef] [PubMed]
- Pokushalov, E.; Romanov, A.; Corbucci, G.; Artyomenko, S.; Baranova, V.; Turov, A.; Shirokova, N.; Karaskov, A.; Mittal, S.; Steinberg, J.S. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J. Am. Coll. Cardiol. 2012, 60, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Remo, B.F.; Preminger, M.; Bradfield, J.; Mittal, S.; Boyle, N.; Gupta, A.; Shivkumar, K.; Steinberg, J.S.; Dickfeld, T. Safety and efficacy of renal denervation as a novel treatment of ventricular tachycardia storm in patients with cardiomyopathy. Heart Rhythm 2014, 11, 541–546. [Google Scholar] [CrossRef]
- Hawson, J.; Harmer, J.A.; Cowan, M.; Virk, S.; Campbell, T.; Bennett, R.G.; Anderson, R.D.; Kalman, J.; Lee, G.; Kumar, S. Renal Denervation for the Management of Refractory Ventricular Arrhythmias: A Systematic Review. JACC Clin. Electrophysiol. 2021, 7, 100–108. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Fudim, M.; Sobotka, A.A.; Yin, Y.H.; Wang, J.W.; Levin, H.; Esler, M.; Wang, J.; Sobotka, P.A. Selective vs. Global Renal Denervation: A Case for Less Is More. Curr. Hypertens. Rep. 2018, 20, 37. [Google Scholar] [CrossRef]
| Study | N, Population | Clinical Findings |
|---|---|---|
| Gao et al. (2019) [36] | 60, Single-center RCT, EF < 40% | 30 patients in RDN group. At 6 months, compared to control group, RDN was associated with significant increase in LVEF, decrease in NT-ProBNP, BP, and NYHA class. No significant changes in glomerular filtration between two groups. |
| Drożdż et al. (2019) [37] | 20, Single-center RCT, EF < 35% | There were no significant differences in LVEF, BP, 6MWT and NT-proBNP concentration at 6 and 12 months after RD or control. |
| Chen et al. (2017) [35] | 60, Single-center RCT, EF < 40% | 30 patients RDN group. At 6 months, compared to control group, RDN was associated with significant improvement in symptoms, BP, quality of life, LVEF, NT-ProBNP, and NYHA class. No significant changes in glomerular filtration nor complication of renal artery stenosis were observed. |
| Gao et al. (2017) [38] | 14, Single-arm, EF < 45% | There was a significant decrease in symptoms and improvement in 6-min walk test with increase in LVEF at 6 months follow up. No RDN-related complications were observed during the follow-up period. Additionally, there was significant improvement in BP and GFR remained stable. |
| Hopper et al. (2017) [39] | 39, Multi-center, Single-arm, EF < 40% | RDN was associated with reductions in NT-proBNP and 120-min glucose tolerance test in HF patients 12 months after RDN treatment. No significant change in LVEF, 6 min walk test of GFR. |
| Dai et al. (2015) [40] | 20, Single-center, Single-arm, EF < 40% | No obvious change in heart rate or blood pressure was recorded. Symptoms of heart failure were improved in patients after RDN. No complications were recorded in the study. |
| Davis et al. (2013) [34] | 7, Single-center, Single-arm, EF < 40% | Over 6 months there was a non-significant trend in blood pressure reduction. No hypotensive or syncopal episodes were reported. Renal function remained stable. There was a significant improvement in symptoms and a 6-min walk test. |
| Xia et al. (2022) [26] | 220, meta-analysis of the above studies | Bilateral RDN increased the LVEF, decreased the LVESD, and decreased the LVEDD. In addition, RDN significantly decreased systolic and diastolic BP and decreased HR. RDN did not significantly change GFR or serum creatinine levels. The mean 6-min walk test was increased and NT-pro BNP was decreased. |
| Study | N, Population | Clinical Findings |
|---|---|---|
| Brandt et al. (2012) [46] | 64, Single-center non-randomized study, EF > 55% | 46 patients and 18 controls. RDN significantly reduced BP, and LV mass and improved diastolic function at 1 and 6 months. |
| Mahfoud et al. (2014) [47] | 16, Multi-center non-randomized study, EF > 55% | Significant improvement in global longitudinal strain at 6 months. Reduction in left ventricular mass index suggesting an improved diastolic function. |
| Kresoja et al. (2021) [27] | 66, Single center, single-arm, EF > 55% | Patients with HFpEF undergoing RDN showed reduced BP, and increased stroke volume index. LV diastolic stiffness and LV filling pressures as well as NT-proBNP decreased. |
| Study | N, Population | Clinical Outcomes |
|---|---|---|
| UNLOAD-HFpEF | 68, randomized, sham-controlled double-blind design, EF > 55% | Assess the hemodynamic effects of RDN in patients with HFpEF. Effect of RDN on a combination of death, increase in diuretic therapy, hospitalization for heart failure, worsening NYHA-class, change in pulmonary pressure parameters. |
| RE-ADAPT-HF | 144, Prospective, randomized, double-blind, sham-controlled, multicenter. EF < 45% | 6-min walk test, Change in NT-pro-BNP, e-GFR, KCCQ at 6 months. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassab, K.; Soni, R.; Kassier, A.; Fischell, T.A. The Potential Role of Renal Denervation in the Management of Heart Failure. J. Clin. Med. 2022, 11, 4147. https://doi.org/10.3390/jcm11144147
Kassab K, Soni R, Kassier A, Fischell TA. The Potential Role of Renal Denervation in the Management of Heart Failure. Journal of Clinical Medicine. 2022; 11(14):4147. https://doi.org/10.3390/jcm11144147
Chicago/Turabian StyleKassab, Kameel, Ronak Soni, Adnan Kassier, and Tim A. Fischell. 2022. "The Potential Role of Renal Denervation in the Management of Heart Failure" Journal of Clinical Medicine 11, no. 14: 4147. https://doi.org/10.3390/jcm11144147
APA StyleKassab, K., Soni, R., Kassier, A., & Fischell, T. A. (2022). The Potential Role of Renal Denervation in the Management of Heart Failure. Journal of Clinical Medicine, 11(14), 4147. https://doi.org/10.3390/jcm11144147






