Assessment of Antibiotic Pharmacokinetics, Molecular Biomarkers and Clinical Status in Critically Ill Adults Diagnosed with Community-Acquired Pneumonia and Receiving Intravenous Piperacillin/Tazobactam and Hydrocortisone over the First Five Days of Intensive Care: An Observational Study (STROBE Compliant)
Abstract
:1. Introduction
1.1. Background
1.2. Objectives of the Observational Study
2. Methods and Analysis
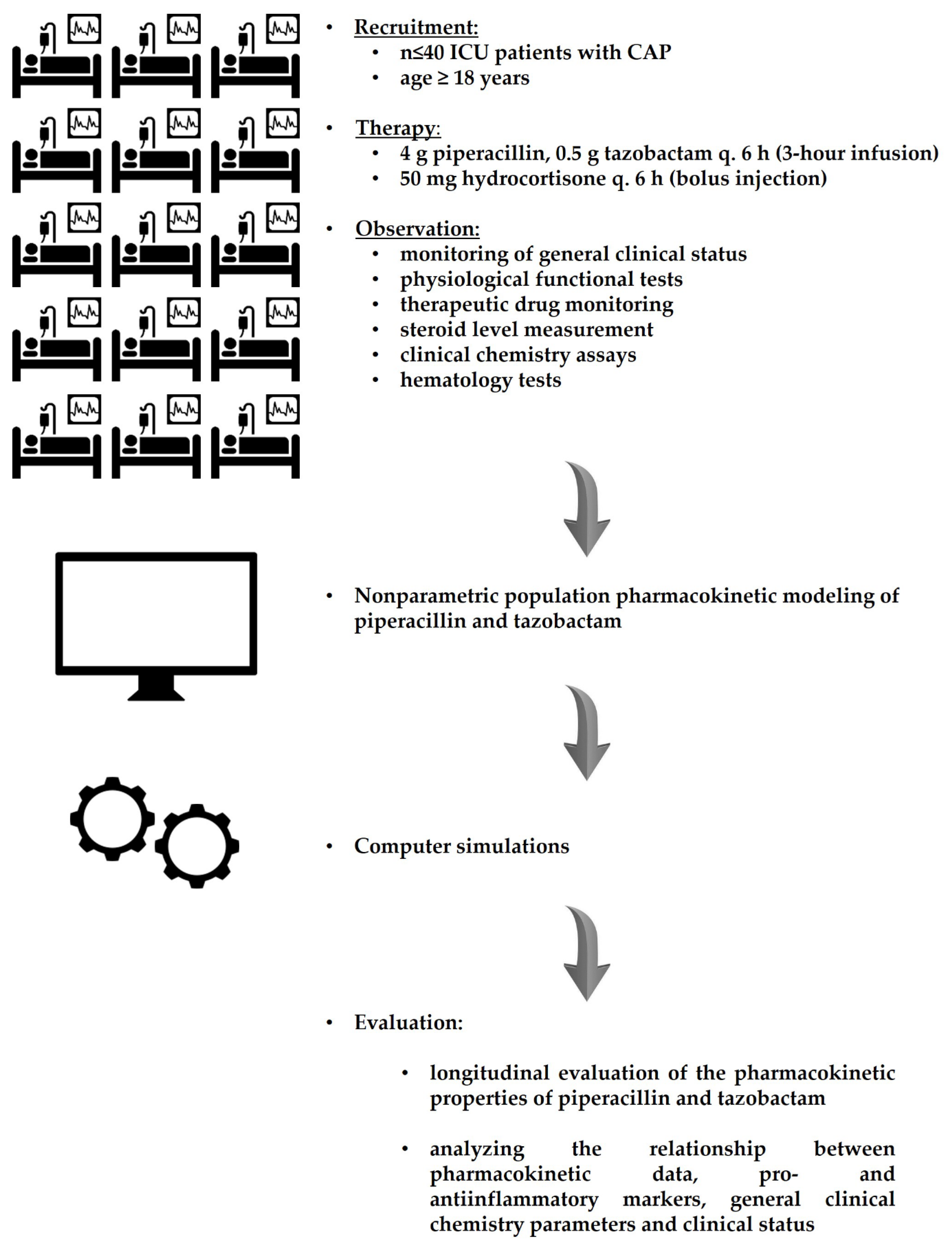
2.1. Observational Study Design
2.2. Participants, Eligibility Criteria and Exit Events
2.3. Protocol of Treatment
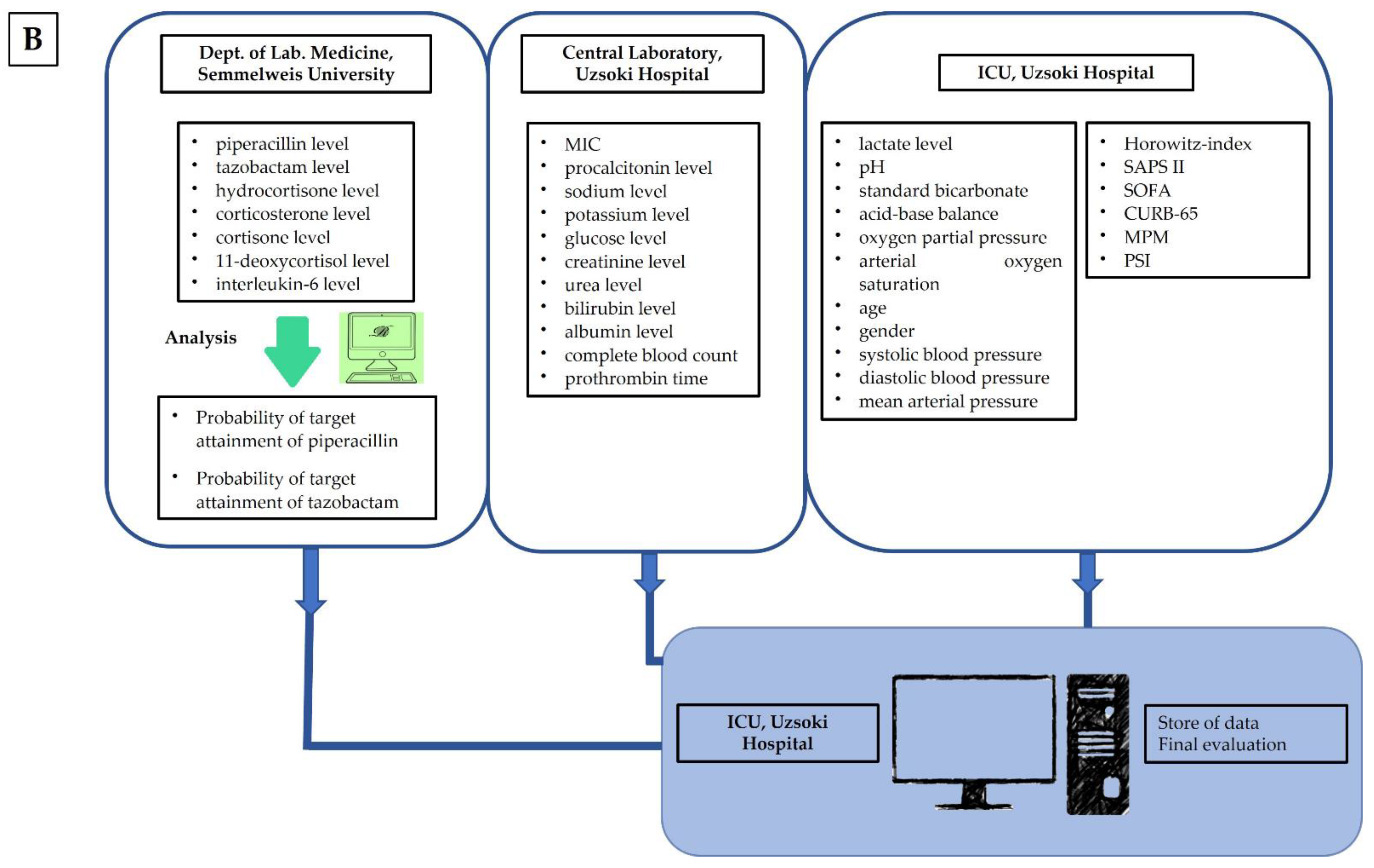
2.4. Sample Collection
2.4.1. Sample ‘A’: Tracheal Secretions
2.4.2. E-Test Method of Antibiotic Susceptibility, MIC Value
2.4.3. Samples ‘B/1-1′–‘B/1-6′: Native Blood Sample
2.4.4. Sample ‘C’: Native Blood
2.4.5. Sample ‘D’: Blood Anticoagulated with Ion-Balanced Heparin
2.4.6. Sample ‘E’: Blood Anticoagulated with K3-EDTA
2.4.7. Sample ‘F’: Blood Anticoagulated with Sodium Citrate
2.5. Disposal of Samples
2.6. Protocols of Intervention
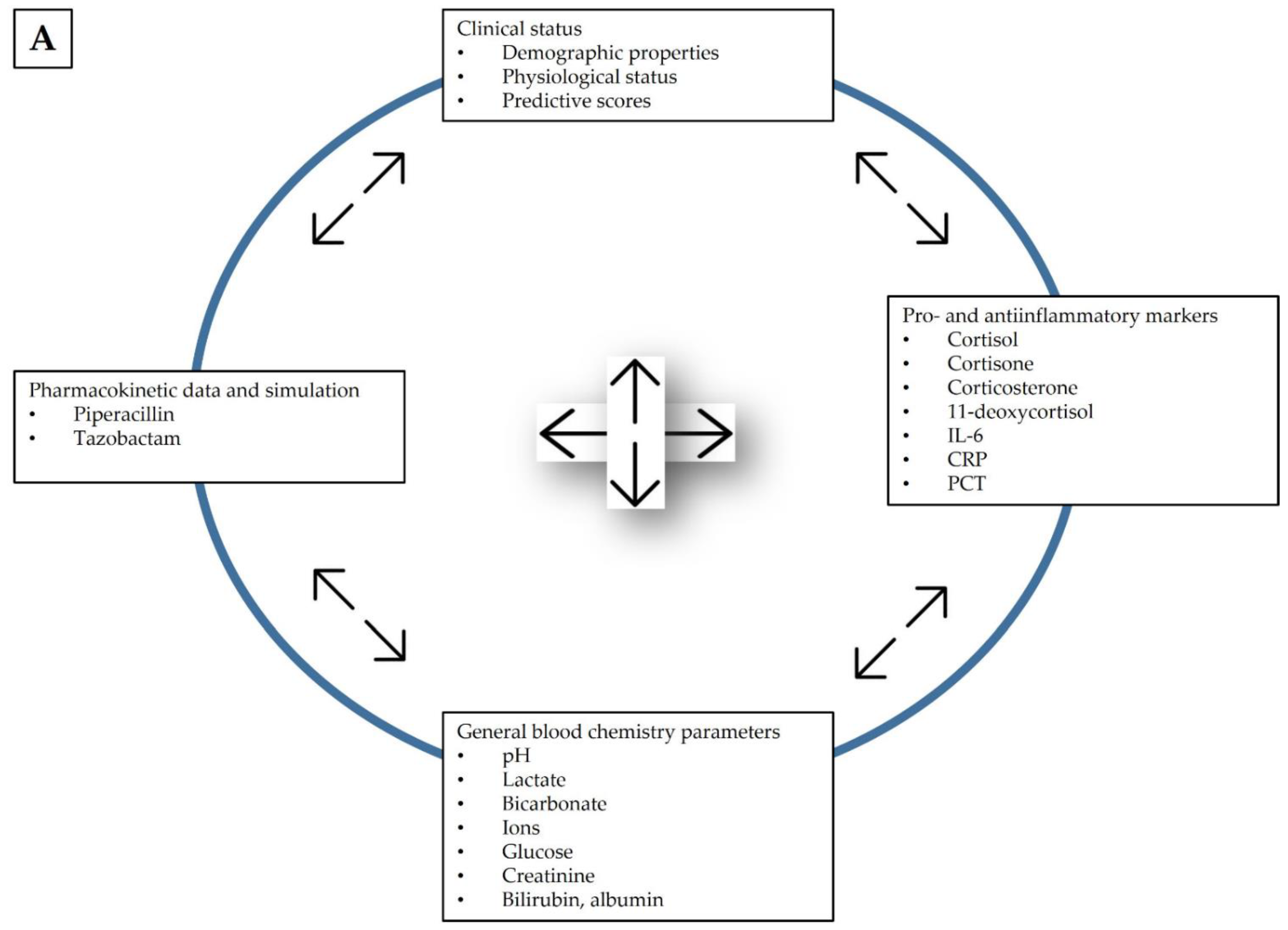
2.7. Observed Parameters
2.8. Data Evaluation
2.9. Endpoints of the Observational Study
- Primary endpoints: (1) PK parameters of piperacillin and tazobactam and (2) the possible changes in concentrations of the monitored endogenous steroids during the five treatment days.
- Secondary endpoints: Based on Monte-Carlo simulation the probability target attainment (PTA) of piperacillin and tazobactam.
- Tertiary endpoints: the association between individual pharmacokinetic parameters of piperacillin and tazobactam and individual laboratory parameters that characterize physiological status.
2.10. Sample Size
2.11. Adverse Events
3. Ethics and Dissemination
3.1. Ethical Considerations
3.2. Score of Observations and Presentation Format
3.3. Data Availability
3.4. Limitations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cillóniz, C.; Ewig, S.; Ferrer, M.; Polverino, E.; Gabarrús, A.; Puig de la Bellacasa, J.; Mensa, J.; Torres, A. Community-acquired polymicrobial pneumonia in the intensive care unit: Aetiology and prognosis. Crit. Care. 2011, 15, R209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sligl, W.I.; Eurich, D.T.; Marrie, T.J.; Majumdar, S.R. Age still matters prognosticating short- and long-term mortality for critically ill patients with pneumonia. Crit. Care Med. 2010, 38, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Woodhead, M.; Welch, C.A.; Harrison, D.A.; Bellingan, G.; Ayres, J.G. Community-acquired pneumonia on the intensive care unit: Secondary analysis of 17,869 cases in the ICNARC Case Mix Programme Database. Crit. Care. 2006, 10 (Suppl. S2), S1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Confalonieri, M.; Urbino, R.; Potena, A.; Piattella, M.; Parigi, P.; Puccio, G.; Della Porta, R.; Fiorgio, C.; Blasi, F.; Umberger, R.; et al. Hydrocortisone infusion for severe community-acquired pneumonia: A preliminary randomized study. Am. J. Respir. Crit. Care Med. 2005, 171, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Kellum, J.A.; Kong, L.; Fink, M.P.; Weissfeld, L.A.; Yealy, D.M.; Pinsky, M.R.; Fine, J.; Krichevsky, A.; Delude, R.L.; Angus, D.C.; et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch. Intern. Med. 2007, 167, 1655–1663. [Google Scholar] [CrossRef] [Green Version]
- Stern, A.; Skalsky, K.; Avni, T.; Carrara, E.; Leibovici, L.; Paul, M. Corticosteroids for pneumonia. Cochrane Database Syst. Rev. 2017, 12, Cd007720. [Google Scholar] [CrossRef]
- Annane, D.; Sébille, V.; Charpentier, C.; Bollaert, P.E.; François, B.; Korach, J.M.; Capellier, G.; Cohen, Y.; Azoulay, E.; Troché, G.; et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002, 288, 862–871. [Google Scholar] [CrossRef] [Green Version]
- Oppert, M.; Schindler, R.; Husung, C.; Offermann, K.; Gräf, K.J.; Boenisch, O.; Barckow, O.; Frei, D.; Eckardt, T.; Kai, U. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit. Care Med. 2005, 33, 2457–2464. [Google Scholar] [CrossRef]
- Sprung, C.L.; Annane, D.; Keh, D.; Moreno, R.; Singer, M.; Freivogel, K.; Weiss, Y.G.; Benbenishty, Y.; Kalenka, A.; Forst, H.; et al. Hydrocortisone therapy for patients with septic shock. N. Engl. J. Med. 2008, 358, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Perry, C.M.; Markham, A. Piperacillin/tazobactam: An updated review of its use in the treatment of bacterial infections. Drugs 1999, 57, 805–843. [Google Scholar] [CrossRef]
- Sörgel, F.; Kinzig, M. Pharmacokinetic characteristics of piperacillin/tazobactam. Intensive Care Med. 1994, 20 (Suppl. S3), S14–S20. [Google Scholar] [CrossRef]
- Sörgel, F.; Kinzig, M. The chemistry, pharmacokinetics and tissue distribution of piperacillin/tazobactam. J. Antimicrob. Chemother. 1993, 31 (Suppl. SA), 39–60. [Google Scholar] [CrossRef] [PubMed]
- Honoré, P.; Jacobs, R.; Regt, J.; Rose, T.; Spapen, H.; Waele, E. Steroid pharmacokinetics: An “overlooked” issue of steroid metabolism in acute and chronic disease. J. Transl. Intern. Med. 2014, 2, 51. [Google Scholar] [CrossRef]
- Sibila, O.; Agustí, C.; Torres, A. Corticosteroids in severe pneumonia. Eur. Respir. J. 2008, 32, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udy, A.A.; Lipman, J.; Jarrett, P.; Klein, K.; Wallis, S.C.; Patel, K.; Kirkpatrick, C.M.; Kruger, P.S.; Paterson, D.L.; Roberts, M.S.; et al. Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit. Care. 2015, 19, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiga, R.P.; Paiva, J.A. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care. 2018, 22, 233. [Google Scholar] [CrossRef] [Green Version]
- Mouton, J.W.; Brown, D.F.; Apfalter, P.; Cantón, R.; Giske, C.G.; Ivanova, M.; MacGowan, A.P.; Rodloff, A.; Soussy, C.-J.; Steinbakk, M.; et al. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: The EUCAST approach. Clin. Microbiol. Infect. 2012, 18, E37–E45. [Google Scholar] [CrossRef] [Green Version]
- EMA. Guideline on the Use of Pharmacokinetics and Pharmacodynamics in the Development of Antimicrobial Medicinal Products; EMA/CHMP/594085/2015; EMA: Amsterdam, The Netherlands, 2016.
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.; Michels, G.; Kluge, S.; et al. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: A randomized controlled trial. Intensiv. Care Med. 2022, 48, 311–321. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Strobe Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [Green Version]
- Kim, V.; Aaron, S.D. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. Eur. Respir. J. 2018, 52, 1801261. [Google Scholar] [CrossRef]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44 (Suppl. S2), S27–S72. [Google Scholar] [CrossRef] [PubMed]
- Pletz, M.W.; Blasi, F.; Chalmers, J.D.; Dela Cruz, C.S.; Feldman, C.; Luna, C.M.; Ramirez, J.A.; Shindo, Y.; Stolz, D.; Torres, A.; et al. International Perspective on the New 2019 American Thoracic Society/Infectious Diseases Society of America Community-Acquired Pneumonia Guideline: A Critical Appraisal by a Global Expert Panel. Chest 2020, 158, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Babu, B.L.; Reyes, L.F.; Chalmers, J.D.; Soni, N.J.; Sibila, O.; Faverio, P.; Cilloniz, C.; Rodriguez-Cintron, W.; Aliberti, S. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: A multinational point prevalence study of hospitalised patients. Eur. Respir. J. 2018, 52, 1701190. [Google Scholar] [CrossRef] [Green Version]
- Baquero, F.; Cantón, R.; Martinez-Beltrán, J.; Bolmström, A. The E-Test as an epidemiologic tool. Diagn. Microbiol. Infect. Dis. 1992, 15, 483–487. [Google Scholar] [CrossRef]
- Chromsystems, Instruments & Chemicals GmbH. Antibiotics in Serum/Plasma—HPLC. 2022. Available online: https://chromsystems.com/en/antibiotics-in-serum-plasma-hplc-61000.html (accessed on 7 April 2022).
- Karvaly, G.; Kovacs, K.; Meszaros, K.; Kocsis, I.; Patocs, A.; Vasarhelyi, B. The comprehensive characterization of adrenocortical steroidogenesis using two-dimensional ultra-performance liquid chromatography—Electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2018, 153, 274–283. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensiv. Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Lim, W.S.; van der Eerden, M.M.; Laing, R.; Boersma, W.G.; Karalus, N.; Town, G.I.; Lewis, S.A.; Macfarlane, J.T. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003, 58, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Fine, M.J.; Auble, T.E.; Yealy, D.M.; Hanusa, B.H.; Weissfeld, L.A.; Singer, D.E.; Coley, C.M.; Marrie, T.J.; Kapoor, W.N. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 1997, 336, 243–250. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Karvaly, G.B.; Neely, M.N.; Kovacs, K.; Vincze, I.; Vasarhelyi, B.; Jelliffe, R.W. Development of a methodology to make individual estimates of the precision of liquid chromatography-tandem mass spectrometry drug assay results for use in population pharmacokinetic modeling and the optimization of dosage regimens. PLoS ONE 2020, 15, e0229873. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Liu, G.; Wang, J.; Aubry, A.F.; Arnold, M.E. Selecting the correct weighting factors for linear and quadratic calibration curves with least-squares regression algorithm in bioanalytical LC-MS/MS assays and impacts of using incorrect weighting factors on curve stability, data quality, and assay performance. Anal. Chem. 2014, 86, 8959–8966. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Neely, M.N.; van Guilder, M.G.; Yamada, W.M.; Schumitzky, A.; Jelliffe, R.W. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther. Drug Monit. 2012, 34, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Leary, R.; Jelliffe, R.; Schumitzky, A.; Guilder, M. An adaptive grid non-parametric approach to pharmacokinetic and dynamic (PK/PD) population models2001. In Proceedings of the 14th IEEE Symposium on Computer-Based Medical Systems, Bethesda, MD, USA, 26–27 July 2001; pp. 389–394. [Google Scholar]
- Wallenburg, E.; Ter Heine, R.; Schouten, J.A.; Raaijmakers, J.; Ten Oever, J.; Kolwijck, E.; Burger, D.M.; Pickkers, P.; Frenzel, T.; Brüggemann, R.J.M. An Integral Pharmacokinetic Analysis of Piperacillin and Tazobactam in Plasma and Urine in Critically Ill Patients. Clin. Pharmacokinet. 2022, 61, 907–918. [Google Scholar] [CrossRef] [PubMed]
 ). Sample and aliquot identifiers are displayed in colored boxes.
). Sample and aliquot identifiers are displayed in colored boxes.
 ). Sample and aliquot identifiers are displayed in colored boxes.
). Sample and aliquot identifiers are displayed in colored boxes.
| Level of Significance | Description |
|---|---|
| Minor | Respiratory rate ≥ 30 breaths/min PaO2/FiO2 ratio ≤ 250 Multilobar infiltrates Confusion/disorientation Uremia (blood urea nitrogen (BUN)) level ≥ 7.14 mmol/L Leukopenia (white blood cell (WBC) count < 4 G/L) Thrombocytopenia (platelet count < 100 G/L) Hypothermia (core temperature < 36 °C) Hypotension requiring aggressive fluid resuscitation |
| Major | Invasive mechanical ventilation Septic shock with the need for vasopressors |
| Test | Method | Days of Sampling | Time of Sampling | Sample Identifier |
|---|---|---|---|---|
| MIC | IST (E-test) | 1; 4 | ordered by principal investigator | A |
| piperacillin level | HPLC-UV | 1–5 | 3.25, 3.5, 4, 4.5, 5, and 5.5 h after administration of the 1st infusion of the day | B/1 |
| tazobactam level | HPLC-UV | 1–5 | 3.25, 3.5, 4, 4.5, 5, and 5.5 h after administration of the 1st infusion of the day | B/1 |
| hydrocortisone (cortisol) level | LC-MS/MS | 1–5 | 5.5 h after administration of the 1st infusion of the day | B/2 |
| corticosterone level | LC-MS/MS | 1–5 | 5.5 h after administration of the 1st infusion of the day | B/2 |
| cortisone | LC-MS/MS | 1–5 | 5.5 h after administration of the 1st infusion of the day | B/2 |
| 11-deoxycortisol level | LC-MS/MS | 1–5 | 5.5 h after administration of the 1st infusion of the day | B/2 |
| interleukin-6 level | CA | 1–5 | before administration of the 1st infusion of the day | C/1 |
| procalcitonin level | CA | 1–5 | before administration of the 1st infusion of the day | C/2 |
| sodium level | CA | 1–5 | before administration of the 1st infusion of the day | C/2 |
| potassium level | CA | 1–5 | before administration of the 1st infusion of the day | C/2 |
| glucose level | CA | 1–5 | before administration of the 1st infusion of the day | C/2 |
| creatinine level | CA | 1–5 | before administration of the 1st infusion of the day | C/2 |
| urea level | CA | 1–5 | before administration of the 1st infusion of the day | C/2 |
| bilirubin level | CA | 1–5 | before administration of the 1st infusion of the day | C/2 |
| albumin level | CA | 1–5 | before administration of the 1st infusion of the day | C/2 |
| lactate level | BGA | 1–5 | ordered by principal investigator | D |
| pH | BGA | 1–5 | ordered by principal investigator | D |
| standard bicarbonate | BGA | 1–5 | ordered by principal investigator | D |
| acid-base balance | BGA | 1–5 | ordered by principal investigator | D |
| oxygen partial pressure | BGA | 1–5 | ordered by principal investigator | D |
| arterial oxygen saturation | BGA | 1–5 | ordered by principal investigator | D |
| complete blood count | hematology blood analyser | 1–5 | before administration of the 1st infusion of the day | E |
| prothrombin time | coagulometer | 1–5 | before administration of the 1st infusion of the day | F |
| Variable | Category | Variable Type | Source/Method/Instrument/Stand |
|---|---|---|---|
| Age (Years) | Discrete | Descriptive | Patient record |
| Gender (Male/female) | Dichotomous | Descriptive | Patient record |
| Body mass index (kg m−2) | Continuous | Descriptive | Observation (height, weight) and calculation |
| Systolic blood pressure (mmHg) | Continuous | Descriptive | Observation |
| Diastolic blood pressure (mmHg) | Continuous | Descriptive | Observation |
| Mean arterial pressure (mmHg) | Continuous | Descriptive | Observation |
| Horowitz-index (no unit) | Continuous | Descriptive | Calculation |
| SAPS II (no unit) | Discrete | Predictive | Calculation |
| SOFA (no unit) | Discrete | Predictive | Calculation |
| CURB-65 (no unit) | Discrete | Predictive | Calculation |
| MPM (no unit) | Discrete | Predictive | Calculation |
| PSI (no unit) | Discrete | Predictive | Calculation |
| Serum IL-6 (pg mL−1) | Continuous | Descriptive | Observation (Laboratory measurement) |
| Procalcitonin (µg mL−1) | Continuous | Descriptive | Observation (Laboratory measurement) |
| Serum cortisol concentration (ng mL−1) | Continuous | Primary outcome | Observation (Laboratory measurement) |
| Serum cortisone concentration (ng mL−1) | Continuous | Primary outcome | Observation (Laboratory measurement) |
| Serum corticosterone concentration (ng mL−1) | Continuous | Primary outcome | Observation (Laboratory measurement) |
| Serum 11-deoxicortisol concentration (ng mL−1) | Continuous | Primary outcome | Observation (Laboratory measurement) |
| PTA of piperacillin (%) | Complex | Primary outcome | Observation (piperacillin concentration, minimal inhibitory concentration) and calculation |
| PTA of tazobactam (%) | Complex | Primary outcome | Observation (tazobactam concentration, minimal inhibitory concentration) and calculation |
| 5-day ICU mortality (%) | Continuous | Secondary outcome | Observation |
| Length of period without ventilation (Days) | Discrete | Descriptive | Observation |
| Length of catecholamine treatment-free period (Days) | Discrete | Descriptive | Observation |
| Length of insulin therapy (Days) | Discrete | Descriptive | Observation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincze, I.; Czermann, R.; Nagy, Z.; Kovács, M.; Neely, M.; Farkas, R.; Kocsis, I.; Karvaly, G.B.; Kopitkó, C. Assessment of Antibiotic Pharmacokinetics, Molecular Biomarkers and Clinical Status in Critically Ill Adults Diagnosed with Community-Acquired Pneumonia and Receiving Intravenous Piperacillin/Tazobactam and Hydrocortisone over the First Five Days of Intensive Care: An Observational Study (STROBE Compliant). J. Clin. Med. 2022, 11, 4140. https://doi.org/10.3390/jcm11144140
Vincze I, Czermann R, Nagy Z, Kovács M, Neely M, Farkas R, Kocsis I, Karvaly GB, Kopitkó C. Assessment of Antibiotic Pharmacokinetics, Molecular Biomarkers and Clinical Status in Critically Ill Adults Diagnosed with Community-Acquired Pneumonia and Receiving Intravenous Piperacillin/Tazobactam and Hydrocortisone over the First Five Days of Intensive Care: An Observational Study (STROBE Compliant). Journal of Clinical Medicine. 2022; 11(14):4140. https://doi.org/10.3390/jcm11144140
Chicago/Turabian StyleVincze, István, Rita Czermann, Zsuzsanna Nagy, Mária Kovács, Michael Neely, Róbert Farkas, Ibolya Kocsis, Gellért Balázs Karvaly, and Csaba Kopitkó. 2022. "Assessment of Antibiotic Pharmacokinetics, Molecular Biomarkers and Clinical Status in Critically Ill Adults Diagnosed with Community-Acquired Pneumonia and Receiving Intravenous Piperacillin/Tazobactam and Hydrocortisone over the First Five Days of Intensive Care: An Observational Study (STROBE Compliant)" Journal of Clinical Medicine 11, no. 14: 4140. https://doi.org/10.3390/jcm11144140
APA StyleVincze, I., Czermann, R., Nagy, Z., Kovács, M., Neely, M., Farkas, R., Kocsis, I., Karvaly, G. B., & Kopitkó, C. (2022). Assessment of Antibiotic Pharmacokinetics, Molecular Biomarkers and Clinical Status in Critically Ill Adults Diagnosed with Community-Acquired Pneumonia and Receiving Intravenous Piperacillin/Tazobactam and Hydrocortisone over the First Five Days of Intensive Care: An Observational Study (STROBE Compliant). Journal of Clinical Medicine, 11(14), 4140. https://doi.org/10.3390/jcm11144140








