Bone Metabolism Alteration in Patients with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical, Sociodemographic, and Laboratory Variables
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. Baseline Assessment of Calcium-Phosphate Metabolism Indicators in the Whole Study Group
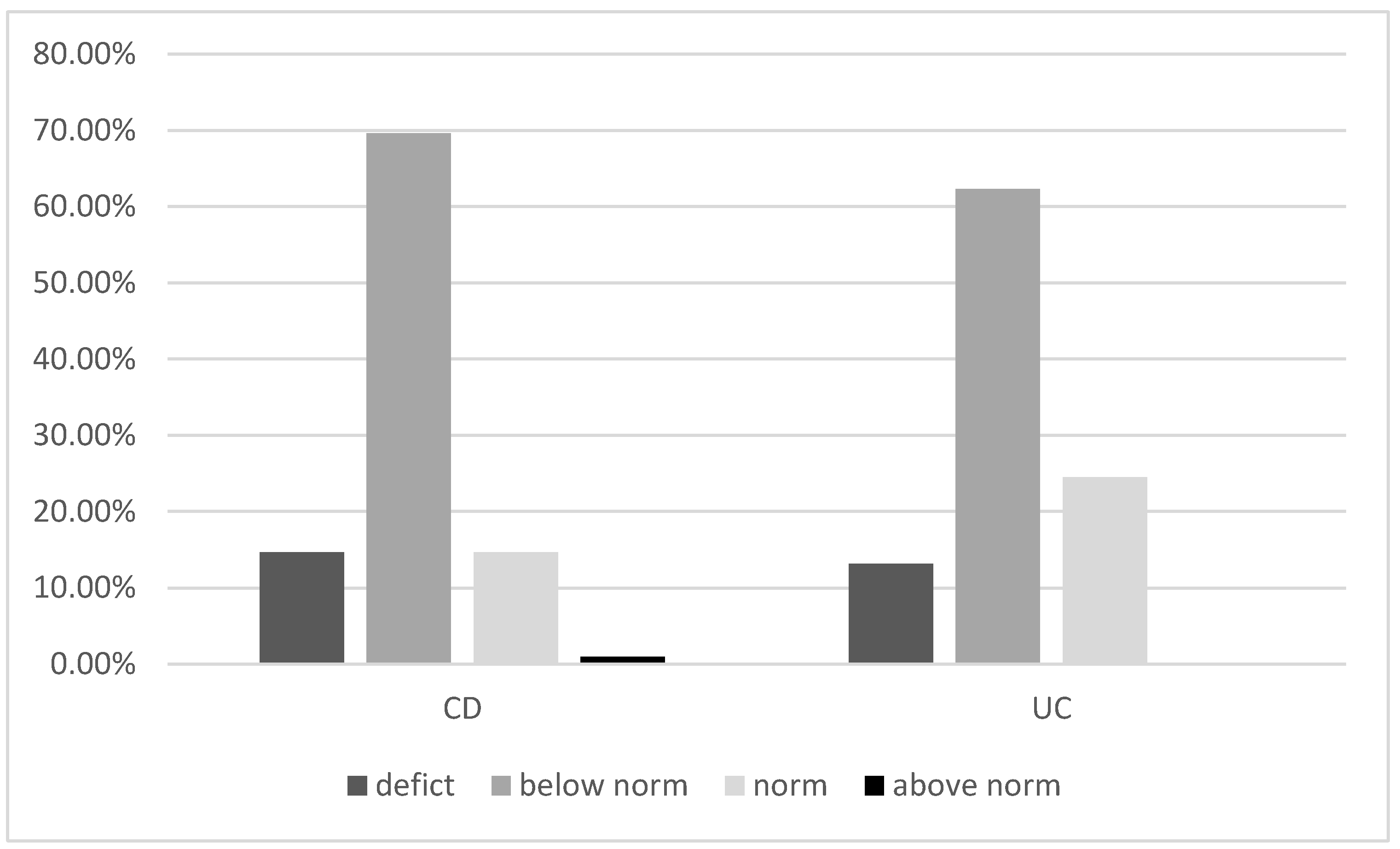
3.2. Vitamin D Deficiency and PTH Levels
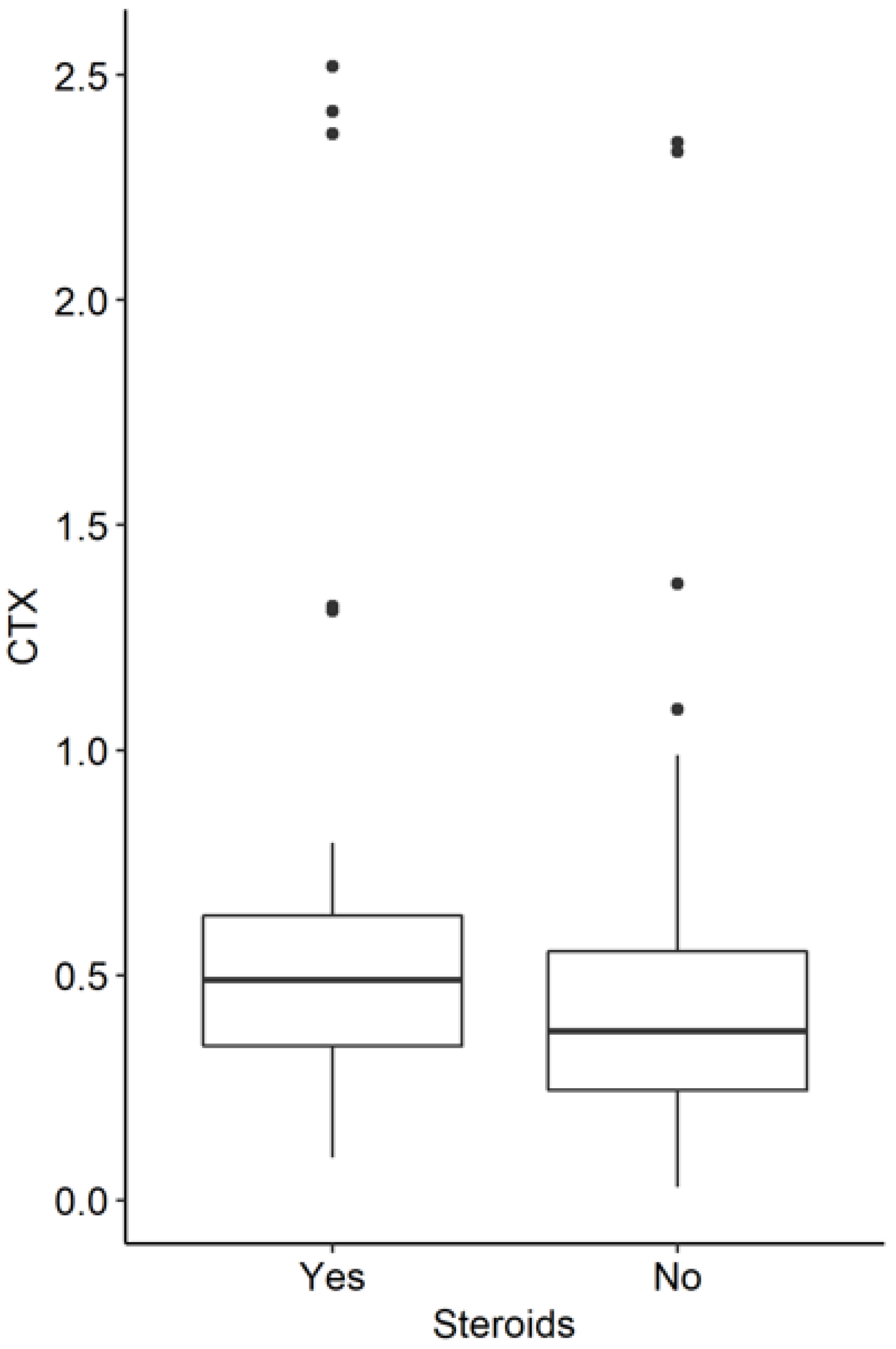
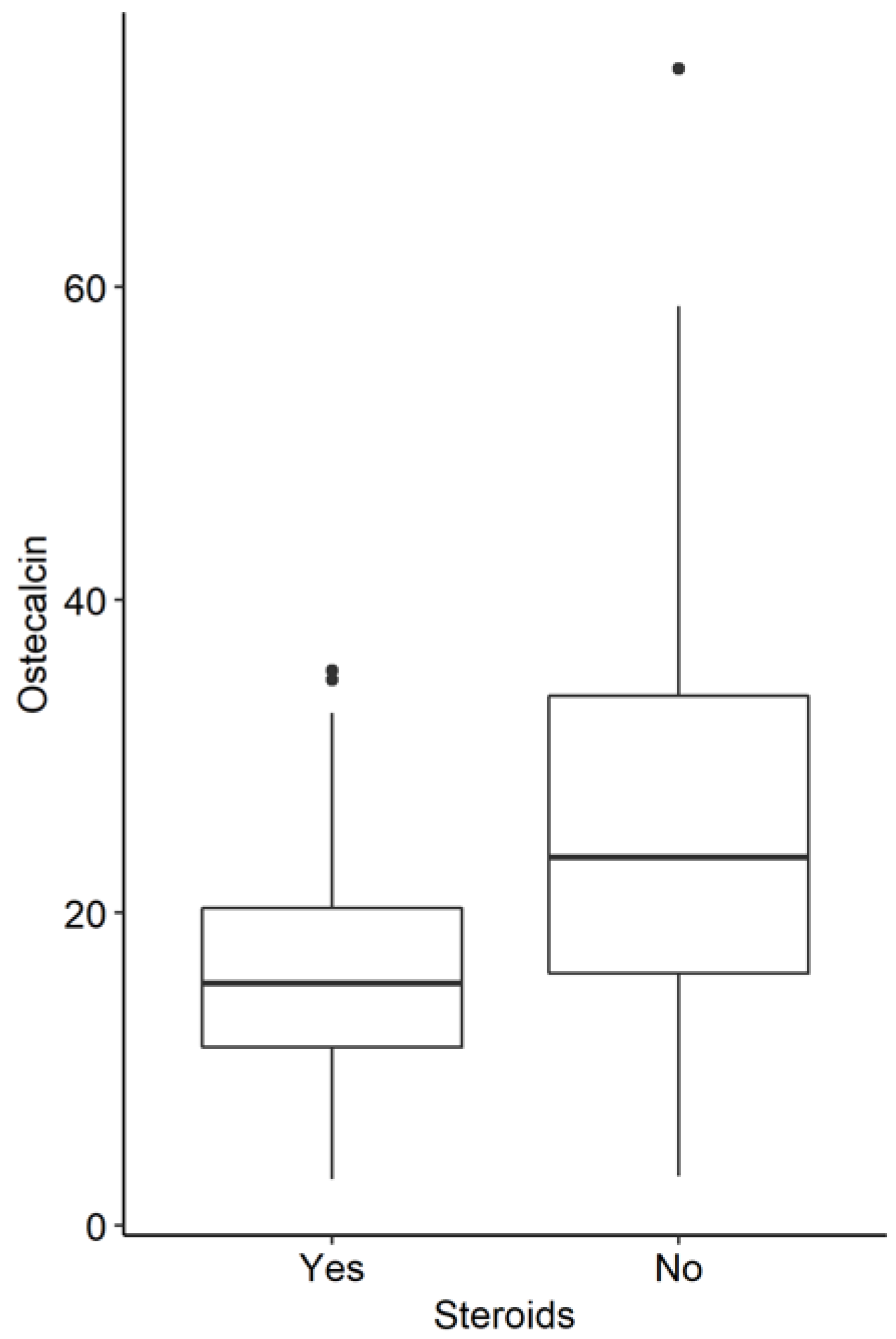
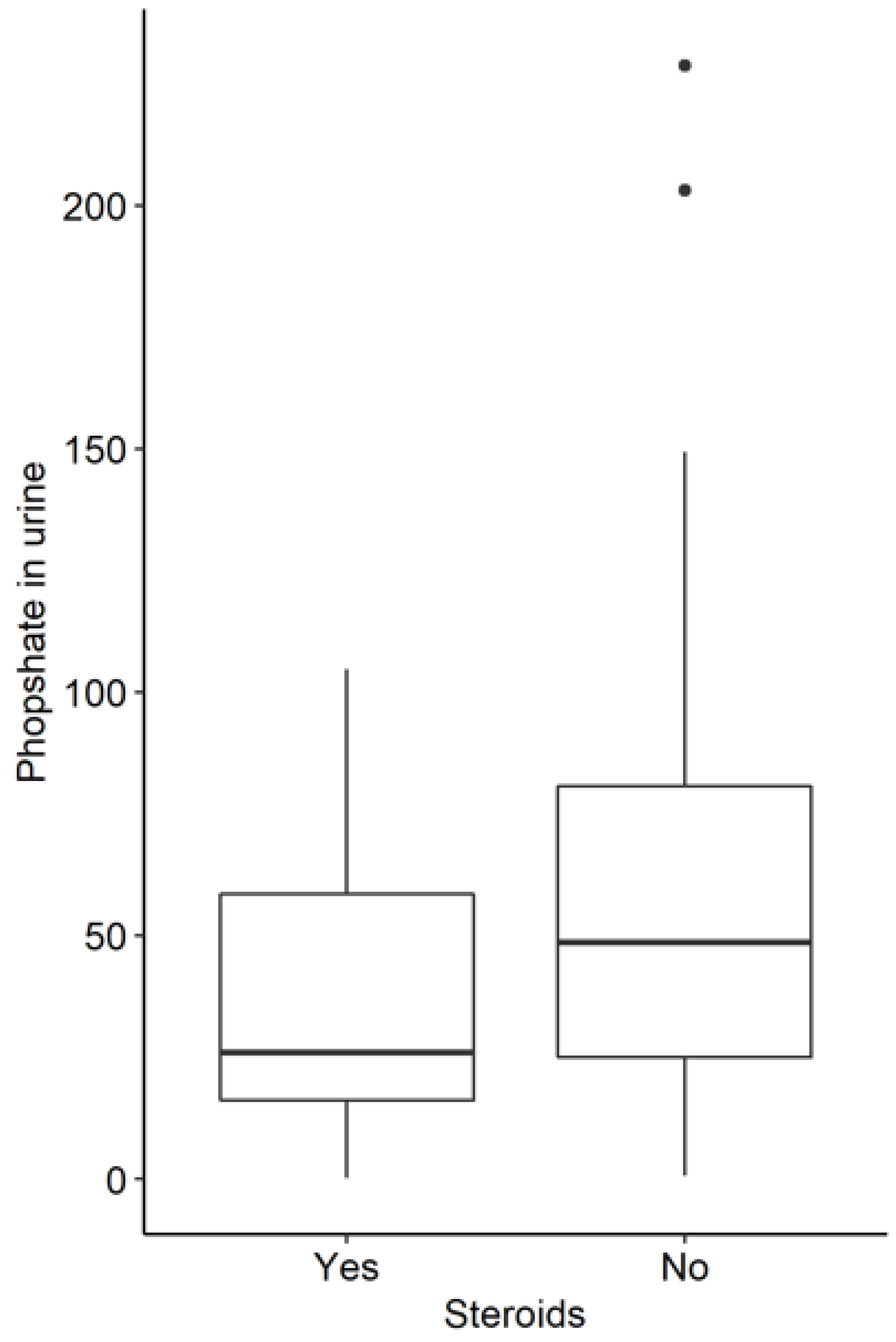
3.3. Influence of Corticoid Therapy in the Whole Study Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaplan, G.G.; Bernstein, C.N.; Coward, S.; Bitton, A.; Murthy, S.K.; Nguyen, G.C.; Lee, K.; Cooke-Lauder, J.; Benchimol, E.I. The Impact of Inflammatory Bowel Disease in Canada 2018: Epidemiology. J. Can. Assoc. Gastroenterol. 2019, 2 (Suppl. S1), S6–S16. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, K.B.; Barańska, D.; Grzelak, P.; Czkwianianc, E.; Szabelska-Zakrzewska, K. Up-to-date overview of imaging techniques in the diagnosis and management of inflammatory bowel diseases. Prz. Gastroenterol. 2019, 14, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42; quiz e30. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, S.; Matuszczyk, M.; Osuch, M.; Meglicka, M.; Szymanska, E.; Bierła, J.; Kierkuś, J. Inflammatory bowel disease—One entity with many molecular faces. Prz. Gastroenterol. 2019, 14, 228–232. [Google Scholar] [CrossRef]
- Lo, B.; Holm, J.P.; Vester-Andersen, M.K.; Bendtsen, F.; Vind, I.; Burisch, J. Incidence, Risk Factors and Evaluation of Osteoporosis in Patients with Inflammatory Bowel Disease: A Danish Population-Based Inception Cohort with 10 Years of Follow-Up. J. Crohns. Colitis. 2020, 14, 904–914. [Google Scholar] [CrossRef]
- Gionchetti, P.; Dignass, A.; Danese, S.; Magro Dias, F.J.; Rogler, G.; Lakatos, P.L.; Adamina, M.; Ardizzone, S.; Buskens, C.J.; Sebastian, S.; et al. Third European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J. Crohns. Colitis. 2017, 11, 135–149. [Google Scholar] [CrossRef]
- Oh, H.J.; Ryu, K.H.; Park, B.J.; Yoon, B.H. Osteoporosis and Osteoporotic Fractures in Gastrointestinal Disease. J. Bone Metab. 2018, 25, 213–217. [Google Scholar] [CrossRef]
- Bravenboer, N.; Oostlander, A.E.; van Bodegraven, A.A. Bone loss in patients with inflammatory bowel disease: Cause, detection and treatment. Curr. Opin. Gastroenterol. 2021, 37, 128–134. [Google Scholar] [CrossRef]
- Tan, B.; Li, P.; Lv, H.; Li, Y.; Wang, O.; Xing, X.P.; Qian, J.M. Vitamin D levels and bone metabolism in Chinese adult patients with inflammatory bowel disease. J. Dig. Dis. 2014, 15, 116–123. [Google Scholar] [CrossRef]
- Lima, C.A.; Lyra, A.C.; Rocha, R.; Santana, G.O. Risk factors for osteoporosis in inflammatory bowel disease patients. World J. Gastrointest. Pathophysiol. 2015, 6, 210–218. [Google Scholar] [CrossRef]
- Johansson, H.; Odén, A.; Kanis, J.A.; McCloskey, E.V.; Morris, H.A.; Cooper, C.; Vasikaran, S.; IFCC-IOF Joint Working Group on Standardisation of Biochemical Markers of Bone Turnover. A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcif. Tissue Int. 2014, 94, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Vasikaran, S.; Eastell, R.; Bruyère, O.; Foldes, A.J.; Garnero, P.; Griesmacher, A.; McClung, M.; Morris, H.A.; Silverman, S.; Trenti, T.; et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011, 22, 391–420. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Herrmann, A.; Göke, M.; Manns, M.P.; von zur Mühlen, A.; Brabant, G. Altered bone metabolism in inflammatory bowel disease. Am. J. Gastroenterol. 1997, 92, 1157–1163. [Google Scholar]
- Gilman, J.; Shanahan, F.; Cashman, K.D. Altered levels of biochemical indices of bone turnover and bone-related vitamins in patients with Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2006, 23, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.; Duggan, P.; O’Brien, M.; Kiely, M.; McCarthy, J.; Shanahan, F.; Cashman, K.D. Seasonality of vitamin D status and bone turnover in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2005, 21, 1073–1083. [Google Scholar] [CrossRef]
- Duggan, P.; O’Brien, M.; Kiely, M.; McCarthy, J.; Shanahan, F.; Cashman, K.D. Vitamin K status in patients with Crohn’s disease and relationship to bone turnover. Am. J. Gastroenterol. 2004, 99, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Łodyga, M.; Eder, P.; Gawron-Kiszka, M.; Dobrowolska, A.; Gonciarz, M.; Hartleb, M.; Kłopocka, M.; Małecka-Wojciesko, E.; Radwan, P.; Reguła, J.; et al. Guidelines for the management of patients with Crohn’s disease. Recommendations of the Polish Society of Gastroenterology and the Polish National Consultant in Gastroenterology. Prz. Gastroenterol. 2021, 16, 257–296. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Płudowski, P.; Ducki, C.; Konstantynowicz, J.; Jaworski, M. Vitamin D status in Poland. Pol. Arch. Med. Wewn. 2016, 126, 530–539. [Google Scholar] [CrossRef]
- Frigstad, S.O.; Høivik, M.; Jahnsen, J.; Dahl, S.R.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, Ø.; Torp, R.; et al. Vitamin D deficiency in inflammatory bowel disease: Prevalence and predictors in a Norwegian outpatient population. Scand. J. Gastroenterol. 2017, 52, 100–106. [Google Scholar] [CrossRef]
- Domislović, V.; Vranešić Bender, D.; Barišić, A.; Brinar, M.; Ljubas Kelečić, D.; Rotim, C.; Novosel, M.; Matašin, M.; Krznarić, Ž. High prevalence of untreated and undertreated vitamin d deficiency and insufficiency in patients with inflammatory bowel disease. Acta Clin. Croat. 2020, 59, 109–118. [Google Scholar]
- Dumitrescu, G.; Mihai, C.; Dranga, M.; Prelipcean, C.C. Serum 25-hydroxyvitamin D concentration and inflammatory bowel disease characteristics in Romania. World J. Gastroenterol. 2014, 20, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, E.; Sadeghi, N.; Parsi, A.; Hashemi, S.J.; Nayebi, M.; Shayesteh, A. Relationship Between Vitamin D Deficiency and Disease Activity in Patients with Inflammatory Bowel Disease in Ahvaz, Iran. Clin. Exp. Gastroenterol. 2020, 13, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, L.; Peng, N.; Xu, S.; Zhang, M.; Zhang, S.; Li, H.; Zhuang, H.; Gong, M.; Wu, D.; et al. Serum concentrations of 25-hydroxyvitamin D and its association with bone mineral density and serum parathyroid hormone levels during winter in urban males from Guiyang, Southwest China. Br. J. Nutr. 2016, 115, 960–966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bregenzer, N.; Erban, P.; Albrich, H.; Schmitz, G.; Feuerbach, S.; Schölmerich, J.; Andus, T. Screening for osteoporosis in patients with inflammatory bowel disease by using urinary N-telopeptides. Eur. J. Gastroenterol. Hepatol. 2002, 14, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Jahnsen, J.; Falch, J.A.; Mowinckel, P.; Aadland, E. Bone mineral density in patients with inflammatory bowel disease: A population-based prospective two-year follow-up study. Scand J. Gastroenterol. 2004, 39, 145–153. [Google Scholar] [CrossRef]
- Silvennoinen, J. Relationships between vitamin, D.; parathyroid hormone and bone mineral density in inflammatory bowel disease. J. Intern. Med. 1996, 239, 131–137. [Google Scholar] [CrossRef]
- Prosnitz, A.R.; Leonard, M.B.; Shults, J.; Zemel, B.S.; Hollis, B.W.; Denson, L.A.; Baldassano, R.N.; Cohen, A.B.; Thayu, M. Changes in vitamin D and parathyroid hormone metabolism in incident pediatric Crohn’s disease. Inflamm. Bowel Dis. 2013, 19, 45–53. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Cano, D.; Ruiz-Villaverde, R.; Callejas-Rubio, J.L.; Pérez, C.C.; García, M.G.; Ortego Centeno, N. Déficit de vitamina D y densidad mineral ósea en la enfermedad de Crohn [Vitamin D deficiency and bone mineral density in Crohn’s disease]. Med. Clin. (Barc) 2011, 137, 62–65. [Google Scholar] [CrossRef]
- Compston, J.E.; Creamer, B. Plasma levels and intestinal absorption of 25-hydroxyvitamin D in patients with small bowel resection. Gut 1977, 18, 171–175. [Google Scholar] [CrossRef]
- Gubatan, J.; Moss, A.C. Vitamin D in inflammatory bowel disease: More than just a supplement. Curr. Opin. Gastroenterol. 2018, 34, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Bollani, S.; Bettica, P.; Bevilacqua, M.; Molteni, P.; Bianchi Porro, G. Altered bone metabolism in inflammatory bowel disease: There is a difference between Crohn’s disease and ulcerative colitis. J. Intern. Med. 2000, 247, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Schoon, E.J.; Geerling, B.G.; Van Dooren, I.M.; Schurgers, L.J.; Vermeer, C.; Brummer, R.J.; Stockbrügger, R.W. Abnormal bone turnover in long-standing Crohn’s disease in remission. Aliment Pharmacol. Ther. 2001, 15, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Kantorovich, V.; Vasiliauskas, E.A.; Gruntmanis, U.; Matuk, R.; Daigle, K.; Chen, S.; Zehnder, D.; Lin, Y.C.; Yang, H.; et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut 2004, 53, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Szathmári, M.; Prónai, L.; Tulassay, Z. Altered bone metabolism in inflammatory bowel disease. Am. J. Gastroenterol. 1998, 93, 848–849. [Google Scholar] [CrossRef]
- Chetcuti Zammit, S.; Ellul, P.; Girardin, G.; Valpiani, D.; Nielsen, K.R.; Olsen, J.; Goldis, A.; Lazar, D.; Shonová, O.; Nováková, M.; et al. Vitamin D deficiency in a European inflammatory bowel disease inception cohort: An Epi-IBD study. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1297–1303. [Google Scholar] [CrossRef]
- Schäffler, H.; Schmidt, M.; Huth, A.; Reiner, J.; Glass, Ä.; Lamprecht, G. Clinical factors are associated with vitamin D levels in IBD patients: A retrospective analysis. J. Dig. Dis. 2018, 19, 24–32. [Google Scholar] [CrossRef]

| CD n =119 | UC n = 68 | |
|---|---|---|
| Sex (Female, n (%)) | 49 (41%) | 41(60%) |
| Median age (years) (SD) | 34 (±12.67) | 31(±10.84) |
| Median therapy period (years) | 6 | 6 |
| Disease activity | CDAI < 150: 62 patients CDAI 150–220: 31 patients CDAI 220–450: 25 patients CDAI > 450: 1 patient | Truelove Witts scale remission: 9 mild: 39 moderate:16 severe:4 |
| Current use of medications | ||
| 5-ASA (mesalasine or suphalasalsine) | 99 (83%) | 64 (94%) |
| Corticosteroids Prednisone or methylprednisolone | 9 (7.5%) | 15 (22%) |
| Budesonide | 11 (9.2%) | 13 (19.1%) |
| Immunosuppressants | ||
| Azathioprine | 10 (8.4%) | 29 (42.6%) |
| Mercaptopurine | 10 (8.4%) | 3 (4.4%) |
| Methotrexate | 5 (4.2%) | |
| Biologic agents | ||
| Infliximab | 12 (10%) | 3 (4%) |
| Adalimumab | 12 (10%) | |
| Vedolizumab | 3 (2.5%) | 5 (7.3%) |
| Ustekinumab | 9 (7.8%) |
| M | Whole Study Group Median | SD | Normal Range | |
|---|---|---|---|---|
| PTH (pg/mL) | 40.4 | 42.90 | 39.37 | 14.9–56.9 |
| Vitamin D (ng/mL) | 22.40 | 20.3 | 20.45 | 30–100 |
| Ca (mmol/L) | 2.40 | 2.33 | 0.68 | 2.09–2.54 |
| Phosphate serum (mg/dL) | 4.62 | 3.44 | 7.98 | 2.5–4.5 |
| Phosphate urine (mg/dL) | 51.00 | 42.90 | 39.37 | |
| Osteocalcin (ng/mL) | 24.92 | 20.55 | 26.19 | women: before menopause 11–46; after menopause 15–46; men 14–46 |
| CTX (ng/mL) | 1.83 | 0.40 | 17.38 | women: before menopause < 0.573; after menopause < 1.008 men: age 30–50 <0.584; >50 0.704 |
| Albumins (g/dL) | 5.92 | 4.25 | 10.54 | 3.5–5.20 |
| ALP (U/L) | 87.53 | 68 | 113.58 | 35–129 |
| CRP (mg/L) | 14.42 | 3.50 | 49.61 | <5 |
| CD (n = 116) Median (IQR) | UC (n = 67) Median (IQR) | p | Normal Range | |
|---|---|---|---|---|
| PTH (pg/mL) | 36.90 (21.85) | 29.90 (20.20) | 0.085 | 14.9–56.9 |
| Vitamin D (ng/mL) | 20.70 (13.35) | 20.05 (15.55) | 0.618 | 30–100 |
| Ca (mmol/L) | 2.33 (0.19) | 2.34 (0.17) | 0.353 | 2.09–2.54 |
| Phosphate serum (mg/dL) | 3.43 (0.63) | 3.50 (0.80) | 0.421 | 2.5–4.5 |
| Phosphate urine (mg/dL) | 51.20 (56.40) | 31.25 (44.25) | 0.003 | |
| Osteocalcin (ng/mL) | 22.60 (17.85) | 17.80 (17.60) | 0.029 | women: before menopause 11–46; after menopause 15–46; men 14–46 |
| CTX (ng/mL) | 0.40 (0.34) | 0.42 (0.33) | 0.266 | women: before menopause < 0.573; after menopause < 1.008 men: age 30–50 <0.584; >50 0.704 |
| Albumins (g/dL) | 4.27 (0.55) | 4.20 (0.69) | 0.866 | 3.5–5.20 |
| ALP (U/L) | 68.50 (25.75) | 66.0 (28.0) | 0.369 | 35–129 |
| Calprotectin | ||
|---|---|---|
| PTH | r | −0.09 |
| p | 0.431 | |
| CTX | r | 0.32 |
| p | 0.005 | |
| Osteocalcin | r | 0.25 |
| p | 0.035 |
| No Steroids (n = 135) | Steroids (n = 48) | ||||||
|---|---|---|---|---|---|---|---|
| Medium Range | Me | IQR | Medium Range | Me | IQR | p | |
| Albumins | 88.40 | 4.27 | 0.54 | 83.13 | 4.18 | 0.62 | 0.541 |
| ALP | 90.08 | 68.50 | 27.75 | 82.34 | 66.00 | 22.00 | 0.370 |
| CTX | 79.86 | 0.38 | 0.33 | 101.18 | 0.49 | 0.29 | 0.013 |
| Ca | 86.98 | 2.33 | 0.16 | 96.53 | 2.34 | 0.22 | 0.275 |
| CRP | 86.89 | 2.20 | 8.00 | 106.36 | 6.45 | 13.83 | 0.029 |
| Osteocalcin | 96.37 | 23.80 | 18.20 | 58.63 | 15.50 | 9.95 | <0.001 |
| Phosphate | 84.43 | 3.41 | 0.64 | 96.04 | 3.52 | 0.76 | 0.180 |
| Phosphate (urine) | 84.49 | 48.60 | 57.48 | 62.14 | 26.00 | 45.55 | 0.005 |
| PTH | 93.35 | 36.05 | 23.85 | 72.56 | 29.50 | 14.45 | 0.018 |
| Vitamin D | 85.64 | 21.00 | 14.18 | 75.02 | 18.10 | 15.00 | 0.218 |
| Ca | Vitamin D | ||
|---|---|---|---|
| PTH | r | 0.74 | −0.19 |
| p | <0.001 | 0.013 | |
| Ca | r | 0.04 | |
| p | 0.588 |
| Disease Activity | n | Median | IQR | p | |
|---|---|---|---|---|---|
| Remission | 54 | 50.96 | 0.33 | 0.350 | |
| CTX | Mild active disease | 32 | 55.81 | 0.39 | |
| Moderately active | 21 | 63.91 | 0.27 | ||
| Remission | 54 | 60.08 | 20.60 | ||
| Osteocalcin | Mild active disease | 33 | 52.62 | 17.50 | 0.326 |
| Moderately active | 22 | 48.36 | 13.25 | ||
| Remission | 56 | 55.62 | 19.18 | ||
| PTH | Mild active disease | 32 | 58.66 | 27.23 | 0.906 |
| Moderately active | 24 | 55.69 | 27.10 |
| Disease Activity | n | Median | IQR | p | |
|---|---|---|---|---|---|
| CTX | Remission | 9 | 24.11 | 0.21 | 0.378 |
| Mild active disease | 35 | 30.81 | 0.28 | ||
| Moderately active | 15 | 35.83 | 0.60 | ||
| Severe | 3 | 40.00 | |||
| Osteocalcin | Remission | 9 | 33.22 | 18.20 | 0.853 |
| Mild active disease | 35 | 31.61 | 18.30 | ||
| Moderately active | 16 | 28.90 | 18.30 | ||
| Severe | 3 | 38.00 | |||
| PTH | Remission | 9 | 29.61 | 17.25 | 0.299 |
| Mild active disease | 35 | 30.79 | 19.80 | ||
| Moderately active | 16 | 38.38 | 27.10 | ||
| Severe | 3 | 19.33 |
| Budesonide (n = 24) | Prednisone/Methylprednisolone (n = 24) | ||||
|---|---|---|---|---|---|
| Me | SD | Me | SD | p | |
| Ca | 2.62 | 0.77 | 2.34 | 0.17 | 0.098 |
| Phosphate | 8.80 | 16.97 | 3.56 | 0.57 | 0.144 |
| Phosphate (urine) | 41.09 | 29.88 | 31.50 | 23.82 | 0.245 |
| PTH | 37.93 | 26.67 | 30.11 | 9.70 | 0.211 |
| Vitamin D | 20.39 | 16.01 | 19.21 | 8.31 | 0.770 |
| CTX | 0.68 | 0.72 | 0.57 | 0.22 | 0.476 |
| Osteocalcin | 18.07 | 9.80 | 15.04 | 5.89 | 0.210 |
| CRP | 36.60 | 126.36 | 11.60 | 13.68 | 0.340 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tulewicz-Marti, E.M.; Lewandowski, K.; Rydzewska, G. Bone Metabolism Alteration in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2022, 11, 4138. https://doi.org/10.3390/jcm11144138
Tulewicz-Marti EM, Lewandowski K, Rydzewska G. Bone Metabolism Alteration in Patients with Inflammatory Bowel Disease. Journal of Clinical Medicine. 2022; 11(14):4138. https://doi.org/10.3390/jcm11144138
Chicago/Turabian StyleTulewicz-Marti, Edyta Maria, Konrad Lewandowski, and Grażyna Rydzewska. 2022. "Bone Metabolism Alteration in Patients with Inflammatory Bowel Disease" Journal of Clinical Medicine 11, no. 14: 4138. https://doi.org/10.3390/jcm11144138
APA StyleTulewicz-Marti, E. M., Lewandowski, K., & Rydzewska, G. (2022). Bone Metabolism Alteration in Patients with Inflammatory Bowel Disease. Journal of Clinical Medicine, 11(14), 4138. https://doi.org/10.3390/jcm11144138






