Primary Thrombophilia XVII: A Narrative Review of Sticky Platelet Syndrome in México
Abstract
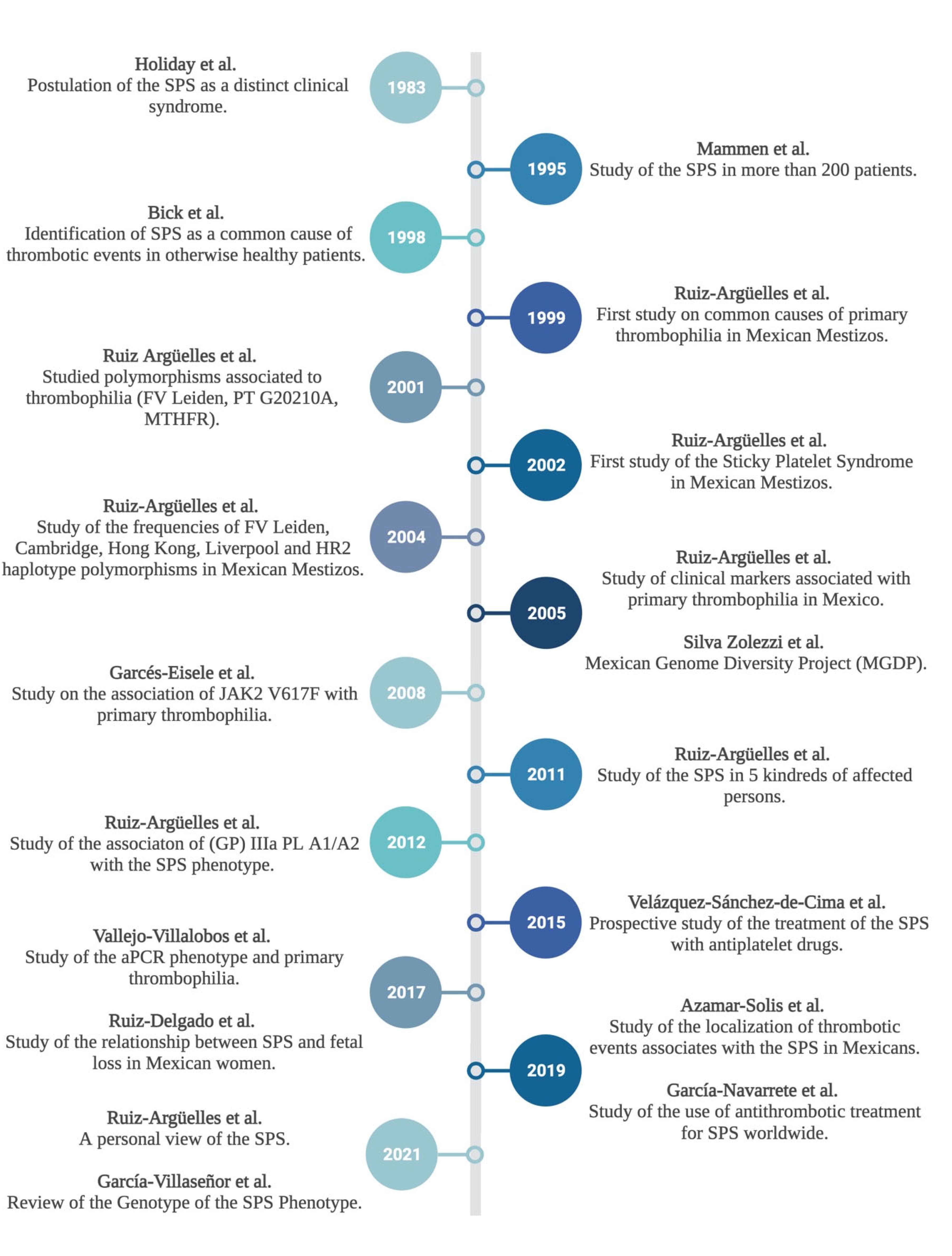
1. Introduction
1.1. Primary Thrombophilia and Sticky Platlet Syndrome: The Mexican Experience
1.2. Multifactorial Thrombophilia
1.3. Subtypes of SPS and Inheritance
1.4. Insights on the Treatment of SPS
1.5. Concluding Remarks
- (1)
- SPS is a phenotype of platelet hyperaggregability, defined by increased in vitro platelet aggregation after the addition of very low concentrations of adenosine diphosphate and/or epinephrine. The concentrations and dilutions of the agents are relatively well standardized.
- (2)
- The genotype is currently unknown, but several observations on the genes of platelets proteins are being studied: platelet glycoprotein IIIa PLA1/A2; platelet glycoprotein 6, growth arrest specific 6, coagulation factor V, integrin subunit beta 3, platelet endothelial aggregation receptor 1, serpin family C member 1, serpin family E member 1.
- (3)
- The SPS phenotype is probably the expression of genetic conditions interacting with other medical conditions or environmental factors, such as diabetes mellitus, hormonal therapy, and pregnancy.
- (4)
- SPS may lead into both arterial and venous thrombosis, the latter being more frequent.
- (5)
- SPS is a hereditary autosomal dominant trait.
- (6)
- SPS is the most frequent cause of hereditary thrombophilia in México, and probably in other countries.
- (7)
- Patients with SPS have been identified and treated in all continents of the world.
- (8)
- SPS is a frequent cause of miscarriages and obstetric complications.
- (9)
- SPS usually needs another thrombophilic condition to fully express as a thrombotic episode. It has recently been described as a risk factor for thrombosis during COVID-19.
- (10)
- The hyperaggregability of SPS reverts to employing antiplatelet drugs and the re-thrombosis rate of persons with the syndrome is very low while being on treatment. Most patients revert the hyperaggregability with aspirin, but around one quarter need two antiplatelet drugs. It is therefore advisable to assess the SPS phenotype after starting the antiplatelet drug, in order to define further treatment. Treating persons with SPS with oral anticoagulants does not reduce the re-thrombosis rate
- (11)
- Claiming that SPS is a non-entity indicates that it is not being assessed properly and may also be detrimental for patients. The treatment is cheap, available and effective, as well as tolerated by most persons, which is the use of low-doses of aspirin and other antiplatelet drugs.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mammen, E.F. Ten Years’ Experience with the “Sticky Platelet Syndrome”. Clin. Appl. Thromb. 1995, 1, 66–72. [Google Scholar] [CrossRef]
- Kubisz, P.; Ruiz-Argüelles, G.J.; Stasko, J.; Holly, P.; Ruiz-Delgado, G.J. Sticky Platelet Syndrome: History and Future Per-spectives. Semin. Thromb. Hemost. 2014, 40, 526–534. [Google Scholar]
- Velázquez-Sánchez-de-Cima, S.; Zamora-Ortiz, G.; Hernández-Reyes, J.; Vargas-Espnosa, J.; García-Chavez, J.; Rosa-les-Padrón, J. Primary Thrombophilia in México X: A Prospective Study of the Treatment of the Sticky Platelet Syndrome. Clin. Appl. Thromb. Hemost. 2015, 21, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Bick, R.L. Sticky Platelet Syndrome: A Common Cause of Unexplained Arterial and Venous Thrombosis. Clin. Appl. Thromb. 1998, 4, 77–81. [Google Scholar] [CrossRef]
- Pons-Estel, B.A.; Catoggio, L.J.; Cardiel, M.H.; Soriano, E.R.; Gentiletti, S.; Villa, A.R.; Abadi, I.; Caeiro, F.; Alvarellos, A.; Alarcón-Segovia, D. The GLADEL Multinational Latin American Prospective Inception Cohort of 1214 Patients with Systemic Lupus Erythematosus: Ethnic and disease heterogeneity among “Hispanics”. Medicine 2004, 83, 1–17. [Google Scholar] [CrossRef]
- Silva-Zolezzi, I.; Hidalgo-Miranda, A.; Estrada-Gil, J.; Fernandez-Lopez, J.C.; Uribe-Figueroa, L.; Contreras, A.; Balam-Ortiz, E.; del Bosque-Plata, L.; Velazquez-Fernandez, D.; Lara, C.; et al. Analysis of genomic diversity in Mexican Mestizo populations to develop genomic medicine in Mexico. Proc. Natl. Acad. Sci. USA 2009, 106, 8611–8616. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Argüelles, G.J.; González-Estrada, S.; Garcés-Eisele, J.; Ruiz-Argüelles, A. Primary Thrombophilia in Mexico: A Pros-pective Study. Am. J. Hematol. 1999, 60, 1–5. [Google Scholar] [CrossRef]
- Ruiz-Argüelles, G.J.; Garcés-Eisele, J.; Reyes-Núñez, V.; Ramírez-Cisneros, F.J. Primary Thrombophilia in Mexico. II. Factor V G1691A (Leiden), Prothrombin G20210A, and Methylenetetrahydrofolate Reductase C677T Polymorphism in Thrombophilic Mexican Mestizos. Am. J. Hematol. 2001, 66, 28–31. [Google Scholar] [CrossRef]
- Ruiz-Argúelles, G.J.; López-Martinez, B.; Cruz-Cruz, D.; Esparza-Silva, L.; Reyes-Aulis, M.B. Primary Thrombophilia in Mexico III: A Prospective Study of the Sticky Platelet Syndrome. Clin. Appl. Thromb. 2002, 8, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Argüelles, G.J.; Poblete-Naredo, I.; Reyes-Núñez, V.; Garcés-Eisele, J.; López-Martínez, B.; Gómez-Rangel, J.D. Primary thrombophilia in Mexico IV: Frequency of the Leiden, Cambridge, Hong Kong, Liverpool and HR2 haplotype polymorphisms in the factor V gene of a group of thrombophilic Mexican Mestizo patients. Rev. Investig. Clin. 2004, 56, 600–604. [Google Scholar]
- Ruiz-Argüelles, G.J.; López-Martínez, B.; Valdés-Tapia, P.; Gómez-Rangel, J.D.; Reyes-Núñez, V.; Garcés-Eisele, J. Primary thrombo-philia in Mexico. V. A comprehensive prospective study indicates that most cases are multifactorial: Multifactorial Throm-bophilia in Mexicans. Am. J. Hematol. 2005, 78, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Villalobos, M.F.; Gomez-Cruz, G.B.; Cantero-Fortiz, Y.; Olivares-Gazca, J.C.; Olivares-Gazca, M.; Murrieta-Alvarez, I.; Reyes-Nuñez, V.; Ruiz-Argüelles, G.J. Primary Thrombophilia XIV: Worldwide Identification of Sticky Platelet Syndrome. Semin. Thromb. Hemost. 2019, 45, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.I.; Levine, M.N.; Konkle, B.A.; Kearon, C. Thrombotic Disorders: Diagnosis and Treatment. Hematology 2003, 2003, 520–539. [Google Scholar] [CrossRef]
- Ruiz-Argüelles, G.J.; González-Carrillo, M.L.; Estrada-Gómez, R.; Valdés-Tapia, P.; Parra-Ortega, I.; Porras-Juárez, A. Trombofilia primaria en México Parte VI: Falta de asociación estadística entre las condiciones trombofílicas heredadas. Gac. Méd. Méx. 2007, 4, 317–322. [Google Scholar]
- Garcés-Eisele, J.; González-Carrillo, M.L.; Reyes-Núñez, V.; Ruiz-Argüelles, G.J. Primary thrombophilia in México VII: The V617F mutation of JAK2 is not a frequent cause of thrombosis. Hematology 2008, 13, 244–246. [Google Scholar] [CrossRef]
- Ruiz-Delgado, G.J.; Cantero-Fortiz, Y.; Mendez-Huerta, M.A.; Leon-Gonzalez, M.; Nuñez-Cortes, A.K.; Leon-Peña, A.A.; Olivares-Gazca, J.C.; Ruiz-Argüelles, G.J. Primary Thrombophilia in Mexico XII: Miscarriages are More Frequent in People with Sticky Platelet Syndrome. Turk. J. Hematol. 2017, 34, 239–243. [Google Scholar]
- Sokol, J.; Kubisz, P.; Stasko, J. Comment on: Inherited Thrombophilia and Pregnancy Complications: Should We Test? Semin. Thromb. Hemost. 2019, 46, 501. [Google Scholar] [CrossRef]
- Ruiz-Argüelles, G.J.; Alarcón-Urdaneta, C.; Calderón-García, J.; Ruiz-Delgado, G.J. Primary thrombophilia in México VIII: Description of five kindreds of familial platelet syndrome phenotype. Rev. Hematol. Mex. 2011, 12, 73–78. [Google Scholar]
- Ruiz-Argüelles, G.J.; Garcés-Eisele, J.; Camacho-Alarcón, C.; Moncada-Gonzalez, B.; Valdés-Tapia, P.; León-Montes, N.; Ruiz-Delgado, G.J. Primary thrombophilia in Mexico IX: The Glycoprotein IIIa PL A1/A2 Polymorphism is Not Associated With the Sticky Platelet Syndrome Phenotype. Clin. Appl. Thromb. Hemost. 2013, 19, 689–692. [Google Scholar] [CrossRef]
- Skerenova, M.; Jedinakova, Z.; Simurda, T.; Skornova, I.; Stasko, J.; Kubisz, P.; Sokol, J. Progress in the Understanding of Sticky Platelet Syndrome. Semin. Thromb. Hemost. 2016, 43, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Azamar-Solis, B.; Cantero-Fortiz, Y.; Olivares-Gazca, J.C.; Olivares-Gazca, J.M.; Gomez-Cruz, G.B.; Murrieta-Álvarez, I.; Ruiz-Delgado, G.J.; Ruiz-Argüelles, G.J. Primary Thrombophilia in Mexico XIII: Localization of the Thrombotic Events in Mexican Mestizos with the Sticky Platelet Syndrome. Clin. Appl. Thromb. 2019, 25, 1–4. [Google Scholar] [CrossRef]
- Kubisz, P. Response to “Comment on Sticky Platelet Syndrome”. Semin. Thromb. Hemost. 2014, 40, 274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mikovic, D.; Stanciakova, L.; Sinzinger, H.; Kubisz, P. Meeting Report: 18th International Meeting of the Danubian League against Thrombosis and Haemorrhagic Disorders. Semin. Thromb. Hemost. 2015, 41, 903–906. [Google Scholar] [CrossRef] [PubMed][Green Version]
- García-Navarrete, Y.I.; Vallejo-Villalobos, M.F.; Olivares-Gazca, J.M.; Cantero-Fortiz, Y.; León-Peña, A.A.; Olivares-Gazca, J.C.; Murrieta-Álvarez, I.; Ruiz-Delgado, G.J.; Ruiz-Argüelles, G.J. Primary thrombophilia XV: Antithrombotic treatment of sticky platelet syndrome worldwide. Ann. Blood 2019, 4, 15. [Google Scholar] [CrossRef]
- Ruiz-Argüelles, G.J. A personal view of the sticky platelet syndrome. Rev. Hematol. Mex. 2021, 22, 199–201. [Google Scholar]
- Ruiz-Argüelles, G.J.; Ruiz-Delgado, G.J. Algunas reflexiones sobre el síndrome de plaquetas pegajosas en 2019. Rev. Hematol. Mex. 2019, 20, 243–246. [Google Scholar] [CrossRef]
- García-Villaseñor, E.; Bojalil-Álvarez, L.; Murrieta-Álvarez, I.; Cantero-Fortiz, Y.; Ruiz-Delgado, G.J.; Ruiz-Argüelles, G.J. Primary Thrombophilia XVI: A Look at the Genotype of the Sticky Platelet Syndrome Phenotype. Clin. Appl. Thromb. 2021, 27, 1076029619841700. [Google Scholar] [CrossRef] [PubMed]
- Moncada, B.; Ruíz-Arguelles, G.J.; Castillo-Martínez, C. The sticky platelet syndrome. Hematology 2013, 18, 230–232. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minutti-Zanella, C.; Villarreal-Martínez, L.; Ruiz-Argüelles, G.J. Primary Thrombophilia XVII: A Narrative Review of Sticky Platelet Syndrome in México. J. Clin. Med. 2022, 11, 4100. https://doi.org/10.3390/jcm11144100
Minutti-Zanella C, Villarreal-Martínez L, Ruiz-Argüelles GJ. Primary Thrombophilia XVII: A Narrative Review of Sticky Platelet Syndrome in México. Journal of Clinical Medicine. 2022; 11(14):4100. https://doi.org/10.3390/jcm11144100
Chicago/Turabian StyleMinutti-Zanella, Claudia, Laura Villarreal-Martínez, and Guillermo J. Ruiz-Argüelles. 2022. "Primary Thrombophilia XVII: A Narrative Review of Sticky Platelet Syndrome in México" Journal of Clinical Medicine 11, no. 14: 4100. https://doi.org/10.3390/jcm11144100
APA StyleMinutti-Zanella, C., Villarreal-Martínez, L., & Ruiz-Argüelles, G. J. (2022). Primary Thrombophilia XVII: A Narrative Review of Sticky Platelet Syndrome in México. Journal of Clinical Medicine, 11(14), 4100. https://doi.org/10.3390/jcm11144100





