Abstract
Background: Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is associated with poor prognosis in cardiovascular diseases. However, the predictive value of TRAIL for the short-term outcome and risk stratification of acute pulmonary embolism (PE) remains unknown. Methods: This study prospectively included 151 normotensive patients with acute PE. The study outcome was a composite of 30-day adverse events, defined as PE-related death, shock, mechanical ventilation, cardiopulmonary resuscitation, and major bleeding. Results: Overall, nine of 151 (6.0%) patients experienced 30-day adverse composite events. Multivariable logistic regression showed that TRAIL was an independent predictor of study outcome (OR 0.19 per SD; 95% CI 0.04–0.90). An ROC curve revealed that TRAIL’s area under the curve (AUC) was 0.83 (95% CI 0.76–0.88). The optimal cut-off value for TRAIL was 18 pg/mL, with a sensitivity, specificity, negative predictive value, positive predictive value, positive likelihood ratio, and negative likelihood ratio of 89%, 69%, 99%, 15%, 2.87, and 0.16, respectively. Compared with the risk stratification algorithm outlined in the 2019 ESC guidelines, our biomarker-based risk stratification strategy (combining TRAIL and hs-cTnI) has a similar risk classification effect. Conclusion: Reduced plasma TRAIL levels predict short-term adverse events in normotensive patients with acute PE. The combination of the 2019 ESC algorithm and TRAIL aids risk stratification in normotensive patients with acute PE.
1. Introduction
Venous thromboembolism (VTE), including deep vein thrombosis and pulmonary embolism, contributes a significant burden on health and survival and ranks third among life-threatening cardiovascular diseases [1]. Acute pulmonary embolism (PE) is the most severe clinical manifestation of VTE. Most patients with acute PE are normotensive, and early mortality ranges from 3–7% [2,3,4]. Early prognostic assessment and risk stratification for normotensive patients with acute PE is essential for determining appropriate treatment management approaches. The 2019 European Society of Cardiology (ESC) guidelines suggested that the extensively validated and broadly used simplified pulmonary embolism severity index (sPESI), combined with right ventricular (RV) dysfunction and laboratory biomarkers, can be used to classify acute PE patients without hemodynamic instability into intermediate- or low-risk groups. In addition to clinical parameters and scores, patients in the intermediate-risk group who display RV dysfunction and elevated cardiac troponin levels are classified into the intermediate-high-risk category [5]. Previous evidence demonstrated that a subgroup of normotensive patients with acute PE (i.e., intermediate-risk group) might benefit from aggressive treatment strategies [6]. Thus, optimizing risk stratification in normotensive PE is essential to enhance clinical practice.
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), which is also known as Apo-2 ligand (Apo-2L) or TNF superfamily 10 (TNFSN10), is a member of the TNF superfamily of cytokines, which is broadly expressed in various tissues of the human body [7]. TRAIL is selectively expressed in vascular smooth muscle cells of the pulmonary artery and aorta [8]. Soluble TRAIL mainly appears to be released by activated leukocytes such as monocytes and neutrophils [9]. TRAIL is a pro-apoptotic protein which has broad biological functions. TRAIL may play a crucial role in the pathway linking coagulation and inflammation elicited by thrombin and mediates the amplification of pro-coagulant endothelial microparticles released by thrombin and the inflammatory process [10]. Several clinical studies have shown that reduced TRAIL levels are associated with poor prognosis in patients with acute myocardial infarction or heart failure, suggesting that TRAIL has predictive effects in cardiovascular diseases [11,12,13].
In this study, we hypothesized that TRAIL may be involved in the pathophysiological mechanism of PE through the interplay between coagulation and inflammation and might assist in the prognostic assessment of patients with acute PE. Thus, our study aimed to identify the short-term prognostic assessment and risk stratification of TRAIL in normotensive patients with acute PE.
2. Materials and Methods
2.1. Study Design and Setting
We conducted a prospective study of normotensive patients with acute pulmonary embolism from 2015 to 2017 at Beijing Anzhen Hospital in China (NCT 04118634). Based on the amended Declaration of Helsinki, the study protocol was approved by the Ethics Committee of Beijing Anzhen Hospital (No. 2018048X), and all patients provided written informed consent.
2.2. Selection of Participants
As shown in Figure 1, normotensive patients (defined as SBP ≥ 90 mmHg) were consecutively enrolled if they had acute PE, were aged ≥ 18 years, and the onset of the illness was ≤14 days ago. Patients with acute PE were objectively confirmed by computed tomography pulmonary angiography (CTPA) and a ventilation-perfusion lung scan. The exclusion criteria were the following: [14,15,16] (1) hemodynamic instability: (A) cardiac arrest: cardiopulmonary resuscitation required; (B) obstructive shock: systolic blood pressure (BP) < 90 mmHg or vasopressors required to achieve a BP ≥ 90 mmHg despite adequate filling status and end-organ hypoperfusion (altered mental status; cold, clammy skin; oliguria/anuria); (C) persistent hypotension: systolic BP < 90 mmHg or systolic BP drop ≥ 40 mmHg lasting longer than 15 min and not caused by new-onset arrhythmia, hypovolaemia, or sepsis; (2) recurrence of PE; (3) chronic thromboembolic pulmonary hypertension; (4) life expectancy <3 months (i.e., the end stage of diseases); (5) ongoing pregnancy; (6) renal insufficiency (estimated glomerular filtration rate <30 mL/ min*1.73 m2) or hepatic dysfunction (Child–Pugh class B or C); (7) withdrawal of written consent for participation in this study; and (8) missing blood samples and troponin data.
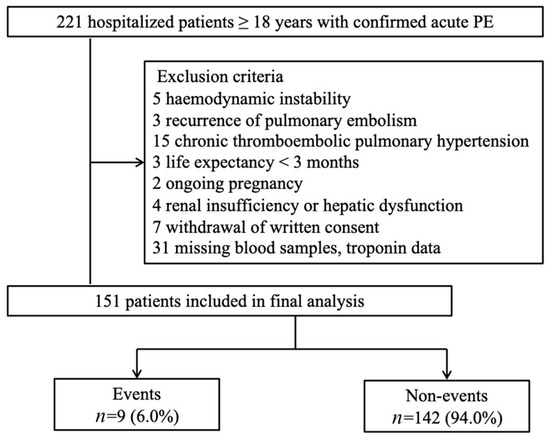
Figure 1.
Study participants flow diagram. PE, pulmonary embolism.
2.3. Methods of Measurement
The diagnosis of acute PE was assessed using the Wells clinical probability rule, D-dimer, and imaging tests by the diagnostic algorithm outlined in the 2019 ESC guidelines [5]. All patients underwent transthoracic echocardiography within 24 h after diagnosis of PE. The diagnosis of RV dysfunction was based on the following diagnostic criteria [5]: (1) RV dilatation at the apical four-chamber view (RV end-diastolic diameter/left ventricular end-diastolic diameter >1.0), (2) depressed contractility of the RV free wall, (3) tricuspid regurgitation velocity acceleration, and (4) decreased tricuspid annular systolic excursion (<17 mm). The electronic medical record system obtained other clinical data, laboratory findings, and treatment details. According to the risk stratification strategy proposed in the 2019 ESC guidelines, all normotensive patients with acute PE were classified into the intermediate-high-, intermediate-low-, and low-risk groups according to their sPESI score, RV dysfunction, and troponin level. The physicians made treatment decisions while being unaware of TRAIL levels after carefully considering each patient’s clinical symptoms, laboratory findings, and imaging tests.
Venous plasma samples were collected from patients within 24 h after admission in vacuum tubes and immediately frozen at −80 °C after centrifugation at 3000× g for 10 min. Plasma TRAIL concentrations were determined using an ELISA kit (Ray Biotech, Inc. Norcross, GA, USA). Other laboratory tests were completed by the laboratory department of Beijing Anzhen Hospital.
2.4. Outcome Measures
The study outcome was 30-day adverse composite events, defined as PE-related death or at least one of the following complications: (1) the need for mechanical ventilation assistance, (2) the need for catecholamine administration for treatment or prevention, (3) cardiopulmonary resuscitation, or (4) major bleeding. PE-related death was determined by (1) autopsy, (2) clinically severe acute PE, and (3) in cases where other causes were excluded. Major bleeding was defined as clinically overt bleeding accompanied by at least one of the following: (1) fatal bleeding or bleeding that occurred at critical sites or organs (intracranial, intraspinal, retroperitoneal, intraocular, and pericardial bleeding); (2) hemodynamic instability due to bleeding and/or a fall in the hemoglobin level ≥20 g/L, or bleeding that led to the transfusion of at least two units of blood [17].
All patients were followed up by pre-trained research staff. We determined the occurrence of the study outcome by using data collected through a review of the electronic medical records, clinical visits, and telephone follow-up interviews for up to 30 days.
2.5. Biomarker-Based Risk Algorithm
In the 2019 ESC prognostic strategy, risk assessment for early mortality consists of seven clinical parameters (sPESI rule), two relevant imaging modalities (TTE or CTPA), and four cardiac biomarkers (troponin, NT-proBNP, H-FABP, and copeptin). Objective assessments are relatively time-consuming, labor-intensive, and cost-intensive. Thus, in this study, a biomarker-based risk algorithm was developed to evaluate the risk assessment of normotensive patients with acute PE. This biomarker-based stratification strategy was established using TRAIL combined with hs-cTnI levels. According to previous studies [18,19,20], hs-cTnI possessed superior negative predictive values (NPV) for short-term adverse events and could be used as the first step in risk stratification to classify patients with low-risk acute PE.
2.6. Statistical Analyses
The Kolmogorov–Smirnov test for normal distribution was used for continuous variables. Skewed continuous variables were expressed as medians (interquartile range [IQR]). Categorical variables were expressed as absolute numbers or percentages. Comparisons of continuous variables were analyzed using unpaired Student’s t-tests or Mann–Whitney U tests, and comparisons of categorical variables were analyzed using Chi-squared or Fisher’s exact tests. Correlations between continuous variables were analyzed using Spearman’s rank correlation coefficient. The prognostic relevance of clinical variables, cardiac biomarkers, TRAIL levels, and sPESI scores for 30-day adverse events was calculated using univariate (unadjusted) and multivariate (adjusted) logistic regression analysis, producing odds ratios (OR) and 95% confidence intervals (CIs). Factors for inclusion in the multivariate analysis were determined after considering the findings from previous publications and the latest ESC guidelines and significant predictors (p < 0.05) from the univariate analysis. Receiver operating characteristic (ROC) curve analysis was performed to determine the area under the curve (AUC) of TRAIL cut-off values for the study outcomes. Youden’s index was used to identify optimal cut-off values. Sensitivity, specificity, negative predictive values (NPV), positive predictive values (PPV), negative likelihood ratios (−LR), positive likelihood ratios (+LR), and the corresponding 95% CIs were calculated. The McNemar–Bowker test was used to compare the distribution of patients in different risk stratification strategies (2019 ESC algorithm and biomarker-based approach). Two-tailed p values < 0.05 were considered statistically significant. All statistical analyses were conducted using SPSS (version 25.0; IBM, Chicago, IL, USA).
3. Results
3.1. Characteristics of Study Subjects
Between January 2015 and December 2017, 221 patients were screened, of whom 70 met the exclusion criteria (flow chart shown as Figure 1). Among the 151 patients who participated in this study, nine (6%) experienced 30-day adverse composite events. One patient died directly due to PE; seven patients required catecholamine administration for treatment or prevention. Two patients required mechanical ventilation, two required cardiopulmonary resuscitation, and one suffered major bleeding. The clinical and demographic characteristics of study participants with and without study events are presented in Table 1. The event group more frequently experienced syncope, RV dysfunction, higher BNP and hs-cTnI concentrations, and sPESI scores ≥ 1 compared to the non-event group. Additionally, nine (6.0%) patients received thrombolytic therapy and five (55.6%) experienced adverse outcomes.

Table 1.
Baseline characteristics of normotensive patients with acute pulmonary embolism.
3.2. Association between TRAIL Levels and Short-Term Prognosis
The median TRAIL concentration was 23.1 pg/mL (IQR 15.0–32.3) in all patients. Patients in the events group had significantly lower TRAIL levels (median, 10.1 pg/mL [IQR 3.6–16.4]) than patients in the non-event group (median 23.5 pg/mL [IQR 16.1–32.6], p = 0.001). The TRAIL concentrations were weakly correlated with BNP (r = −0.28, p = 0.001) and hs-cTnI (r = −0.24, p = 0.003). The predictors of 30-day adverse composite events were investigated using a univariate logistic regression analysis (Table 2). Significant predictors of 30-day adverse composite events in the univariate analysis included syncope (OR = 9.83; 95% CI 2.30–42.08, p = 0.002), RV dysfunction (OR = 16.5; 95% CI 3.82–71.30, p = 0.000), BNP (OR = 3.60 per SD; 95% CI 1.91–6.78, p = 0.000), TRAIL (OR = 0.18 per SD; 95% CI 0.06–0.56, p = 0.003), and a sPESI score ≥ 1 (OR = 16.17; 95% CI 1.95–133.11, p = 0.010). Considering the findings from previous publications and the latest ESC guidelines, significant predictors from the univariate analysis and cardiac troponin (hs-cTnI) were included in the multivariate logistic regression analysis (Table 2). After adjustment, TRAIL was independently and significantly associated with 30-day adverse composite events in normotensive patients with acute PE (OR = 0.19 per SD; 95% CI 0.04–0.90, p = 0.036). As shown in Figure 2, ROC analysis revealed that the AUC of TRAIL was 0.83 (95% CI 0.76–0.88, p < 0.001) for the prediction of short-term adverse outcomes, and the optimal cut-off value for TRAIL based on Youden’s index was 18 pg/mL, at which point the sensitivity, specificity, NPV, PPV, +LR, and −LR were 89%, 69%, 99%, 15%, 2.87, and 0.16, respectively.

Table 2.
Predictors of an adverse 30-day outcome.
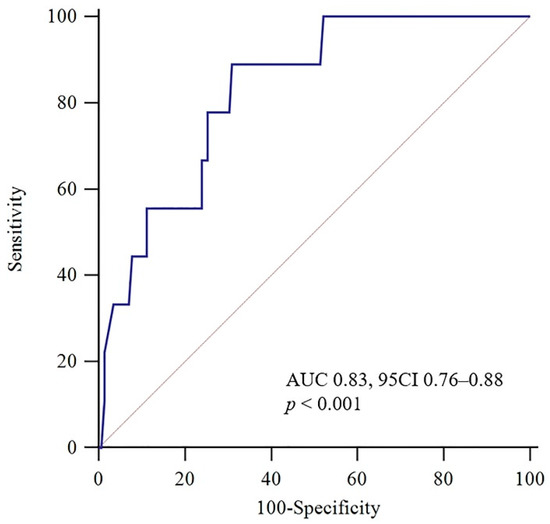
Figure 2.
Receiver operating characteristic (ROC) curve for TRAIL concerning an adverse 30-day outcome. AUC: area under the curve; CI: confidence interval.
3.3. TRAIL’s Role in Risk Stratification
According to the 2019 ESC risk algorithm (Figure 3), 10 (6.6%) patients were classified into the intermediate-high risk group, 78 (51.7%) into the intermediate-low risk group, and 63 (41.7%) into the low-risk group. During the follow-up, the 30-day adverse composite events occurred in 5 (50%), 4 (5.1%), and 0 (0%) patients, respectively. The risk assessment using the biomarker-based strategy based on hs-cTnI and TRAIL is shown in Figure 3. As with the 2019 ESC risk algorithm, the stepwise biomarker-based strategy demonstrated strong predictive performance in identifying intermediate-high- and low-risk group patients (Table 3). Both the biomarker-based strategy and the 2019 ESC algorithm showed high sensitivity (100%) and NPV (100%) in identifying low-risk patients, while the biomarker-based strategy had higher specificity than the 2019 ESC algorithm (65% vs. 44%, p < 0.001). When identifying intermediate-high-risk group patients, both strategies had high specificity (88% vs. 96%, p < 0.001) and the biomarker-based strategy had a superior trend of sensitivity (89% vs. 56%, p = 0.375). To combine the performance of the biomarker-based strategy and the 2019 ESC algorithm, we tested whether TRAIL may improve patients re-classified as belonging to the intermediate-high risk group, as shown in Figure 4. Using TRAIL < 18 pg/mL to further stratify patients in the intermediate-low risk group, 28 patients were identified as being at higher risk, with four adverse events. The prognostic performance of risk assessment using the 2019 ESC algorithm and TRAIL for the prediction of an adverse 30-day outcome is shown in Table 3, for which the sensitivity, specificity, NPV, PPV, +LR, and −LR were 100%, 80%, 100%, 24%, 5, and 0, respectively.
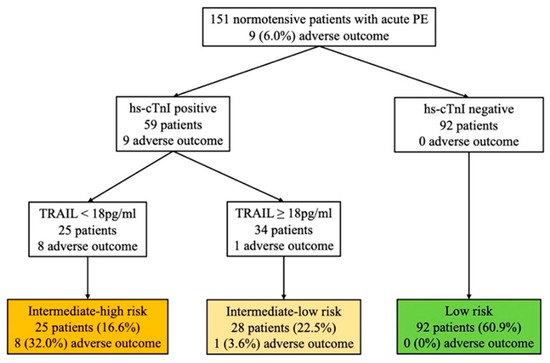
Figure 3.
Risk assessment using the biomarker-based strategy based on hs-cTnI and TRAIL. The number (%) of patients with an adverse 30-day outcome is shown for each strategy. Hs-cTnI levels >0.04 ng/mL are defined as positive. PE: pulmonary embolism; hs-cTnI, high-sensitivity cardiac troponin I; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.

Table 3.
Prognostic performance of risk assessment strategies for the prediction of an adverse 30-day outcome.
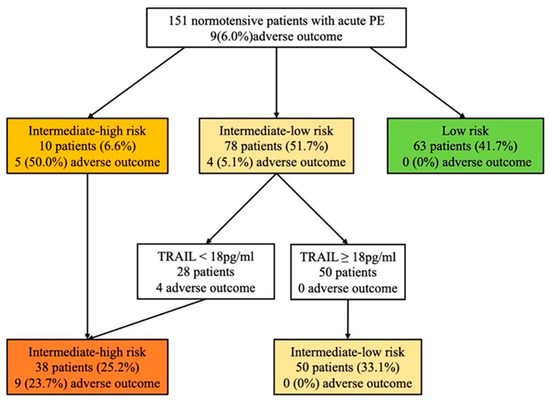
Figure 4.
Risk assessment using the 2019 ESC algorithm and TRAIL. The number (%) of patients with an adverse 30-day outcome is shown for each strategy. Hs-cTnI levels > 0.04 ng/mL are defined as positive. PE: pulmonary embolism; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
4. Discussion
This study investigated the relationship between plasma TRAIL concentrations and short-term adverse outcomes and whether TRAIL can optimize the current risk stratification. Using a cut-off value of 18 pg/mL, we found that decreased plasma TRAIL levels had an independently prognostic performance for 30-day adverse outcomes. A stepwise biomarker-based risk assessment strategy combining hs-cTnI and TRAIL improves predictive performance in identifying intermediate-high- and low-risk group patients. The combination of the 2019 ESC algorithm and TRAIL aids risk stratification in normotensive patients with acute PE.
4.1. The Potential Role of TRAIL in PE
TRAIL exists as either a type II membrane protein or a soluble protein. TRAIL receptors are expressed in the cardiovascular system in vascular smooth cells and cardiomyocytes, including osteoprotegerin (OPG). TRAIL has been found to play a role in ischemic vascular diseases and cardiovascular disease (CVD) [20,21,22,23,24]. Several prospective studies have demonstrated that lower TRAIL concentrations predicted poor prognosis in patients with CVD [13,25,26]. In our study, lower TRAIL concentrations were associated with short-term adverse outcomes. Low levels of TRAIL tend to represent poor prognosis. This is similar to the findings of several previous studies, in which serum TRAIL levels were negatively related to the severity of coronary heart disease [27], lower serum TRAIL levels were associated with worse outcomes in patients with acute myocardial infarction [28], and higher TRAIL levels in patients with advanced heart failure were associated with an improved prognosis [12,29]. Despite this, it is unclear how TRAIL can clinically influence the thrombosis and inflammation process during acute PE. However, it is plausible that the interaction between TRAIL and its receptors modulates the progression of thromboembolism. The role of inflammation-modulating maladaptive RV remodeling and dysfunction has been demonstrated. Acute PE leads to a cascade of inflammatory response which might be followed by leukocyte recruitment to the lesion. TRAIL recruits activated leukocytes to a particular tissue and initiates apoptosis to terminate the immune response. TRAIL promotes the proliferation of vascular smooth muscle cells and neovascularization [28,29]. TRAIL also enhances endothelial nitric oxide synthase phosphorylation, NOS activity, and NO synthesis; thus, it causes vasodilation [30,31]. Interestingly, there is a negative correlation between TRAIL and hsCRP, which provides further support for the protective role of TRAIL in the development of atherosclerosis and acute coronary disease [32].
4.2. The Combination of TRAIL and the 2019 ESC Algorithm for Risk Assessment in Normotensive Patients with Acute PE
Based on the 2019 ESC guidelines, treatment decisions for normotensive patients with acute PE need to be based on a risk stratification strategy, with low-risk patients being considered for early discharge and home treatment, intermediate-low or intermediate-high risk patients being closely monitored and offered reperfusion therapy if deterioration occurs. Recent cohort studies developed combination models for the identification of intermediate-high-risk PE patients (e.g., PREP score, FAST score, and Bova score) [33,34,35], and several studies investigated the prognostic value of biomarkers on risk stratification (e.g., Copeptin and Lipocalin-2) [16,19]. Due to the relatively limited performance of the 2019 ESC algorithm, we developed a novel and simple stepwise biomarker-based strategy using TRAIL and hs-cTnI. More patients were re-classified into the low-risk and intermediate-high risk groups using a biomarker-based algorithm. To combine the performance of the biomarker-based strategy and the 2019 ESC algorithm, we also tested whether TRAIL may improve patients re-classified as belonging to the intermediate-high risk group. As shown in Table 3 and Figure 4, the prognostic performance of risk assessment was improved using the 2019 ESC algorithm and TRAIL to predict an adverse 30-day outcome.
There are some limitations in this study that merit mentioning here. First, the included population came from a single center, and the number of people who experienced an outcome was low. However, adverse outcomes (6%) were similar to those reported in other studies [14,15,18,19]. Second, of the 221 patients screened, 31 (14.0%) were excluded due to missing data. Given the small size and the low event rate of this study, we could not evaluate if TRAIL has additional value on top of the existing risk stratification. Further large-scale studies are required in future using independent study cohorts. This study also lacked multiple consecutive measurements for TRAIL. The mechanism and pathophysiological process throughout the pulmonary embolism need to be further explored and validated.
5. Conclusions
In conclusion, reduced plasma TRAIL levels predict short-term adverse events in normotensive patients with acute PE. The combination of the 2019 ESC algorithm and TRAIL aids risk stratification to assist physicians in the making of treatment decisions and care of patients.
Author Contributions
H.Y. (Haixu Yu) and W.R. contributed equally as the first authors. Y.L., Z.-C.J. and J.D. conceived the study and its design. Y.L. and J.D. obtained research funding. H.Y. (Hui Yuan), L.X., Y.L., Z.-C.J. and J.D. supervised the conducting of the trial and data collection. J.Y., J.L., K.M. and Z.L. undertook the recruitment of patients and managed the data, including quality control. H.Y. (Haixu Yu), W.R. and J.Y. provided statistical advice on the study design and analyzed the data. H.Y. (Haixu Yu) and W.R. drafted the manuscript. All authors contributed substantially to article revisions and approved the final version for submission. J.D. and Z.-C.J. take responsibility for the paper as a whole. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Natural Science Foundation of China (grant number 81930014) and the Beijing Collaborative Innovative Research Centre for Cardiovascular Diseases, Beijing, China.
Institutional Review Board Statement
This was a prospective study in a single center in China from 2015 to 2017. (NCT 04118634). The study protocol was approved by the Ethics Committee of Beijing Anzhen Hospital (No. 2018048X), and all patients provided written informed consent.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying the results presented in the study are available from Beijing Anzhen Hospital.
Acknowledgments
We would like to thank the Natural Science Foundation and Beijing Collaborative Innovative Research Centre for Cardiovascular Diseases for their funding support to the studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, T.L.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A major contributor to global disease burden. Semin. Thromb. Hemost. 2014, 40, 724–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naess, I.A.; Christiansen, S.C.; Romundstad, P.; Cannegieter, S.C.; Rosendaal, F.R.; Hammerstrom, J. Incidence and mortality of venous thrombosis: A population-based study. J. Thromb. Haemost. 2007, 5, 692–699. [Google Scholar] [CrossRef]
- Alotaibi, G.S.; Wu, C.; Senthilselvan, A.; McMurtry, M.S. Secular Trends in Incidence and Mortality of Acute Venous Thromboembolism: The AB-VTE Population-Based Study. Am. J. Med. 2016, 129, 879.e19–879.e25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lei, J.; Shao, X.; Dong, F.; Wang, J.; Wang, D.; Wu, S.; Xie, W.; Wan, J.; Chen, H.; et al. Trends in Hospitalization and In-Hospital Mortality From VTE, 2007 to 2016, in China. Chest 2019, 155, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimenez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazza, G. Advanced Management of Intermediate- and High-Risk Pulmonary Embolism: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 76, 2117–2127. [Google Scholar] [CrossRef]
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A.; et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Gochuico, B.R.; Zhang, J.; Ma, B.Y.; Marshak-Rothstein, A.; Fine, A. TRAIL expression in vascular smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 278, L1045–L1050. [Google Scholar] [CrossRef]
- Tecchio, C.; Huber, V.; Scapini, P.; Calzetti, F.; Margotto, D.; Todeschini, G.; Pilla, L.; Martinelli, G.; Pizzolo, G.; Rivoltini, L.; et al. IFNα-stimulated neutrophils and monocytes release a soluble form of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on leukemic cells. Blood 2004, 103, 3837–3844. [Google Scholar] [CrossRef] [Green Version]
- Simoncini, S.; Njock, M.S.; Robert, S.; Camoin-Jau, L.; Sampol, J.; Harle, J.R.; Nguyen, C.; Dignat-George, F.; Anfosso, F. TRAIL/Apo2L mediates the release of procoagulant endothelial microparticles induced by thrombin in vitro: A potential mechanism linking inflammation and coagulation. Circ. Res. 2009, 104, 943–951. [Google Scholar] [CrossRef]
- Secchiero, P.; Corallini, F.; Ceconi, C.; Parrinello, G.; Volpato, S.; Ferrari, R.; Zauli, G. Potential prognostic significance of decreased serum levels of TRAIL after acute myocardial infarction. PLoS ONE 2009, 4, e4442. [Google Scholar] [CrossRef] [PubMed]
- Niessner, A.; Hohensinner, P.J.; Rychli, K.; Neuhold, S.; Zorn, G.; Richter, B.; Hulsmann, M.; Berger, R.; Mortl, D.; Huber, K.; et al. Prognostic value of apoptosis markers in advanced heart failure patients. Eur. Heart J. 2009, 30, 789–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, B.; Koller, L.; Hohensinner, P.J.; Zorn, G.; Brekalo, M.; Berger, R.; Mortl, D.; Maurer, G.; Pacher, R.; Huber, K.; et al. A multi-biomarker risk score improves prediction of long-term mortality in patients with advanced heart failure. Int. J. Cardiol. 2013, 168, 1251–1257. [Google Scholar] [CrossRef]
- Lankeit, M.; Jimenez, D.; Kostrubiec, M.; Dellas, C.; Hasenfuss, G.; Pruszczyk, P.; Konstantinides, S. Predictive value of the high-sensitivity troponin T assay and the simplified Pulmonary Embolism Severity Index in hemodynamically stable patients with acute pulmonary embolism: A prospective validation study. Circulation 2011, 124, 2716–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanni, S.; Jimenez, D.; Nazerian, P.; Morello, F.; Parisi, M.; Daghini, E.; Pratesi, M.; Lopez, R.; Bedate, P.; Lobo, J.L.; et al. Short-term clinical outcome of normotensive patients with acute PE and high plasma lactate. Thorax 2015, 70, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Liu, Z.; Lu, J.; Yang, X.; Yan, X.X.; Mi, Y.; Hua, L.; Li, Y.; Jing, Z.C.; Du, J. Lipocalin-2 Predicts Long-Term Outcome of Normotensive Patients with Acute Pulmonary Embolism. Cardiovasc. Toxicol. 2020, 20, 101–110. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Hellenkamp, K.; Schwung, J.; Rossmann, H.; Kaeberich, A.; Wachter, R.; Hasenfuß, G.; Konstantinides, S.; Lankeit, M. Risk stratification of normotensive pulmonary embolism: Prognostic impact of copeptin. Eur. Respir. J. 2015, 46, 1701–1710. [Google Scholar] [CrossRef] [Green Version]
- Hellenkamp, K.; Pruszczyk, P.; Jimenez, D.; Wyzgal, A.; Barrios, D.; Ciurzynski, M.; Morillo, R.; Hobohm, L.; Keller, K.; Kurnicka, K.; et al. Prognostic impact of copeptin in pulmonary embolism: A multicentre validation study. Eur. Respir. J. 2018, 51, 1702037. [Google Scholar] [CrossRef] [Green Version]
- Nash, M.; McGrath, J.P.; Cartland, S.P.; Patel, S.; Kavurma, M.M. Tumour necrosis factor superfamily members in ischaemic vascular diseases. Cardiovasc. Res. 2019, 115, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Harper, E.; Forde, H.; Davenport, C.; Rochfort, K.D.; Smith, D.; Cummins, P.M. Vascular calcification in type-2 diabetes and cardiovascular disease: Integrative roles for OPG, RANKL and TRAIL. Vascul. Pharmacol. 2016, 82, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Forde, H.; Harper, E.; Davenport, C.; Rochfort, K.D.; Wallace, R.; Murphy, R.P.; Smith, D.; Cummins, P.M. The beneficial pleiotropic effects of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) within the vasculature: A review of the evidence. Atherosclerosis 2016, 247, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Auria, F.; Centurione, L.; Centurione, M.A.; Angelini, A.; Di Pietro, R. Tumor Necrosis Factor Related Apoptosis Inducing Ligand (Trail) in endothelial response to biomechanical and biochemical stresses in arteries. J. Cell. Biochem. 2015, 116, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Ther. 2018, 182, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Ferrucci, L.; Secchiero, P.; Corallini, F.; Zuliani, G.; Fellin, R.; Guralnik, J.M.; Bandinelli, S.; Zauli, G. Association of tumor necrosis factor-related apoptosis-inducing ligand with total and cardiovascular mortality in older adults. Atherosclerosis 2011, 215, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Osmancik, P.; Teringova, E.; Tousek, P.; Paulu, P.; Widimsky, P. Prognostic value of TNF-related apoptosis inducing ligand (TRAIL) in acute coronary syndrome patients. PLoS ONE 2013, 8, e53860. [Google Scholar] [CrossRef] [Green Version]
- Ajala, O.; Zhang, Y.; Gupta, A.; Bon, J.; Sciurba, F.; Chandra, D. Decreased serum TRAIL is associated with increased mortality in smokers with comorbid emphysema and coronary artery disease. Respir. Med. 2018, 145, 21–27. [Google Scholar] [CrossRef]
- Di Bartolo, B.A.; Cartland, S.P.; Prado-Lourenco, L.; Griffith, T.S.; Gentile, C.; Ravindran, J.; Azahri, N.S.; Thai, T.; Yeung, A.W.; Thomas, S.R.; et al. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Promotes Angiogenesis and Ischemia-Induced Neovascularization Via NADPH Oxidase 4 (NOX4) and Nitric Oxide-Dependent Mechanisms. J. Am. Heart Assoc. 2015, 4, e002527. [Google Scholar] [CrossRef] [Green Version]
- Cartland, S.P.; Genner, S.W.; Zahoor, A.; Kavurma, M.M. Comparative Evaluation of TRAIL, FGF-2 and VEGF-A-Induced Angiogenesis In Vitro and In Vivo. Int. J. Mol. Sci. 2016, 17, 2025. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Xiang, G.; Lu, J.; Xiang, L.; Dong, J.; Mei, W. TRAIL protects against endothelium injury in diabetes via Akt-eNOS signaling. Atherosclerosis 2014, 237, 718–724. [Google Scholar] [CrossRef]
- Manuneedhi Cholan, P.; Cartland, S.P.; Dang, L.; Rayner, B.S.; Patel, S.; Thomas, S.R.; Kavurma, M.M. TRAIL protects against endothelial dysfunction in vivo and inhibits angiotensin-II-induced oxidative stress in vascular endothelial cells in vitro. Free Radic. Biol. Med. 2018, 126, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Michowitz, Y.; Goldstein, E.; Roth, A.; Afek, A.; Abashidze, A.; Ben Gal, Y.; Keren, G.; George, J. The involvement of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in atherosclerosis. J. Am. Coll. Cardiol. 2005, 45, 1018–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bova, C.; Sanchez, O.; Prandoni, P.; Lankeit, M.; Konstantinides, S.; Vanni, S.; Jimenez, D. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur. Respir. J. 2014, 44, 694–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, O.; Trinquart, L.; Caille, V.; Couturaud, F.; Pacouret, G.; Meneveau, N.; Verschuren, F.; Roy, P.M.; Parent, F.; Righini, M.; et al. Prognostic factors for pulmonary embolism: The prep study, a prospective multicenter cohort study. Am. J. Respir. Crit. Care Med. 2010, 181, 168–173. [Google Scholar] [CrossRef]
- Lankeit, M.; Friesen, D.; Schafer, K.; Hasenfuss, G.; Konstantinides, S.; Dellas, C. A simple score for rapid risk assessment of non-high-risk pulmonary embolism. Clin. Res. Cardiol. 2013, 102, 73–80. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).